Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.10 no.52 México Mar./Abr. 2019
https://doi.org/10.29298/rmcf.v10i52.414
Articles
Glucose injections in Jacaranda mimosifolia D. Don in urban areas of Texcoco de Mora
1Posgrado en Ciencias Forestales. Colegio de Postgraduados. México.
2Instituto de Ciencias Agropecuarias, Universidad Autónoma del Estado de Hidalgo. México.
3Posgrado en Estadística. Colegio de Postgraduados. México.
Jacaranda trees are common in urban areas of Valle de Mexico and often grows under nutritional and water stress conditions, which affects its growth. The supply of carbohydrates through the vascular system has recently been recommended as an option to improve growth and vitality. Therefore, the objective of this work is to assess the effect of glucose injected to the trunk of Jacaranda mimosifolia trees.in for concentrations: 0 (control), 30, 55 and 80 g L-1. Diameter (cm) and height (m) increments were determined, the canopy condition and foliage production were monitored and the content of carbohydrate in shoots, trunks and roots as well as the chlorophyll fluorescence (Fv/Fm). Significant differences were found (P≤0.05) in the increase in diameter and height with the application of 80 g L-1 glucose. The crown condition showed significantly higher values in density and lower in transparency, as well as an adequate foliage production, with the highest concentration (80 g L-1). Carbohydrate content showed significant differences (P≤0.05) in trunk, not in buds, roots, or chlorophyll fluorescence. The injections of glucose to the trunk have a significant effect on the growth of Jacaranda trees and affecting vitality to a lesser extent.
Key words: Urban trees; soluble sugars; chlorophyll; stress; phenology; Jacaranda mimosifolia D. Don
Los árboles de jacaranda son comunes en áreas urbanas del valle de México y se desarrollan frecuentemente bajo condiciones de estrés nutricional o hídrico, lo que restringe el crecimiento del arbolado. El suministro de carbohidratos a través del sistema vascular recientemente se ha recomendado como una opción para mejorar el crecimiento y vitalidad. Por lo anterior, el objetivo del trabajo fue evaluar el efecto de la glucosa sobre crecimiento y vitalidad de árboles de Jacaranda mimosifolia inyectada al tronco en cuatro concentraciones: 0 (testigo), 30, 55 y 80 g L-1 Se midieron incrementos en diámetro (cm) y altura (m), se monitoreó la condición de copa y producción de follaje y además se evaluó el contenido de carbohidratos en brotes, troncos y raíces, así como la fluorescencia de la clorofila (Fv/Fm). Se encontraron diferencias significativas (P≤0.05) en el incremento en diámetro y altura con la aplicación de 80 g L-1 de glucosa. La condición de copa mostró valores significativamente más altos en densidad y bajos en transparencia, así como una buena producción de follaje, con la concentración más alta del carbohidrato (80 g L-1). Su contenido presentó diferencias significativas (P≤0.05) en tronco, no así en brotes, raíces, ni en valores de fluorescencia de la clorofila. Las inyecciones de glucosa al tronco tienen un efecto significativo en el crecimiento de árboles de esta especie y afectan en menor medida la vitalidad del arbolado.
Palabras clave: Árboles urbanos; azúcares solubles; clorofila; estrés; fenología; Jacaranda mimosifolia D. Don
Introduction
Urban trees under optimal healthy conditions provide ecosystem services such as shade, recreation, carbon capture, interception of air pollutants and positive impact on human well-being (Moser et al., 2018; Scholz et al., 2018). However, in many cases, the environment is not favorable for the growth of trees (Martínez et al., 2010, Moser et al., 2017). due to space limitations, nutritional deficiencies, soil compaction, lack of water, extreme temperatures, pollution and vandalism (Stojnic et al., 2016, Allen et al., 2017). This stresses the tree and results in poor growth that has an impact on vitality and high mortality rates (Koeser et al., 2014).
