Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.10 no.56 México nov./dic. 2019 Epub 30-Abr-2020
https://doi.org/10.29298/rmcf.v10i56.567
Review article
Response mechanisms to abiotic stress: towards a forest species perspective
1Centro Nacional de Investigación Disciplinaria en Conservación y Mejoramiento de Ecosistemas Forestales, INIFAP, México.
Abiotic stress is the leading threat to the productivity and survival of the most important crops and forest ecosystems worldwide, as current climate change patterns indicate likely increases in the severity and frequency of extreme weather events such as drought, water and soil salinity, and extreme temperatures. Through evolution, plants have developed diverse molecular, morphological and physiological mechanisms to respond to adverse environments. Understanding such mechanisms is essential to develop and apply conservation and breeding strategies for plant species, with the larger aim of protecting biodiversity and producing tolerant/resistant organisms capable of overcoming abiotic stress conditions. However, most of the available knowledge about stress recognition, signaling and response in plants come from the study of annual species. Even though there are some studies regarding forest taxa like Populus, Eucalyptus, Picea, or Pinus, Mexican species are absent from the literature. With its vast biological richness and diversity, Mexico is a privileged country; nevertheless, the lack of knowledge regarding the susceptibility of its forest resources is the main obstacle to enacting policies oriented towards mitigating the negative consequences of this type of stress.
Key Words: Climate change; drought; forest ecosystems; abiotic stress; salinity; signaling transduction
El estrés de origen abiótico tiene un importante impacto negativo para la productividad y supervivencia de los principales cultivos agrícolas y ecosistemas forestales del mundo. Las tendencias actuales del cambio climático pronostican un aumento en la severidad y frecuencia de fenómenos climáticos y condiciones ambientales adversas como la sequía, salinidad del agua y suelo, así como temperaturas extremas. A lo largo de la evolución, las plantas han desarrollado diversos mecanismos moleculares, morfológicos y fisiológicos para responder a las condiciones desfavorables del entorno. La comprensión de dichos mecanismos es esencial para implementar estrategias de conservación y mejoramiento genético de especies vegetales, a fin de proteger la biodiversidad y producir organismos tolerantes / resistentes al daño ocasionado por el estrés abiótico. Sin embargo, la mayoría de los estudios disponibles sobre identificación, transducción de señales y procesos de respuesta al estrés en plantas deriva de investigaciones en especies anuales. Aunque existen algunos trabajos realizados en taxones forestales de los géneros Populus, Eucalyptus, Picea y Pinus, se carece de estudios dedicados a especies mexicanas. Por su alta riqueza y diversidad biológica, México es un país privilegiado; no obstante, la falta de conocimiento sobre la susceptibilidad de sus recursos forestales es el principal obstáculo para diseñar planes de acción orientados a mitigar las consecuencias perjudiciales del estrés abiótico.
Palabras clave Cambio climático; deficiencia hídrica; ecosistemas forestales; estrés abiótico; salinidad; transducción de señales
Introduction
Abiotic stress refers to the environmental factors that alter the physiological and metabolic processes of plants (Taiz and Zeiger, 2010). It is considered to be the central cause of the loss of over 50 % of the main crops of agricultural interest in the world (Boyer, 1982; Bray et al., 2000), as well as the reduction of vegetal growth, development and yield (Sah et al., 2016), which affects over 95 % of the agricultural surface (Cramer et al., 2016). This situation is worsened by climate change, which impacts the frequency of fires, the rapid increase in the temperature, and disturbances in the rain precipitation patterns, among other factors (IPCC, 2007).
Consequently, the genotypes and species that are sensitive to these fluctuations may disappear and be replaced by other taxa (Alfaro et al., 2014). Such is the case of Oreomunnea mexicana (Standl.) J. F. Leroy, which is a relict species (Palacios-Chávez and Rzendowski, 1993) classified as threatened (González-Espinosa et al., 2011). Its potential distribution was predicted for several different climate change scenarios that suggested a reduction of 36 to 55 % of its habitat, as its ecological niche is highly specialized and sensitive to environmental changes (Alfonso-Corrado et al., 2017).
The incidence of adverse environmental conditions involves significant changes in the net productivity of ecosystems (Thornley and Cannell, 1996; Wang et al., 2012). The forest biomes and their communities and dependent industries do not escape the effects of such disturbances (Hof et al., 2017), as they influence the distribution and the growth rate of forest species (Hamann and Wang, 2006).
The forests provide timber and non-timber products, as well as an important diversity of environmental services, such as the regulation of the water cycle and the biochemical cycles, and the control of the soil erosion and soil formation, among others (Ninan and Inoue, 2013). As for the former, the total value of the global exportation of forest products has been estimated in 262 billion USD (FAOSTAT, 2018). Nevertheless, many of the ecosystem services do not have a market, and therefore their economic value cannot be estimated (Pearce, 2001).
Forest ecosystems occupy nearly 30 % of the earth’s land surface, i.e. 42 million km2 (Bonan, 2008); for this reason, their management and conservation is an essential strategy for mitigating the effects of climate change (Schimel et al., 2001), as the trees and woody plants work as primary carbon (C) sequestration mechanisms (Thomas and Martin, 2012), capturing the atmospheric C and storing it in the trunk, branches and leaves, as part of their growth process (Justine et al., 2015). Water deficiency, salinity, and extreme temperatures are the main abiotic factors that limit the survival and productivity of tree species (Choat et al., 2012; Anderegg et al., 2013; Harfouche et al., 2014).
Furthermore, the negative consequences of abiotic stress are expected to worsen due to climate change (Fedoroff et al., 2010). For example, one of the main causes of mortality and of reduced productivity is hydraulic failure during water deficiency, as gas embolisms are generated in the xylem, preventing the efficient flow of water to the leaves for photosynthesis (Choat et al., 2012) and eventually causing death, as in the case of Picea abies (L.)H. Karst. (Solber, 2004), Cedrus atlanticad (Endl.)Manetti ex Carrière (Bentouati, 2008) and Populus tremuloides Michx. (Hogg et al., 2008). For a long list of forest species experiencing mortality due to drought, see Allen et al. (2010).
During the evolutionary process, the plants have developed mechanisms of tolerance and resistance to stress which allow regulatory responses to reestablish cellular homeostasis or act to reduce the harmful effects (Mickelbrat et al., 2015). Stress due to environmental conditions causes a series of reactions in the plants, such as the inhibition of the meristematic growth of aerial structures in order to favor root elongation, disturbances of carbon metabolism (Xiong and Zhu, 2002), detriment in the production of viable pollen (Alqudah et al., 2011), and even dysfunctions in the phenology that might cause asynchronism between the floral development and the availability of pollinizers (Dawson et al., 2001). For example, Cedrela odorata L. is a native species of Mexico and one of the neotropical species with the highest economic importance (Patiño, 1997). Its pollination depends on insects (Cavers et al., 2013), so that an asynchrony with these would result in a reduced production of seeds. However, there are no studies assessing the consequences of this type of alterations in C. odorata.
Knowledge of the effects of abiotic stress is based mainly on studies performed in model species and in agricultural species such as Arabidopsis thaliana (L.) Heynh. (arabidopsis), Nicotiana tabacum L. (tobacco), Oryza sativa L. (rice), Triticum aestivum L. (wheat), Zea mays L. (corn or maize), among others. However, the information about the molecular biology and physiology of forest species under conditions of stress is limited, even non-existent for a large number of species, as in the case of the forest resources of Mexico. In this regard, a small number of species has been the object of studies related to the response to abiotic stress. For example, sensitivity to hydric deficiency was assessed in Pinus engelmannii Carrière and P. lumholtzii Robinson and Fernald of northern Mexico in a range of altitudes through a dendrochronological study for the 1945-2004 period (Bickford et al., 2011). The sensitivity of growth to the drought was determined, particularly at a low altitude, and P. engelmannii turned out to be the species with the least tolerance.