From photosynthesis, the synthesis of carbohydrates is essential for the tree, since these compounds maintain their metabolism, accumulate reserves and promote the growth of new tissues (Maselli and Silveira, 2017). If the production of sugar depends on the availability of minerals in the soil and environmental factors (Gamboa and Marín, 2012, Valenzuela et al., 2013), then, environmental stress conditions, derived from the urban environment, cause a reduction in the tree's carbohydrate reserves (Martínez et al., 2010; Koeser et al., 2014; Moser et al., 2018). Vitality is directly related to the quantity of reserves, so vigorous individuals are able to resist in stressful situations (Johnstone et al., 2013; Callow et al., 2018; Ramírez et al., 2018).
The exogenous supply of carbohydrates to the tree vascular system has a positive effect on its development, due to the increase in energy reserves (Percival et al., 2004; Martínez et al., 2009; Martínez et al., 2013). Although injections to the trunk were first used for the chemical treatment of diseases, fungi and pests (Costonis, 1981; Perry et al., 1991; Dal Maso et al., 2014; Acimovic et al., 2015), They have recently been used as the most viable option to improve the state of urban trees (Martínez et al., 2009; Percival and Sacre, 2014; Suryanto et al., 2018). The advantage of the injection to the trunk is that the applied compound is used in its entirety when incorporated into the vascular system (Costonis, 1981; Wise et al., 2014; Acimovic et al., 2016) and it is distributed throughout the tree (Kobza et al., 2011).
The jacaranda tree (Jacaranda mimosifolia D. Don), is an arboreal species belonging to the Bignonaceae family (Sharma et al., 2016). Its ornamental use in urban spaces has increased in recent decades (Miyajima et al., 2013; Zaouchi et al., 2015) because it is a medium-sized species with abundant and colorful flowering (Miyajima et al., 2013). It is important to improve the vitality condition of jacarandas that grows in environments that are not always favorable. Therefore, the objective of this work was to evaluate the effect of glucose injected into the trunk on the growth and vitality of Jacaranda mimosifolia trees located.
Materials and Methods
The work was carried out in the trees of the Texcoco-Chapingo Boulevard (19°30'04.19'' N and 98°53'00.85'' W) in Texcoco de Mora municipality, State of Mexico. The area has an average altitude of 2 240 m, a temperate semi-dry climate, an average annual temperature of 15.9 °C and an average precipitation of 686 mm per year; the soil is clay-textured Vertisol (Gutiérrez and Ortiz, 1999). A total of 36 Jacaranda mimosifolia trees with a normal diameter of 27 cm and a height of 7 m on average were selected, aligned with a 6 m spacing.
The application of glucose was made through macro injections at the base of the trunk of each tree (Costonis, 1981). Drillings of 4.4 mm in diameter were made at the base of the tree at a depth of approximately 25.4 mm (Acimovic et al., 2016) perpendicular to the trunk; a 550 rpm cordless drill DW130V (DeWalt Industrial Tool Co.) and a manual pump (H. D. Hudson Industries TM) were used at a constant pressure of 0.13 MPa, to apply a total of 10 L of solution per tree in July 2017.
To know the growth of the trees 11.5 months after the application of the treatments, the trunk diameter (cm) was measured at the beginning and at the end of the experiment at a height of 10 cm above the ground with a diametric tape 283D (Forestry suppliers Inc.); by the frequent bifurcation of the trunk below 1.30 m in height and the measurement point was marked with indelible ink. The total height (m) was measured with a clinometer (BruntonTM). From the above variables, the increase of 11.5 months in diameter (ID) and in height (IA) was estimated.
To determine the vitality of the trees, information was recorded on the crown condition of each tree through the crown density (Dnc) and crown transparency (Trc); this was carried out visually by two people located at a horizontal distance proportional to the height of each tree, it was determined with a scale divided into 5 % classes (Westfall et al., 2009; Saavedra et al., 2016). The amount of foliage production of each tree was assessed by the 6-class method used to evaluate infestation by mistletoe (Hawksworth, 1977). The crown of each tree was divided into three thirds, each third was evaluated separately assigning a grade of 0, 1 or 2, for each amount of foliage, where: 0 is non-visible or low production, 1 is an adequate production and 2 is a high production. In the actual study, the assessment of the amount of foliage produced in the different treated trees was made.