Due to its relevance in commercial forest plantations of the tropical and subtropical region of America, Pinus oocarpa Schiede ex Schltdl, Pinus patula Schiede ex Schltdl and Pinus pseudostrobus Lindl. were subjected to two contrasting hydric regimes in order to assess their tolerance to drought (Flores et al., 2018). However, the three species exhibited a similar response mechanism, i.e. an allometric adjustment of the shoot/root biomass ratio; intraspecies variation was also identified in the phenotypic plasticity of growth in height, biomass of the needles and stem, and specific leaf area. Finally, the difference in the proportions of seedling survival was evidenced: 4 % for P. pseudostrobus, 12 % or P. patula, and 30 % for P. oocarpa.
These studies on Mexican species are valuable; however, they do not address the molecular and physiological origin of the response to stress. Therefore, the objective of this contribution is to present an overview of the signaling processes and the response mechanisms to three types of abiotic stress: water deficiency, high salinity and high temperatures. Emphasis is made on the available knowledge regarding the impact of each one of the types of stress mentioned on forest species.
General mechanisms of recognition, signal transduction, and response to stress in plants
The plants have developed adaptation, as well as defense mechanisms against the damage caused by the environmental conditions; this involves a large diversity of chemical compounds that regulate the transporters and the biochemical reactions, and modulate the gene expression (Tuteja and Sopory, 2008). These mechanisms comprise three phases (Figure 1): the recognition of the stress, the signal transduction, and the response (Biswal et al., 2011).
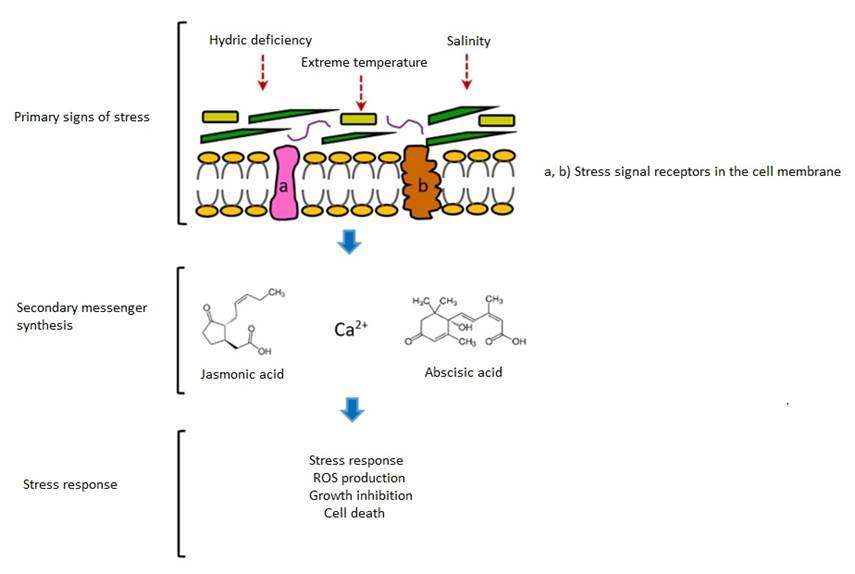
Figure 1 General mechanism of induction of response to abiotic stress in plants. The primary signs of stress are perceived by the receptors of the cell membrane that activate the production of second messengers. This signaling cascade results in the expression of response genes that induce a series of biochemical and physiological changes that will lead to tolerance, adaptation or death.
During the recognition phase, the primary signs of stress are processed via the receptors of the cell membrane (Tuteja et al., 2011). For example, the primary sign for water deficiency is hyperosmolarity, while the primary signs for high salinity are altered osmotic pressure and ionic toxicity (Zhu, 2016). As for high temperature, four putative sensors have been proposed: a plasma membrane channel that triggers an influx of Ca2+, a sensor histone sensor in the nucleus, and two protein sensors in the endoplasmic reticule and the cytosol (Mittler et al., 2012). In general, despite all the resources allotted to the study of this topic, so far only a few potential receptors have been identified (Zhu, 2016), including G protein coupled receptors, ionic channels, kinase-type receptors or histidine kinase (Tuteja, 2007).
Signal transduction (also known as cell signaling or transmembrane signaling) is part of the process whereby the cells communicate with their environment and which allows them to respond to external stimuli through changes in the gene expression (Bradshaw and Dennis, 2010) that will eventually culminate in adaptation or in death (Tuteja, 2007). Thus, the reception of primary signals causes the production of second messengers (Dixit and Jayabaskaran, 2013), which are mainly non-protein molecules such as calcium, cyclic nucleotides, polyphosphoinositides, nitric oxide, sugars, abscisic acid, jasmonic acid, salicylic acid and polyamines (Tuteja and Sopory, 2008). The plants face various types of stress simultaneously, which suggests that the signaling routs interact with one another so that they may be additive, negative or compete for a target (Knight and Knight, 2001).
Vegetal hormones intervene not only in the regulation of vegetal growth and development, but also in signal transduction to respond to the environmental conditions (Sreenivasulu et al., 2012). In this sense, abscisic acid (ABA) is the most important (Hadiarto and Tran, 2011); Sreenivasulu et al., 2012; Sah et al., 2016), and therefore it is also known as the stress hormone (Mehrotra et al., 2014). The relevance of this phytoregulator is such that the stress response mechanisms have been classified into two large groups: ABA-dependent and ABA-independent (Chinnusamy et al., 2004). In general, ABA regulates the increase of transcription factors, kinases and phosphatases that confer adaptation or tolerance to various types of abiotic stress (Tuteja, 2007; Sah et al., 2016). Because the ABA is considered to be the main sign of stress response, it is suggested that it must be a central part of the strategies and programs oriented toward the generation of abiotic stress tolerant crops (Dar et al., 2017). Extensive publications describing in detail the importance of ABA in cell signaling may be consulted in Tuteja (2007), Kim (2014) Sah et al. (2016) and Dar et al. (2017).
Another one of the main secondary messengers of abiotic stress is calcium (Ca2+) (Xiong and Zhu, 2002). Once the plant receives a stimulus, there is an immediate increase in the Ca2+ concentration in the cytosol, nucleus or mitochondria (Ranty et al., 2016). The signs of Ca2+ are differentiated as to their spatial location, duration and frequency; therefore, each specific response is known as a signature of calcium; these are detected by means of various proteins, giving continuity to signaling cascades (Yuan et al., 2017). Comprehensive reviews regarding the function of calcium in signal transduction can be consulted in Sanders et al. (2002), Kim et al. (2010), Dixit and Jayabaskaran (2013), and Ranty et al. (2016).
As for the response mechanisms to abiotic stress, these are based on genes grouped into three major categories: 1) genes involved in signaling cascades and transcription control; 2) genes with direct functions of protection of membranes and proteins, and 3) genes involved in ion transport and consumption (Wang et al., 2003). The response depends on the type and duration of the stimulus. Table 1 shows the general mechanisms of response to water deficiency, salinity and high temperature.
Table 1 Main effects of abiotic stress in plants and general mechanisms of response.
| Type of stress | Effects on the plant | Response mechanisms |
|---|---|---|
| Water deficiency | Reduction of the hydric potential, cell dehydration, reduction of cell expansion and of the metabolism, cavitation, ion cytotoxicity, cell death, inhibition of the photosynthesis (Taiz and Zeiger, 2010). | Accumulation of LEA proteins (Welin et al., 1994), especially of dehydrins (Arumingtyas et al., 2013), reduced root elongation, reduced leaf area (Hu and Xiong, 2014), stomatal closure (Froux et al., 2005). |
| High salinity | Reduction of the hydric potential, cell dehydration, ionic toxicity, inhibition of the photosynthesis (Taiz and Zeiger, 2010). | Ionic compartmentalization in vacuoles, reduction of shoot growth, limited ion flow from the roots to the shoots (Munns and Tester, 2008). |
| High temperature | Destabilization of the cell membrane and the cytoskeleton, various proteins and RNA, inhibition of the photosynthesis and of respiration, leaf senescence, fruit and flower abortion (Taiz and Zeiger, 2010; Mittler et al., 2012). | Production of antioxidants, accumulation of solutes, induction of mitogen-activated kinase proteins (MAPK), induction of kinase protein cascades (CDPK), chaperone activation (Wahid et al., 2007), accumulation of osmotic protectors, stomatal closure, synthesis of secondary metabolites (Mathur and Jajoo, 2014), thermal shock proteins (Iba, 2002) and salicylic acid (Nazar et al., 2017). |
Water deficiency
Water is essential for the biochemical and molecular functioning of living organisms (Xiong and Zhu, 2002). Water deficiency triggers a series of ion, mechanical and osmotic signals recognized by various receptors (Hamisch et al., 2016). In general, cell dehydration, alteration of mitosis, and reduction of metabolism, of the cell volume and of turgidity are observed, and so is a negative hydric potential in the apoplast and a high ionic concentration that can turn out to be cytotoxic (Taiz and Zeiger, 2010; Farooq et al., 2009). One of the main processes affected by hydric stress is photosynthesis. A reduction in the production of biomass, reduced leaf expansion, inefficiency of the function of chloroplasts (Wahid et al., 2007), a low diffusion of CO2 (Pinheiro and Chaves, 2001), and a reduction of the net photosynthesis and of stomatal conductance have been observed (Mena-Petite et al., 2000; Bigras, 2005). Furthermore, a reduction of the leaf area and the total number of leaves, of transpiration, of intercellular CO2 concentration, and of the carboxylation efficiency are also observed in Populus cathayana Rehder (Xu et al., 2008).
Two of the main molecule groups involved in water deficiency resistance and response are LEA (late-embryogenesis abundant) proteins and the growth hormone, abscisic acid (ABA). LEA proteins were discovered in terrestrial plants (Hand et al., 2010) and owe their name to having been identified during the last stages of development of the seeds (Pedrosa et al., 2015). Their main functions are tolerance to dehydration and protection of seminal viability during ex situ storage, as well as resistance to stress due to drought, salinity and cold, as they stabilize other proteins and cell membranes and prevent protein aggregation during stress periods (Close, 1996; Goyal et al., 2005; Hong-Bo et al., 2005).
The genes codifying for the synthesis of LEA proteins are a broad family that has been extensively studied in angiosperms, particularly in those of agricultural interest, of which the only evaluated forest species is Populus trichocarpa Torr. & A.Gray ex Hook. (Lan et al., 2013). However, in relation to gimnosperms, only a few partial studies have been carried out on groups of proteins of this family in Picea glauca (Moench) Voss (Jin-Zhuo and David, 1998; Sena et al., 2017); Pseudotsuga menziessi (Mir.)Franco (Jarvis et al., 1996), Pinus pinaster Aiton (Perdiguero et al., 2014) and Pinus tabuliformis Carrière (Gao and Ting, 2016).
Dehydrins belong to the LEA protein group and accumulate in various ways, according to the type and intensity of the stress in ripe seeds or vegetal vegetative tissues in response to dehydration, salinity, cold and freezing (Close, 1996; Tunnacliffe and Wise, 2007). 41 complete dehydrin codifying sequences were identified in Picea glauca by Sena et al. (2017); these represent more than four times the number of sequences found in angiosperms, and even more times than those found in the species of the genus Pinus studied so far.
The morphological responses to hydric deficiency include the following: leaf cuticular waxes occur on the surface of all terrestrial plants (Jetter et al., 2006) and is one of the first protective barriers against various biotic and abiotic factors (Shepherd and Griffiths, 2006). The increase and change of composition of the cuticular wax under adverse environmental circumstances has been evident in several edible species like wheat, alfalfa and sweet peas (Sánchez et al., 2001; Aharoni et al., 2004; Zhang et al., 2005; Kosma et al., 2009; Seo et al., 2011; Lee and Suh, 2015; Zhang et al., 2013; Zhang et al., 2015). As for forest species, the comparative study of six conifer species ―Picea engelmannii Engelm., Abies lasiocarpa (Hook.) Nutt., Pinus contorta Douglas ex Loudon, Pseudotsuga menziesii, Pinus ponderosa Douglas ex Lawson and Pinus flexilis E.James― is prominent, and although this study does not identify the relationship between the hardness and the composition of the cuticle with resistance to dehydration, it does determine the level of tolerance to drought of each of these taxa (Hadley and Smith, 1990).
Radicular system also responds to hydric stress; for example, in Pinus pinaster, two provenances with differential tolerance to hydric stress were subjected to a high osmotic potential (0.8 MPa) that resulted in a quicker and longer root elongation in the drought-tolerant population than in the susceptible population (Nguyen and Lamant, 1989). This response mechanism is induced by ABA and allows exploring adjoining sites in search of water, as has been observed in corn (Spollen et al., 2000; Sharp et al., 2002).
ABA is the most important plant hormone in signal transduction related to drought stress (Harfouche et al., 2014). One of its functions is the stimulation of stomatal closure in order to reduce water loss due to transpiration (Kim et al., 2010). Stomata consist of two guard cells that detect high levels of ABA, and which therefore reduce their volume and turgidity by eliminating potassium ions and anions (MacRobbie, 1998). In addition, ABA induces the overexpression of nearly 50 % genes associated with the cuticle (Jenks et al., 2007). However, the relationship between the molecular mechanisms of cuticular wax and ABA is yet to be clarified (Xue et al., 2017).
Ionic stress and hyperosmolarity due to hydric deficiency also cause oxidative damage as a result of the excess of reactive oxygen species (ROS) (Xiong and Zhu, 2002). ROS play an important role in plant signaling and are involved in growth, development and response to biotic and abiotic stress, as well as in programmed cell death (Bailey-Serres and Mittler, 2006). Nevertheless, in high concentrations, ROS damage the cell lipids, carbohydrates, proteins and DNA (Das and Roychoudhury, 2014). In most cases, defense against ROS depends on antioxidants such as ascorbic acid, glutathione, thioredoxin and carotenoids, as well as on enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), and guaiacol peroxidase (GPX) (Xiong and Zhu, 2002; Das and Roychoudhury, 2014).
Salinity
Soil salinity is a growing problem for crops across the world, especially on irrigated land (Negrão et al., 2017). The main consequence is yield reduction, as salt alters osmolarity or promotes ion toxicity within vegetal tissues. In such circumstances, plants respond quickly, by readjusting the osmotic potential, or slowly, by reducing salt toxic concentrations in their cells (Munns and Tester, 2008). Adaptation mechanisms in response to salinity have three purposes: to produce tolerance to osmotic stress, to avoid ion excess (particularly Na+) in plant tissues, and to increase tissues tolerance to ion accumulation (Munns and Tester, 2008). These can complement one another and vary the responses, depending on stress severity and on the affected species.
Due to its ability to survive high concentrations of NaCl, Populus euphratica Oliv. has served as a model for studies to elucidate the physiological and molecular mechanisms involved in tree species tolerance to saline stress. Cl- compartmentalization in the vacuoles of the root tissue cells or Na+ in the leaf apoplast; diminution of the NaCl levels in the xylem, and prevention of K+ loss through ion channels in order to maintain the ion balance are examples of the physiological mechanisms of this species (Chen and Polle, 2010). Genomic studies suggest that P. euphratica tolerance to salinity is the result of duplication and/or positive regulation of several families of genes involved in ion transport and maintenance of the homeostasis (Ma et al., 2013).
The best studied example is the HKT1 gene family, whose proteins participate in Na+ transport in different species, functioning as Na+/K+ cotransporters and as high- and low-affinity selective Na+ transporters (Davenport et al., 2007). P. euphratica has four HKT1 paralogues within its genome, unlike P. trichocarpa, which has only one (Ma et al., 2013).
Many of the genes related to saline stress include different proteins that participate in the uptake and transport of salts. However, some genes have an osmoprotective function and antioxidant enzymes, while others are involved in growth and development, including transcription factors, kinase proteins, phosphatase proteins or signaling molecules, such as calmodulin-binding proteins and certain ABA-induced proteins (Munns, 2005).
The technology of recombinant DNA allows evaluating the potential utility of certain genes to confer tolerance to salinity in forest species; an example of these is Pinus taeda L., in which the overexpression of osmoregulatory enzymes increases the survival rate under high salinity conditions (Tang et al., 2005). Tolerance to saline stress has been successfully increased in Populus through the transfer of a transcription factor in this species’ response to ethylene, which plays a role in growth and development of the plant (Li et al., 2009).