The content of carbohydrates, total sugars and reducing sugars in tree tissues was calculated. Samples were taken at random from five branches of the lower crown of each tree; In addition, with an increment hammer (Haglof Company Group TM), five samples were taken near the base of the trunk (4mm × 100 mm) (Martínez et al., 2009). These were macerated in liquid nitrogen and stored at -20 °C until processing in the laboratory. Two alcoholic extractions of sugars from the harvested tissues were performed, each with 40 mL of 80 % ethanol (v/v) for 20 min at 100 °C. The result of the extraction was diluted in 10 mL of distilled water for the quantitative determination (Quentin et al., 2015); samples were worked in triplicate.
The concentration of total sugars was determined by the anthrone method (Witham et al., 1971); a GenesysTM10S Vis & UV-Vis spectrophotometer was used to read the absorbance at 600 nm; total sugars were quantified with a standard glucose curve at a concentration of 20 to 200 μg mL-1. For the reducing sugars, the method of Nelson (1944) and Somogyi (1952) was used; absorbance was recorded on the spectrophotometer at 540 nm and a standard curve containing glucose of 15 to 150 μg mL-1 was used. The results were expressed in mg of glucose per gram of dry weight (mg g-1 ps-1).
Analogously, the content of starch in roots was quantified; for this, approximately 5 g of tissue was collected below the base of the trunk, and the precipitate of the alcohol extraction process described above was hydrolyzed with the diastase enzyme (SIGMA) (Palevitz and Newcomb, 1970). The anthrone method was applied to the resulting hydrolyzate based on the glucose content present in the sample; the result was expressed in mg g-1 ps-1.
Finally, chlorophyll fluorescence (Fv/Fm) was recorded with a Pocket PEA portable fluorometer (Hansatech Instruments Ltd.), with a detection time of 1 s and emitting light at a wavelength of 650 nm, with an intensity of 3500 μmol m-2 s-1 (Zhang et al., 2016). Measurements were made after adapting to the dark for 10 minutes with pocket PEA clips a total of 10 randomly chosen leaves taken from the outside of the low crown of each tree (Martínez et al., 2009).
The experimental design was completely random; the treatments consisted of four glucose concentrations: 0 (control), 30, 55 and 80 g L-1; the control only contained purified water. The distribution of treatments was random with nine repetitions for each treatment (Percival et al., 2004; Martínez et al., 2009).
The assumptions of normality were checked with Shapiro-Wilk (n≤50 and α = 0.05) and the homogeneity of variances (Barttlet test). A multivariate analysis (MANOVA) was performed on those variables that fulfilled the assumptions of normality and homogeneity (P≤0.05), we proceeded with the comparisons of means with the DSH test (significant honest difference of Tukey) and α = 0.05. In those variables that did not meet the assumptions (P> 0.05), the nonparametric method of Kruskal-Wallis was used and the means were compared with the sum of the Wilcoxon ranges. It was analyzed statistically with the RStudio Team® program (R Core Team, 2015).
Results and Discussion
Increase in diameter
Significant differences (P≤0.05) in the increase in diameter of the jacaranda trees were recorded after 11.5 months of the application of the treatments. The highest concentration of glucose (80 g L-1) showed average growth values 200 % higher than those of the specimens injected with the control (Figure 1). However, the application of carbohydrate doses lower than 80 g L-1 of glucose had no effect on growth in diameter, possibly due to the demand of large amounts of carbohydrates (Piper and Fajardo, 2016). Therefore, low glucose concentrations are insufficient to promote growth and its effect is probably diluted by being mobilized to cover the energy requirements of other physiological processes such as photosynthesis, respiration, transpiration, absorption and translocation (Martínez et al., 2013; Ramírez et al., 2018). In some cases, sugars are used to store carbohydrate reserves, all at the expense of vegetative growth (Piper and Fajardo, 2016). Previous studies have shown that the use of carbohydrates has positive effects on the diameter growth of trees such as oak (Quercus virginiana P. Miller), poplar (Populus nigra (Moench) Koehne), English oak (Quercus robur L.) and European beech (Fagus sylvatica L.) (Martínez et al., 2009; Percival and Sacre, 2014), a positive effect has also been observed in the diameter of Ceylon oak branches (Schleicera oleosa Merr.) (Suryanto et al., 2018).