Thermal stress (high temperature)
An increase of 0.85 °C in the combined global mean temperature of the land and ocean surfaces was registered between 1880 and 2012 (IPCC, 2014), and an increase of 0.2 to 0.3 °C per decade has been predicted for the next years (Jones et al., 1999; Fahad et al., 2017). Increases of 10-15 °C in the ambient temperature are regarded as thermal stress or shock, generally causing the denaturalization and aggregation of proteins and an increased fluidity of membrane lipids (Wahid et al., 2007), as well as a disturbance in the organization of the microtubules and in cell division (Smertenko et al., 1997), alterations in photosynthesis and in the primary and secondary metabolism of lipids (Xu et al., 2006), and, therefore, delayed development and growth, or death (Mathur and Jajoo, 2014). For a comprehensive review of signaling and response to high temperatures, we recommend consulting Wahid et al. (2007), Mittler et al. (2012) and Wang et al. (2017).
Although accurate mechanisms for heat detection and signaling in plants are still unknown, molecules such as proteins bound to guanosine triphosphate, nucleoside diphosphate kinase, annexin and brassinosteroid kinases are known to participate in signal transduction (Wang et al., 2017).
Photosynthesis is one of the main metabolic processes affected by high temperatures (Farooq et al., 2009), as these alter photosystem II and the electron transport chains, disrupt the tilacoidal membrane, and inhibit the b6/f cytochrome complex, ribulose-1,5-biphosphate carboxylase/oxygenase, ATP synthesis, and carbon fixation (Biswal et al., 2011; Ashraf and Harris 2013; Mathur and Jajoo, 2014). This may account for limited growth of various species and for early senescence, as has been observed in sugar cane (Saccharum officinarum L.) (Ebrahim et al., 1998). In Picea glauca, temperatures of 42-43 °C impede photophosphorylation by tilacoids and to supply water to photosystem II, while even higher temperatures (44-46 °C) drastically reduce the use of NADPH and ATP in the Calvin cycle (Bigras, 2005).
Root system is more sensitive to heat than certain aerial parts, as its optimal level of growth is 5 to 6 degrees lower (DiPaola, 1992; Paulsen, 1994). For this reason, heat negatively affects the hydraulic conductivity of the root system (Morales et al., 2003), as well as the capacity to uptake and assimilate nutrients (Giri et al., 2017), especially nitrogen, phosphorus and potassium (Fahad et al., 2017). Balance, stability, concentration, biosynthesis and compartmentalization of plant growth hormones are altered under high heat conditions (Maestri et al., 2002). As in the case of water deficiency, extreme temperature results in ROS release (Wang et al., 2017), and the previously mentioned enzyme and non-enzyme mechanisms act in response to damage caused by these free radicals.
In response to high temperature, the main strategy of plants is the synthesis of heat shock proteins (HSP) (Iba, 2002) that confer membrane stability, efficiency in water and nutrients use efficiency, and protect of the photosynthetic apparatus (Camejo et al., 2005; Ahn and Zimmerman, 2006; Momcilovic and Ristic, 2007; Horváth et al., 2008). The HSPs were identified in heat stress conditions; however, today they are known to participate in response to biotic stress (Yu et al., 2016), cold, dehydration, UV light, salinity, heavy metals, and mechanic damage (Swindell et al., 2007). In addition to HSPs, there are other groups of compounds that intervene in the response to heat shock and which have very diverse origins, such as cytokinins, fatty acids, terpenoids, and flavonoids ―the metabolic routes that most influence the response to high temperature in Pinus radiata (Escandón et al., 2018)―, as well as giberelines, ethylene and brassinosteroids (Clarke et al., 2009; Zhang and Wang, 2011; Dubois et al., 2018).
Final considerations
Adaptation to abiotic stress is controlled by molecular networks that trigger a series of morphological and physiological responses. Research on the processes involved in the adaptation and resistance to adverse conditions in forests species advances slowly, as they are highly complex and involve the long life cycles and large genomes. Given the scarcity of information about tree species, it is impossible to formulate a complete overview of the above processes in this group of organisms. However, the constant development of omic tools provides an opportunity to study the functioning of these living beings in an integrative way for the purpose of describing, elucidating, and understanding such processes.
Several authors consider that the mechanisms of response, tolerance and resistance to abiotic stress are difficult to control and utilize in genetic improvement programs, as the response processes are genetically complex. Nevertheless, characterization and understanding of molecular mechanisms and stress physiology will make it possible to explore strategies for achieving genetic improvement through the use of characteristics and organisms with an ecological and productive value, which must necessarily include the main Mexican forest species.
Referencias
Aharoni, A., S. Dixit, R. Jetter, E. Thoenes, G. Van Arkel and A. Pereira. 2004. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16: 2463-2480. Doi: 10.1105/tpc.104.022897. [ Links ]
Ahn, Y. J. and J. L. Zimmerman. 2006. Introduction of the carrot HSP17.7 into potato (Solanum tuberosum L.) enhances cellular membrane stability and tuberization in vitro. Plant CellEnvironment 29: 95-104. Doi: 10.1111/j.1365-3040.2005.01403.x. [ Links ]
Alfaro, R. I., B. Fady, G. G. Vendramin, I. K. Dawson, R. A. Fleming, C. Sáenz-Romero, R. A. Lindig-Cisneros, T. Murdock, B. Vinceti, C. M. Navarro, T. Skroppa, G. Baldinelli, Y. A. El-Kassaby and J. Loo. 2014. The role of forest genetic resources in responding to biotic and abiotic factors in the context of anthropogenic climate change. Forest Ecology and Management 333: 76-87. Doi: 10.1016/j.foreco.2014.04.006. [ Links ]
Alfonso-Corrado, C., F. Naranjo-Luna, R. Clark-Tapia, J. E. Campos, O. R. Rojas-Soto, M. D. Luna-Krauletz, B. Bodenhorn, M. Gorgonio-Ramírez and N. Pacheco-Cruz. 2017. Effects of Environmental Changes on the Occurrence of Oreomunnea mexicana (Juglandaceae) in a Biodiversity Hotspot Cloud Forest. Forests 8: 261. Doi: 10.3390/f8080261. [ Links ]
Alqudah, A. M., N. H. Samarah and R. E. Mullen. 2011. Chapter 6 - Drought stress effects on crop pollination, seed set, yield and quality. In: Lichtfouse, E. (ed.). Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation, Sustainable Agriculture Reviews 6. Springer. Netherlands. 354 p. [ Links ]
Allen, C. D., A. K. Macalady, H. Chenchouni, D. Bachelet, N. Mcdowell, M. Vennetier, T. Kitzberger, A. Rigling and D. D. Breshears. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259 (4): 660-p. 684. Doi: 10.1016/j.foreco.2009.09.001ff. ffhal-00457602f. [ Links ]
Anderegg, W. R. L., J. M. Kane and L. D. L. Anderegg. 2013. Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change 3: 30-36. Doi:10.1038/NCLIMATE1635. [ Links ]
Arumingtyas, E. L., E. S. Savitri and R. D. Purwoningrahayu. 2013. Protein profiles and Dehydrin accumulation in some soybean varieties (Glycine max L. Merr) in drought stress conditions. American Journal of Plant Science 4:134-41. Doi: 10.4236/ajps.2013.41018. [ Links ]
Ashraf, M. and P. J. C. Harris. 2013. Photosynthesis under stressful environments: An overview. Photosynthetica 51: 163-190. Doi: 10.1007/s11099-013-0021-6. [ Links ]
Bailey-Serres, J. and R. Mittler. 2006. The Roles of Reactive Oxygen Species in Plant Cells. Plant Physiology 141: 311. Doi: 10.1104/pp.104.900191. [ Links ]
Bentouati, A. 2008. La situation du cèdre de l’Atlas en Algérie. Forêt Méditerranéenne 29: 203-209. [ Links ]
Bickford, I. N., P. Z. Fulé and T. E. Kolb. 2011. Growth sensitivity to drought of co-occurring Pinus spp. along an elevation gradient in northern Mexico. Western North American Naturalist 71 (3): 338-348. Doi: 10.3398/064.071.0302. [ Links ]
Bigras, F. J. 2005. Photosynthetic response of white spruce families to drought stress. New Forests 29 (2): 135-148. Doi: 10.1007/s11056-005-0245-9. [ Links ]
Biswal, B., P. N. Joshi, M. K. Raval and U. C. Biswal. 2011. Photosynthesis, a global sensor of environmental stress in green plants: stress signaling and adaptation. Current Science 101 (1): 47-56. [ Links ]
Bonan, G. B. 2008. Forestsand climate change: Forcings, feedbacks, and the climate benefits of forests. Science 320: 1444-1449. Doi: 10.1126/science.1155121. [ Links ]
Boyer, J. S. 1982. Plant productivity and environment. Science 218: 443-448. Doi: 10.1126/science.218.4571.443. [ Links ]
Bradshaw, R. A. and E. A. Dennis. 2010. Chapter 1 - Cell Signaling: Yesterday, Today, and Tomorrow. In: Bradshaw, R. A. and E. A. Dennis (eds.). Handbook of Cell Signaling. 2nd edition. Academic Press. San Diego, CA, USA. 3048 p. [ Links ]
Bray, E. A., J. Bailey-Serres and E. Weretilnyk. 2000. Responses to abiotic stresses. In: Gruissem, W., B. Buchannan and R. Jones (eds.). Biochemistry and molecular biology of plants. American Society of Plant Physiologists. Rockville, MD, USA. pp 1158-1249. [ Links ]
Camejo, D., P. Rodríguez, M. A. Morales, J. M. Dell’amico, A. Torrecillas and J. J. Alarcón. 2005. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. Journal of Plant Physiology 162: 281-289. Doi: 10.1016/j.jplph.2004.07.014. [ Links ]
Cavers, S., A. Telford, F. Arenal Cruz, A. J. Pérez C., R. Valencia, C. Navarro, A. Buonamici and A. J. Lowe. 2013. Cryptic species and phylogeographical structure in the tree Cedrela odorata L. throughout the Neotropics. Journal of Biogeography 40: 732-746. Doi: 10.1111/jbi.12086. [ Links ]
Chen, S. and A. Polle. 2010. Salinity tolerance of Populus. Plant Biology 12:317-333. Doi: 10.1111/j.1438-8677.2009.00301.x. [ Links ]
Chinnusamy, V., K. Schumaker and J. K. Zhu. 2004. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signaling in plants. Journal of Experimental Botany 55:225-236. Doi: 10.1093/jxb/erh005. [ Links ]
Choat, B., S. Jansen, T. J. Brodribb, H. Cochard, S. Delzon, R. Bhaskar, S. J. Bucci, T. S. Field, S. M. Gleason, U. G. Hacke, L. Jacobsen, F. Lens, H. Maherali, J. Martínez-Vilalta, S. Mayr, M. Mencuccini, P. J. Mitchell, A. Nardini, J. Pittermann, R. B. Pratt, J. S. Sperry, M. Westoby, I. J. Wright and A. E. Zanne. 2012. Global convergence in the vulnerability of forests to drought. Nature 491: 752-755. Doi: 10.1038/nature11688. [ Links ]
Clarke, S. M., S. M. Cristescu, O. Miersch, F. J. M. Harren, C. Wasternack and L. A. Mur. 2009. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytologist 182: 175-187. Doi: 10.1111/j.1469-8137.2008.02735.x. [ Links ]
Close, T. J. 1996. Dehydrins: emergence of a biochemical role of a fam ily of plant dehydration proteins. Physiologia plantarum 97:795-803. Doi: 10.1111/j.1399-3054.1996.tb00546.x. [ Links ]
Cramer, G. R., K. Urano, S. Delrot, M. Pezzotti and K. Shinozaki. 2011. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biology 11: 163. Doi: 10.1186/1471-2229-11-163. [ Links ]
Dar, N. A., I. Amin, W. Wani, S. A. Wani, A. B. Shikari, S. H. Wani and K. Z. Masoodi. 2017. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gene 11: 106-111. Doi: 10.1016/j.plgene.2017.07.003. [ Links ]
Das, K. and A. Roychoudhury. 2014. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science 2: 53. Doi: 10.3389/fenvs.2014.00053. [ Links ]
Dawson, I. K., B. Vinceti, J. C. Weber, H. Neufeldt, J. Russell, A. G. Lengkeek, A. Kalinganire, R. Kindt, J. P. B. Lillesø, J. Roshetko and R. Jamnadass. 2011. Climate change and tree genetic resource management: maintaining and enhancing the productivity and value of smallholder tropical agroforestry landscapes. A review. Agroforestry Systems 81: 67:tf. Doi: 10.1007/s10457-010-9302-2. [ Links ]
Davenport, R. J., A. Muñoz-Mayor, D. Jha, P. A. Essah, A. Rus and M. Tester. 2007. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant, Cell and Environment 4:497-507. Doi: 10.1111/j.1365-3040.2007.01637.x. [ Links ]
DiPaola, J. 1992. Physiological responses of turfgrasses to heat stress. In: Waddington, D. V., R. N. Carrow and R.C . Shearman (eds.). Turfgrass. Agronomy Society of America. Madison, WI, USA. pp. 231-262. [ Links ]
Dixit, A. K. and C. Jayabaskaran. 2013. Calcium signaling during abiotic stress in plants: roles of calcium dependent protein kinases. In: Hemantaranjan, A. (ed.). Advances in Plant Physiology. Vol. 14. Scientific Publishers. Jodhpur, India. pp. 348-369. [ Links ]
Dubois, M., Van den Broeck, L. and Inzé, D. 2018. The pivotal role of ethylene in plant growth. Trends in Plant Science 23 (4): 311-323. Doi: 10.1016/j.tplants.2018.01.003. [ Links ]
Ebrahim , M. K., O. Zingsheim, M. N. El-Shourbagy, P. H. Moore and E. Komor. 1998. Growth and sugar storage in sugarcane grown at temperature below and above optimum. Journal of Plant Physiology 153: 593-602. Doi: 10.1016/S0176-1617(98)80209-5. [ Links ]
Escandón, M., M. Meijón, L. Valledor, J. Pascual, G. Pinto and M. J. Cañal. 2018. Metabolome Integrated Analysis of High-Temperature Response in Pinus radiata. Frontiers in Plant Science 9: 485. Doi: 10.3389/fpls.2018.00485. [ Links ]
Fahad, S., A. A. Bajwa, U. Nazir, S. A. Anjum, A. Farooq, A. Zohaib, S. Sadia, W. Nasim, S. Adkins, S. Saud, M. Z. Ihsan, H. Alharby, C. Wu, D. Wang and J. Huang. 2017. Crop production under drought and heat stress: Plant responses and management options. Frontiers in Plant Science 8: 1147. Doi: 10.3389/fpls.2017.01147. [ Links ]
Food and Agriculture Organization of United Nations Statistics (FAOSTAT). 2018. Forestry production and trade database. http: http: http://www.fao.org/faostat/en/#data/FO/visualize (28 de agosto de 2019). [ Links ]
Farooq, M., A. Wahid, N. Kobayashi, D. Fujita and S. M. A. Basra. 2009. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29 (1):185-212. Doi: 10.1051/agro:2008021. [ Links ]