Increase in height
The increase in height presented significant differences (P≤0.05) to the glucose injection; the height of the trees showed higher average values for the two highest concentrations of glucose (55 and 80 g L-1), compared to the low concentrations (0 and 30 g L-1) (Figure 2). This may indicate that the former stimulate growth in height (Martínez et al., 2013; Percival and Sacre, 2014). In certain broadleaf species such as willows (Salix sp.), poplar trees (Populus sp.), birches (Betula sp.) and maples (Acer sp.). The growth of the apical bud and flowering is manifested long before the vegetative growth (Percival and Sacre, 2014). Initial stages of vegetative reproduction depend exclusively on the reserves that the tree accumulated in the previous growth cycle (Percival and Sacre, 2014; Ramírez et al., 2018). This supports the idea that the exogenous application of carbohydrates stimulates growth in urban trees (Martínez et al., 2013).
Crown condition
The average values of crown density (Dnc) were significantly different among glucose concentrations (P≤0.05). The highest average value in Cnd occurred at 80 and 55 g L-1 (75 % and 76 %, respectively), while the lowest average was observed in the control (0 g L-1) with 59 % (Figure 3). More than 50 % of the evaluated trees developed 70 % in crown density, which indicates a good amount of foliage (Saavedra et al., 2016). It is well- known that above 75 % is unusual for most urban tree species, unless they grow in open spaces (Westfall et al., 2009). Dnc stand out represent a high potential of growth, and, therefore, of survival, in comparison with those mature specimens with less amount of foliage (Zaragoza et al., 2014; Maselli and Silveira, 2017). Healthy trees are considered to be those with a crown density greater than 50 % (Westfall et al., 2009; Saavedra et al., 2016), while if they are less than 30 % they have a high probability of dying in a year (Steinman, 1998). The assessed jacaranda trees have a better canopy density than other species located in urban spaces such as the San Juan de Aragón Park, Alameda Norte, South and the Eastern Mexico City with averages less than 70 % (Zaragoza et al., 2014; Saavedra et al., 2016).
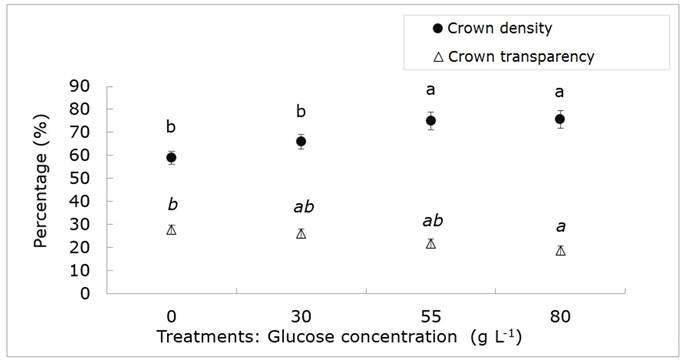
The bars indicate the standard error. Equal letters mean statistically equal means (P≤0.05) using the sum of Wilcoxon ranges.
Figure 3 Density and transparency of the crown of Jacaranda mimosifolia D.Don trees subjected to four glucose concentration treatments.
The average canopy transparency values (Trc) were significantly different (P≤0.05). The highest for this variable was shown by the control trees (0 g L-1), with 28 %, while the lowest corresponds to the treatment with 80 g L-1 of glucose with an average of 19 % (Figure 3). It was also observed that at least 50 % of the studied individuals presented an average of 25 % Trc, which suggests that the amount of foliage in the trees is sufficient to carry out fundamental processes such as photosynthesis and respiration; however, the crown is not dense enough to block the passage of light (Zaragoza et al., 2014).
High Trc values are directly related to stressors, such as pest attack, drought, pruning activities, air pollution, soil compaction or vandalism (Schomaker et al., 2007; Zaragoza et al., 2014); however, the evaluated trees showed compaction and pruning, mainly. Figures below 30 % of this variable in the trees indicate a healthy condition (Saavedra et al., 2016). In general, for the assessed trees in the Texcoco-Chapingo Boulevard are below the mentioned limit so as not to be considered in a state of decline or stress (Figure 3).
The level of foliage production was influenced by the highest glucose concentration (P≤0.05). Despite this, this variable, independently of the glucose concentration, was located between classes 3 and 4 (Hawksworth, 1977); therefore, satisfactory values are considered. The production of foliage is directly related to the Dnc variable described, so it was observed that the higher the value of Dnc, the foliage also increased, as shown by the results for the concentration of 80 g L-1 (Figure 4). This amount of leaves allows the trees to survive and perform their functions correctly (Zaragoza et al., 2014).
Total Sugars and Reducing Sugars
The analysis of carbohydrates showed that both total and reducing sugars in shoots, trunk and starch in roots registered an annual variation, with maximum values in the autumn period, and that decreased gradually in the winter, to finally reach their lowest values during the spring, shortly after the foliage emergence period. This fluctuation is due to the fact that sugars are used in processes such as respiration during winter and the production of new tissues in spring (Valenzuela et al., 2013; Ramírez et al., 2018). This annual variation has been recorded in carbohydrate levels in species such as: semievergreen oak (Quercus virginiana P. Miller), orange (Citrus sinensis L.), tangerine (Citrus reticulata Blanco) and mango (Mangifera indica L.) (Martínez et al., 2009; Gamboa and Marín, 2012; Laskowski, 2014). All the variables presented variations related to the phenological stages of the tree (Laskowski, 2014).
No significant differences were observed (P> 0.05) in total sugars and reducers in shoots, nor in starch from the roots of jacaranda trees. The total sugars in the trunk presented them (P <0.05) in the treatment of 80 g L-1, while the reducing sugars, did not show statistical evidence that indicated an alteration of this variable with the injection of glucose to the trunk; previous studies revealed an increase in reducing sugars, as an indicator of the increase in vitality (Martínez et al., 2009; Laskowski, 2014), which was not confirmed in the current study. The determination of total sugars includes reducing sugars and fractions of polysaccharides such as starch, an important reserve carbohydrate in different organs of the tree (root, stem and branches) used for situations of energy deficiency (Martínez et al., 2013; Piper and Fajardo, 2016). Therefore, it is possible to indirectly estimate the carbohydrate reserve of the trees with a simple difference between the values of these sugars. In this sense, statistical evidence was found (P <0.05) indicating that trees injected with high glucose concentrations maintain more than twice the amount of reserves in the trunk than those treated with low glucose concentrations (Figure 5).
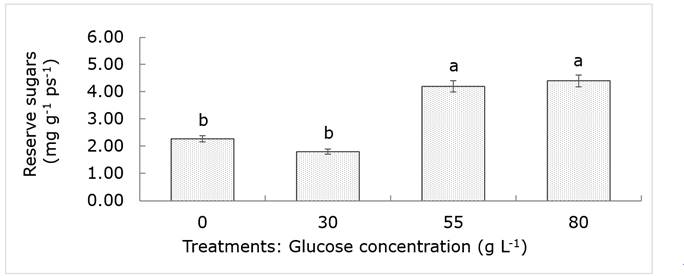
The bars indicate the standard error. Equal letters mean statistically equal means (Tukey: P≤0.05).
Figure 5 Concentration of soluble sugars in the trunk of Jacaranda mimosifolia D.Don trees subjected to four treatments of glucose concentration.
In another study, Ramírez et al. (2018) determined that urban trees such as maple (Acer platanoides L.) have a higher carbohydrate reserve than trees growing under natural conditions; this characteristic improves their ability to face periods of imbalance in carbohydrate levels related to abiotic factors.