Fedoroff, N. V., D. S. Battisti, R. N. Beachy, P. J. Cooper, D. A. Fischhoff, C. N. Hodges, V. C. Knauf, D. Lobell, B. J. Mazur, D. Molden, M. P. Reynolds, P. C. Ronald, M. W. Rosegrant, P. A. Sanchez, A. Vonshak and J. K. Zhu. 2010. Radically rethinking agriculture for the 21st century. Science 327: 833-834. Doi: 10.1126/science.1186834. [ Links ]
Flores, A., J. Climent, V. Pando, J. López-Upton and R. Alía. 2018. Intraspecific variation in Pines from the Trans-Mexican volcanic belt grown under two watering regimes: implications for management of genetic resources. Forests 9(2): 71. Doi: 10.3390/f9020071. [ Links ]
Froux, F., M. E. Ducrey, Dreyer and R. Huc. 2005. Vulnerability to embolism differs in roots and shoots and among three Mediterranean conifers: consequences for stomatal regulation of water loss? Trees 19:137-144. Doi: 10.1007/s00468-004-0372-5. [ Links ]
Gao, J. and L. Ting. 2016. Functional characterization of the late embryogenesis abundant (LEA) protein gene family from Pinus tabuliformis (Pinaceae) in Escherichia coli. Scientific Reports 6: 19467. Doi: 10.1038/srep19467. [ Links ]
Giri, A., S. Heckathorn, S. Mishra and C. Krause. 2017. Heat stress decreases levels of nutrient-uptake and -assimilation proteins in tomato roots. Plants 6 (81): 6. Doi: 10.3390/plants6010006. [ Links ]
González-Espinosa, M., J. A. Meave, F. G. Lorea-Hernández, G. Ibarra-Manríquez, A. C. Newton. 2011. The Red List of Mexican Cloud Forest Trees. 1st edition, Fauna and Flora International. Cambridge, UK. pp. 1-148. [ Links ]
Goyal, K., L. J. Walton and A. Tunnacliffe. 2005. LEA proteins prevent protein aggregation due to water stress. Biochemical Journal 388:151-157. Doi: 10.1042/BJ20041931. [ Links ]
Hadiarto, T. and L. S. P. Tran. 2011. Progress studies of drought-responsive genes in rice. Plant Cell Reports 30: 297-310. Doi: 10.1007/s00299-010-0956-z. [ Links ]
Hadley, J. L. and W. K. Smith. 1990. Influence of leaf Surface wax and leaf area to water content ratio on cuticular transpiration in western conifers, U.S.A. Canadian Journal of Forest Research 20: 1306-1311. Doi: 10.1139/x90-173. [ Links ]
Hamann, A. and T. Wang. 2006. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 87 (11): 2773-2786. Doi: 10.1890/0012-9658(2006)87[2773:PEOCCO]2.0.CO;2. [ Links ]
Hamisch, D., D. Kaufholdt, J. C. Kuchernig, F. Bittner, R. R. Mendel, R. Hänsch and J. Popko. 2016. Transgenic Poplar Plants for the Investigation of ABA-Dependent Salt and Drought Stress Adaptation in Trees. American Journal of Plant Sciences 7: 1337-1356. Doi: 10.4236/ajps.2016.79128. [ Links ]
Hand, S. C., M. A. Menze, M. Toner, L. Boswell and D. Moore. 2010. LEA Proteins During Water Stress: Not Just for Plants Anymore. Annual Review of Physiology 73 (1): 115-134. Doi: 10.1146/annurev-physiol-012110-142203. [ Links ]
Harfouche, A., R. Meilan and A. Altman. 2014. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiology 34: 1181-1198. Doi: 10.1093/treephys/tpu012. [ Links ]
Hof, A., C. C. Dymond and D. J. Mladenoff. 2017. Climate change mitigation through adaptation: the effectiveness of forest diversification by novel tree planting regimes. Ecosphere 8 (11): e01981.10.1002/ecs2.1981. [ Links ]
Hogg, E. H., J. P. Brandt and M. Michaellian. 2008. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestière 38: 1373-1384. Doi:10.1139/X08-001. [ Links ]
Hong-Bo, S., L. Zong-Suo and S. Ming-An. 2005. LEA proteins in higher plants: Structure, function, gene expression and regulation. Colloids and surfaces 45: 131-135. Doi: 10.1016/j.colsurfb.2005.07.017. [ Links ]
Horváth, I., G. Multhoff, A. Sonnleitner and L. Vígh. 2008. Membrane-associated stress proteins: More than simply chaperones. Biochimica et Biophysica Acta 1778: 1653-1664. Doi:10.1016/j.bbamem.2008.02.012. [ Links ]
Hu, H. and L. Xiong. 2014. Genetic engineering and breeding of drought-resistant crops. Annual Review of Plant Biology 65: 715-741. Doi: 10.1146/annurev-arplant-050213-040000. [ Links ]
Iba, K. 2002. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annual Review of Plant Biology 53: 225-245. Doi: 10.1146/annurev.arplant.53.100201.160729. [ Links ]
Intergovernmental Panel on Climate Change (IPCC). 2007. Climate change 2007. In: Pachauri, R. K. and A. Reisinger. (eds.). Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC. Geneva, Switzerland. 104 p. [ Links ]
Intergovernmental Panel on Climate Change (IPCC). 2014. Climate Change 2014: Synthesis Report. In: Core Writing Team, R. K. Pachauri and L. A. Meyer (eds.). Proceedings of the Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland. 151 p. [ Links ]
Jarvis, S. B., M. A. Taylor, M. R. Macleod and H. V. David. 1996. Cloning and characterization of the cDNA clones of three genes that are differentially expressed during dormancy-breakage in the seeds of Douglas fir (Pseudotsuga menziesii). Journal of Plant Physiology 147: 559-566. Doi: 10.1016/S0176-1617(96)80046-0. [ Links ]
Jenks, M. A., P. M. Hasegawa, S. M. Jain, D. K. Kosma and M. A. Jenks. 2007. Eco-physiological and molecular-genetic determinants of plant cuticle function in drought and salt stress tolerance. In: Jenks, M. A., P. M. Hasegawa and S. M. Jain (eds.). Advances in Molecular Breeding toward Drought and Salt Tolerant Crops. Springer. Dordrecht, The Netherlands. pp. 91-120. [ Links ]
Jetter, R., L. Kunst and A. L. Samuels. 2006. Composition of plant cuticular waxes. In: Riederer, M. and C. Müller (eds.). Biology of the Plant Cuticle. Annual Plant Reviews. Vol. 23. Blackwell Publishing. Oxford, UK. pp 145-181. [ Links ]
Jin-Zhuo, D. and I. D. David. 1998. Cloning and characterization of six embryogenesis-associated cDNAs from somatic embryos of Picea glauca and their comparative expression during zygotic embryogenesis. Plant Molecular Biology 39: 859-864. Doi: 10.1023/A:1006146622614. [ Links ]
Jones, P. D., M. New, D. E. Parker, S. Martin and I. G. Rigor. 1999. Surface air temperature and its changes over the past 150 years. Reviews of Geophysics 37: 173-199. Doi: 10.1029/1999RG900002. [ Links ]
Justine, M. F., W. Yang, F. Wu, B. Tan, M. N. Khan and Z. Yeyi. 2015. Biomass stock and carbon sequestration in a chronosequence of Pinus massoniana plantations in the upper reaches of the Yangtze River. Forests 6:3665-3682. Doi: 10.3390/f6103665. [ Links ]
Kim, T. H. 2014. Mechanism of ABA signal transduction: agricultural highlights for improving drought tolerance. Journal of Plant Biology 57: 1-8. Doi: 10.1007/s12374-014-0901-8. [ Links ]
Kim, T. H., M. Böhmer, H. Hu, N. Nishimura and J. I. Schroeder. 2010. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annual Reviews in Plant Biology 61: 561-591. Doi: 10.1146/annurev-arplant-042809-112226. [ Links ]
Knight, H. and Mr. Knight. 2001. Abiotic stress signalling pathways: specificity and cross-talk. Trends in Plant Science 6 (6): 262-267. Doi: 10.1016/S1360-1385(01)01946-X. [ Links ]