Chlorophyll fluorescence
The fluorescence of chlorophyll showed no significant differences in any of the treatments (P> 0.05). This variable kept constant throughout the assessment with average Fv/Fm values of 0.787 for the concentrations of 0 g L-1, 0.810 for 30 g L-1, 0.804 for 55 g L-1, and 0.811 for 80 g L-1 glucose. Trees with Fv/Fm values between 0.78 and 0.85 are considered healthy and free from any type of stress (Johnstone et al., 2013; Uhrin and Supuka, 2016; Callow et al., 2018). Based on this variable, the trees did not exhibit evident signs of stress, as they remained in a condition of constant vitality throughout the experiment. The opposite occurs in the evergreen oak (Quercus virginiana), the sycamore maple (Acer pseudoplatanus L.) and the kusum (Schleicera oleosa Merr), in which the chlorophyll fluorescence was stimulated by the injection of carbohydrates or altered by abiotic factors typical of urban environments such as water stress, compaction or mechanical damage (Martínez et al., 2009; Uhrin and Supuka, 2016; Suryanto et al., 2018).
Conclusions
The injection of 80 g L-1 glucose into the trunk of jacaranda trees positively stimulated the growth in diameter and height. The crown condition kept a profuse density and foliage production, and indicates a good health of the trees; which coincided with a larger amount of reserve carbohydrates. No evidence of an alteration of the vitality condition was found with the use of chlorophyll fluorescence. Therefore, the application of glucose to the trunk promotes vegetative growth and to a lesser extent stimulates the vitality of the trees of the species Jacaranda mimosifolia in urban areas of Texcoco de Mora city.
Acknowledgements
The authors thank the Consejo Nacional de Ciencia y Tecnología (National Council of Science and Technology) (Conacyt) for financing the original project and the municipality of Texcoco de Mora city for allowing to carry out this project in their urban green areas.
REFERENCES
Acimovic, S. G., G. C. McGhee, G. W. Sundin and J. C. Wise. 2015. Evaluation of trunk-injected bactericides and prohexadione-calcium of environmentally friendly control of fire blight (Erwinia amylovora) in apples. Plant Protection Society of Serbia 1:129-134. [ Links ]
Acimovic, S. G. , B. M. Cregg, G. W. Sundin and J. C. Wise. 2016. Comparison of drill-and needle-based tree injection technologies in healing of trunk ports on apple trees. Urban Forestry and Urban Greening 19(1):151-157. [ Links ]
Allen, K. S., R. W. Harper, A. Bayer and N. J. Brazee. 2017. A review of nursery production system and their influence on urban tree survival. Urban Forestry and Urban Greening 21(1):183-191. [ Links ]
Callow, D., P. May and D. M. Johnstone. 2018. Tree vitality assessment in urban landscapes. Forests 9(5):1-7. [ Links ]
Costonis, A. C. 1981. Tree injection: Perspective macro-injection/micro-injection. Journal of Arboriculture 7(10):275-278. [ Links ]
Dal Maso, E., J. Cocking and L. Montecchio. 2014. Efficacy tests on commercial fungicides against ash dieback in vitro and by trunk injection. Urban Forestry & Urban Greening. 13(4):697-703. [ Links ]
Gamboa P., J. R. y W. Marín M. 2012. Fenología, producción y contenido de almidón en árboles de mango en Guanacaste, Costa Rica. Agronomía Mesoamericana 23(1):81-91. [ Links ]
Gutiérrez C., M. C. y C. A. Ortiz S. 1999. Origen y evolución de los suelos en el ex lago de Texcoco, México. Agrociencia 33(2):200-208. [ Links ]
Hawksworth, F. G. 1977. The 6-class dwarf mistletoe rating system. General Technical Report Rm-48. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. Fort Collins, CO USA. 7 p. [ Links ]