Kosma, D. K., B. Bourdenx, A. Bernard, E. P. Parsons, S. Lu, J. Joubes and M. A. Jenks. 2009. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiology 151: 1918-1929. Doi: 10.1104/pp.109.141911. [ Links ]
Lan, T., J. Gao and Q-Y Zeng. 2013. Genome-wide analysis of the LEA (late embryogenesis abundant) protein gene family in Populus trichocarpa. Tree Genetics and Genomes 9 (1): 253-264. Doi: 10.1007/s11295-012-0551-2. [ Links ]
Lee, S. B. and M. C. Suh. 2015. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Reports 34: 557-572. Doi: 10.1007/s00299-015-1772-2. [ Links ]
Li, Y., X. Su, B. Zhang, Q. Huang, X. Zhang and R. Huang. 2009. Expression of jasmonic ethylene responsive factor gene in transgenic poplar tree leads to increased salt tolerance. Tree Physiology 29(2):273-279. Doi: 10.1093/treephys/tpn025. [ Links ]
Ma, T., J. Wang, J. Wang, G. Zhou, Z. Yue, Q. Hu and L. Jianquan. 2013. Genomic insights into salt adaptation in a desert poplar. Nature Communications 4:2797. Doi: 10.1038/ncomms3797. [ Links ]
MacRobbie, E. A. 1998. Signal transduction and ion channels in guard cells. Philosophical Transactions of the Royal Society of London B: Biological Sciences 353:1475-88. Doi: 10.1098/rstb.1998.0303. [ Links ]
Maestri, E., N. Klueva, C. Perrotta, M. Gulli, H. T. Nguyen and N. Marmiroli. 2002. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Molecular Biology 48: 667-681. Doi: 10.1023/A:1014826730024. [ Links ]
Mathur, S. and A. Jajoo. 2014. Photosynthesis: Response to high temperature stress. Journal of Photochemistry and Photobiology B: Biology 137: 116-126. Doi: 10.1016/j.jphotobiol.2014.01.010. [ Links ]
Mehrotra, R., P. Bhalothia, P. Bansal, M. K. Basantani, V. Bharti and S. Mehrotra. 2014. Abscisic acid and abiotic stress tolerance-differenttiers of regulation. Journal of Plant Physiology 171: 486-496. Doi:10.1016/j.jplph.2013.12.007. [ Links ]
Mena-Petite, A., B. González-Moro, C. González-Murua, M. Lacuesta and R. A. Muñoz. 2000. Sequential Effects of Acidic Precipitation and Drought on Photosynthesis and Chlorophyll Fluorescence Parameters of Pinus radiate D. Don Seedlings. Journal of Plant Physiology 156 (1): 84-92. Doi: 10.1016/S0176-1617(00)80276-X. [ Links ]
Mickelbart, M. V., P. M. Hasegawa and J. Bailey-Serres. 2015. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nature Reviews Genetics 16 (4): 237-251. Doi: 10.1038/nrg3901. [ Links ]
Mittler, R., A. Finka and P. Goloubinoff. 2012. How do plants feel the heat? Trends in Biochemical Sciences 37 (3): 118-125. Doi: 10.1016/j.tibs.2011.11.007. [ Links ]
Momcilovic, I. and Z. Ristic. 2007. Expression of chloroplast protein synthesis elongation factor, EF-Tu, in two lines of maize with contrasting tolerance to heat stress during early stages of plant development. Journal of Plant Physiology 164: 90-99. Doi: 10.1016/j.jplph.2006.01.010. [ Links ]
Morales, D., P. Rodríguez, J. Dell’Amico, E. Nicolás, A. Torrecillas and M. J. Sánchez-Blanco. 2003. High-temperature preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato. Biologia Plantarum 47 (2): 203-208. Doi: 10.1023/B:BIOP.0000022252.70836.fc. [ Links ]
Munns, R. 2005. Genes and salt tolerance: Bringing them together. New Phytologist 167(3):645-663. Doi: 10.1111/j.1469-8137.2005.01487.x. [ Links ]
Munns, R. and M. Tester. 2008. Mechanisms of Salinity Tolerance. Annual Review of Plant Biology 59 (1): 651-681. Doi: 10.1146/annurev.arplant.59.032607.092911. [ Links ]
Nazar, R., N. Iqbal and S. Umar. 2017. Heat Stress Tolerance in Plants: Action of Salicylic Acid. In: Nazar, R., N. Iqbal and N. Khan (eds.). Salicylic Acid: A Multifaceted Hormone. Springer. Singapore, Singapore. pp 145-161. Doi: 10.1007/978-981-10-6068-7_8. [ Links ]
Negrão, S., S. M. Schmöckel and M. Tester. 2017. Evaluating physiological responses of plants to salinity stress. Annals of Botany 119 (1): 1-11. Doi: 10.1093/aob/mcw191. [ Links ]
Nguyen, A. and A. Lamant. 1989. Variation in growth and osmotic regulation of roots of water stressed maritime pine (Pinus pinuster Ait.) provenances. Tree Physiology 5: 123-133. Doi: 10.1093/treephys/5.1.123. [ Links ]
Ninan, K. N. and M. Inoue. 2013. Valuing forest ecosystem services: What we know and what we don’t. Ecological Economics 93: 137-149. Doi: 10.1016/j.ecolecon.2013.05.005. [ Links ]
Palacios-Chávez, R. y J. Rzedowski. 1993. Estudio palinológico de las floras fósiles del Mioceno inferior y principios del Mioceno Medio de la región de Pichucalco, Chiapas, México. Acta Botánica Mexicana 24: 1-96. [ Links ]
Patiño V., F. 1997. Recursos genéticos de Swietenia y Cedrela en los neotrópicos: Propuestas para acciones coordinadas. FAO. Roma, Italia. http://www.fao.org/docrep/006/AD111S/AD111S03.htm#ch3 (10 junio 2019). [ Links ]
Paulsen G. M. 1994. High temperature responses of crop plants. In: Boote, K.J., J. M. Bennett, T. R. Sinclair and G. M. Paulsen (eds.). Physiology and Determination of Crop Yield. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America. Madison, WI, USA. pp. 365-389. [ Links ]
Pearce, D. W. 2001. The Economic Value of Forest Ecosystems. Ecosystem Health 7 (4): 284-296. Doi: 10.1046/j.1526-0992.2001.01037.x. [ Links ]
Pedrosa, A. M., Cd. P. S. Martins, L. P. Gonçalves and M. G. C. Costa. 2015. Late Embryogenesis Abundant (LEA) Constitutes a Large and Diverse Family of Proteins Involved in Development and Abiotic Stress Responses in Sweet Orange (Citrus sinensis L. Osb.). PLoS ONE 10 (12): e0145785. Doi:10.1371/journal.pone.0145785. [ Links ]
Perdiguero, P., C. Collada and A. Soto. 2014. Novel dehydrins lacking complete K-segments in Pinaceae, the exception rather than the rule. Frontiers in Plant Science 5: 682. Doi: 10.3389/fpls.2014.00682. [ Links ]
Pinheiro, C. and M. M. Chaves. 2011. Photosynthesis and drought: can we make metabolic connections from available data? Journal of Experimental Botany 62 (3): 869-882. Doi:10.1093/jxb/erq340. [ Links ]
Ranty, B., D. Aldon, V. Cotelle, J. P. Galaud, P. Thuleau and C. Mazars. 2016. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Frontiers in Plant Science 7: 327. Doi: 10.3389/fpls.2016.00327. [ Links ]
Sah, S. K., K. R. Reddy and J. Li. 2016. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Frontiers in Plant Science 7: 571. Doi: 10.3389/fpls.2016.00571. [ Links ]
Sánchez, F. J., M. Manzanares, E. F. De Andrés, J. L. Tenorio and L. Ayerbe. 2001. Residual transpiration rate, epicuticular wax load and leaf color of pea plants in drought conditions. Influence on harvest index and canopy temperature. European Journal of Agronomy 15: 57-70. Doi: 10.1016/S1161-0301(01)00094-6. [ Links ]
Sanders, D., J. Pelloux, C. Browniee and J. F. Harper. 2002. Calcium at the Crossroads of Signaling. The Plant Cell 14: S401-S417. Doi: 10.1105/tpc.002899. [ Links ]
Schimel D. S., J. L. House, K. A. Hibbard, P. Bousquet, P. Ciais, P. Peylin, B. H. Braswell, M. J. Apps, D. Baker and A. Bondeau. 2001. Recent patterns and mechanisms of carbon Exchange by terrestrial ecosystems. Nature 414: 169-172. Doi: 10.1038/35102500. [ Links ]
Sena, J., I. Giguère, P. Rigault, J. Bousquet and J. MacKay. 2017. Expansion of the dehydrin gene family in the Pinaceae is associated with considerable structural diversity and drought-responsive expression. Tree Physiology 38 (3): 1-15. Doi: 10.1093/treephys/tpx125. [ Links ]
Seo, P. J., S. B. Lee, M. C. Suh, M. J. Park, Y. S. Go and C. M. Park. 2011. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 23: 1138-1152. Doi: 10.1105/tpc. 111.083485. [ Links ]
Sharp, R. E. and M. E. LeNoble. 2002. ABA, ethylene and the control of shoot and root growth under water stress. Journal of Experimental Botany 53 (36): 33-37. Doi: 10.1093/jexbot/53.366.33. [ Links ]
Shepherd, T. and W. Griffiths. 2006. The effects of stress on plant cuticular waxes. New Phytologist 171: 469-499. Doi: 10.1111/j.1469-8137.2006.01826.x. [ Links ]
Smertenko, A., P. Draber, V. Viklicky and Z. Opatrny. 1997. Heat stress affects the organization of microtubules and cell division in Nicotiana tabacum cells. Plant Cell and Environment 20: 1534-1542. Doi: 10.1046/j.1365-3040.1997.d01-44.x. [ Links ]
Solberg, S. 2004. Summer drought: a driver for crown condition and mortality of Norway spruce in Norway. Forest Pathology 34: 93-107. Doi: 10.1111/j.1439-0329.2004.00351.x. [ Links ]
Spollen, W. G., M. E. LeNoble, T. D. Samuels, N. Bernstein and R. E. Sharp. 2000. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiology 122: 967-976. Doi:10.1104/pp.122.3.967. [ Links ]
Sreenivasulu, N., V. T. Harshavardhan, G. Govind, C. Seiler and A. Kohli. 2012. Contrapuntal role of ABA: does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506: 265-273. Doi: 10.1016/j.gene.2012.06.076. [ Links ]
Swindell, W. R., M. Huebner and A. P. Weber. 2007. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics 8: 125. Doi: 10.1186/1471-2164-8-125. [ Links ]
Taiz, L. and E. Zeiger. 2010. Plant physiology. 5th edition. Sinauer associates. Sunderland, MA, USA. 782 p. [ Links ]
Tang, W., X. Peng and R. J. Newton. 2005. Enhanced tolerance to salt stress in transgenic loblolly pine simultaneously expressing two genes encoding mannitol-1-phosphate dehydrogenase and glucitol-6-phosphate dehydrogenase. Plant Physiology and Biochemistry 43(2):139-146. Doi: 10.1016/j.plaphy.2005.01.009. [ Links ]
Thomas S. C. and A. R. Martin. 2012. Carbon content of tree tissues: A synthesis. Forests 3(2): 332-352. Doi: 10.3390/f3020332. [ Links ]
Thornley, J. H. M. and M. G. R. Cannell. 1996. Temperate forest responses to carbon dioxide, temperature and nitrogen: a model analysis. Plant, Cell and Environment 19: 1331iron. Doi: 10.1111/j.1365-3040.1996.tb00012.x. [ Links ]
Tunnacliffe, A. and M. J. Wise. 2007. The continuing conundrum of the LEA proteins. Naturwissenschaften 94:791-812. Doi: 10.1007/s00114-007-0254-y. [ Links ]
Tuteja, N. 2007. Mechanisms of High Salinity Tolerance in Plants. In: Häussinger, D. and H. Sies (eds.). Osmosensing and Osmosignaling. Methods in Enzymology. Volume 428. Elsevier. CA, USA. 579 p. [ Links ]
Tuteja, N. and S. K. Sopory. 2008. Chemical signaling under abiotic stress environment in plants. Plan Signaling Behavior 3 (8): 525-536. Doi: doi.org/10.4161/psb.3.8.6186. [ Links ]
Tuteja, N., T. A. Fernandez, A. M. Fortes and D. Bartels. 2011. Plant Abiotic Stress. Plant Signaling and Behavior 6 (2): 173-174. Doi: 10.4161/psb.6.2.15430. [ Links ]
Wahid, A., S. Gelani, M. Ashraf and M. R. Foolad. 2007. Heat tolerance in plants: An overview. Environmental and Experimental Botany 61: 199-223. Doi: 10.1016/j.envexpbot.2007.05.011. [ Links ]
Wang, W., B. Vinocur and A. Altman. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1-14. Doi: 10.1007/s00425-003-1105-5. [ Links ]
Wang, W., C. Peng, D. D. Kneeshaw, G. Larocque, X. Song and X. Zhou. 2012. Quantifying the effects of climate change and harvesting on carbon dynamics of boreal aspen and jack pine forests using the TRIPLEX-Management model. Forest Ecology and Management . 281, 1522log. Doi: 10.1016/j.foreco.2012.06.028. [ Links ]
Wang, X., C. Xu, X. Cai, Q. Wang and S. Dai. 2017. Heat-Responsive Photosynthetic and Signaling Pathways in Plants: Insight from Proteomics. International Journal of Molecular Sciences 18: 2191. Doi: 10.3390/ijms18102191. [ Links ]
Welin, B. V., Å. Olson, M. Nylander and E. T. Palva. 1994. Characterization and dif ferential expression of dhn/lea/rab-like genes during cold acclimation and drought stress in Arabidopsis thaliana. Plant Molecular Biology 26:131-144. [ Links ]
Xiong, L. and J. K. Zhu. 2002. Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell and Environment 25: 131-139. Doi:10.1046/j.1365-3040.2002.00782.x. [ Links ]
Xu, S., J. Li, X. Zhang, H. Wei and L. Cui. 2006. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environmental and Experimental Botany 56: 274-285. Doi: 10.1016/j.envexpbot.2005.03.002. [ Links ]
Xu, X., Q. Peng, C. Wu, H. Korpelainen and C. Li. 2008. Drought inhibits photosynthetic capacity more in females than in males of Populus cathayana. Tree Physiology 28: 1751-1759. Doi: 10.1093/treephys/28.11.1751. [ Links ]
Xue, D., X. Zhang, X. Lu, G. Chen and Z. Chen. 2017. Molecular and Evolutionary Mechanisms of Cuticular Wax for Plant Drought Tolerance. Frontiers in Plant Science 8: 621. Doi: 10.3389/fpls.2017.00621. [ Links ]
Yu, J., Y. Cheng, K. Feng, M. Ruan, Q. Ye, R. Wang, Z. Li, G. Zhou, Z. Yao, Y. Yang and H. Wan. 2016. Genome-Wide Identification and Expression Profiling of Tomato Hsp20 Gene Family in Response to Biotic and Abiotic Stresses. Frontiers in Plant Science 7: 1215. Doi: 10.3389/fpls.2016.01215. [ Links ]
Yuan, P., E. Jauregui, L. Du, K. Tanaka and B. W. Poovaiah. 2017. Calcium signatures and signaling events orchestrate plant-microbe interactions. Current Opinion in Plant Biology 38: 173-183. Doi: 10.1016/j.pbi.2017.06.003. [ Links ]
Zhang, J., C. Broeckling, E. Blancaflor, M. Sledge, L. Sumner and Z. Wang. 2005. Overexpression of WXP1, a putative Medicago truncatula AP2 domain containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). Plant Journal 42: 689-707. Doi: 10.1111/j.1365-313X.2005.02405.x. [ Links ]
Zhang, S. and X. Wang. 2011. Overexpression of GASA5 increases the sensitivity of Arabidopsis to heat stress. Journal of Plant Physiology 168 (17): 2093-2101. Doi: 10.1016/j.jplph.2011.06.010. [ Links ]
Zhang, Z., W. Wang and W. Li. 2013. Genetic interactions underlying the biosynthesis and inhibition of beta-diketones in wheat and their impact on glaucousness and cuticle permeability. PLoS ONE 8:e54129. Doi: 10.1371/journal.pone.0054129. [ Links ]
Zhang, Z., W. Wei, H. Zhu, G. S. Challa, C. Bi, H. N. Trick, W. Li. 2015. W3 is a new wax locus that is essential for biosynthesis of beta-diketone, development of glaucousness, and reduction of cuticle permeability in common wheat. PLoS ONE 10:e0140524. Doi: 10.1371/journal.pone.0140524. [ Links ]
Zhu, J. 2016. Abiotic stress signaling and responses in plants. Cell 167: 313-324. Doi: dx.doi.org/10.1016/j.cell.2016.08.029. [ Links ]
Received: May 15, 2019; Accepted: August 15, 2019











 texto en
texto en 