R Core Team. 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Wien, AT USA. n/p. [ Links ]
Johnstone, D., G. Moore, M. Tausz and M. Nicolas. 2013. The measurement of plant vitality in landscape tress. Arboricultural Journal: The International Journal of Urban Forestry 35(1):18-27. [ Links ]
Kobza, M., G. Juhásová, K. Adamcíková and E. Onrusková. 2011. Tree injection in the management of Horse-Chestnut leaf miner. Cameraria ohridella (Lepidoptera: Gracillariidae). Gesunde Pflanzen 62:139-143. [ Links ]
Koeser, A. K., E. F. Gilman, M. Paz and C. Harchick. 2014. Factors influencing urban tree planting program growth and survival in Florida, United States. Urban Forestry and Urban Greening 13(4):655-661. [ Links ]
Laskowski, L. E. 2014. Contenido de carbohidratos en hojas y raíces de plantas de naranja “Valencia” y mandarina “Dancy” durante un ciclo anual de crecimiento. Revista de la Facultad de Agronomía LUZ 31:1-22. [ Links ]
Martínez T., T., W. T. Watson, M. A. Arnold, L. Lombardini and N. Appel. 2009. Carbohydrate injections as a potential option to improve growth and vitality of live Oaks. Arboriculture and Urban Forestry 35(3):142-147. [ Links ]
Martínez T., T. , W. T. Watson, M. A. Arnold and L. Lombardini. 2010. Microbial activity of a clay soil amended with glucose and starch under live Oaks. Arboriculture and Urban Forestry 36(2):66-72. [ Links ]
Martínez T., T. , F. O. Plascencia E. y L. Islas R. 2013. La relación entre los carbohidratos y la vitalidad en árboles urbanos. Revista Chapingo Serie Ciencias Forestales y del Ambiente 19(3):459-468. [ Links ]
Maselli L., G. and M. Silveira B. 2017. Dendrobiochemistry, a missing link to further understand carbon allocation during growth and decline of trees. Trees 31(6):1745-1758. [ Links ]
Miyajima, I., C. Takemura, N. Kobayashi, M. S. Soto and G. Facciuto. 2013. Flower bud initiation and Development of Jacaranda mimosifolia (Bignoniaceae) in Japan. Acta Horticulturae 1000(7):71-76. [ Links ]
Moser, A., E. Uhl, T. Rötzer, P. Biber, J. Dahlhausen, B. Lefer and H. Pretzsch. 2017. Effects of climate and the urban heat island. Effect on urban tree growth in Houston. Open Journal of Forestry 7(4):428-445. [ Links ]
Moser, A. , E. Uhl, T. Rötzer, P. Biber, J. M. Caldentey and H. Pretzsch. 2018. Effects of climate trends and drought events on urban tree growth in Santiago de Chile. Ciencia e Investigación Agraria 45(1):35-50. [ Links ]
Nelson, N. 1944. A photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry 153:375-380. [ Links ]
Palevitz, B. A. and E. H. Newcomb. 1970. A study of sieve element starch using sequential enzymatic digestion and electron microscopy. The Journal of Cell Biology. 45:383-398. [ Links ]
Percival, G. C., G. A. Fraser and S. Barnes. 2004. Soil injections of carbohydrates improve fine root growth of established urban trees. Arboricultural Journal 28(1-2):95-101. [ Links ]
Percival, G. C. and K. Sacre. 2014. The influence of soluble carbohydrates, slow-release nitrogen and plant growth regulator on transplant survival of trees. Arboricultural Journal 36(3):140-160. [ Links ]
Perry, T. O., F. S. Santamour, R. J. Stipes, T. Shear and A. L. Shigo. 1991. Exploring alternatives to tree injection. Journal of Arboricultural 17(8):217-226. [ Links ]
Piper, F. I. and A. Fajardo. 2016. Carbon dynamics of Acer pseudoplatanus seedlings under drought and complete darkness. Tree Physiology 36(11):1400-1408. [ Links ]
Quentin, A, E. Pinkard, M. Ryan, et al. 2015. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiology 35(11): 1146-1165. [ Links ]
Ramírez, J. A., I. T. Handa, J. M. Posada, S. Delagrange and C. Messier. 2018. Carbohydrate dynamics in roots, stems, and branches after maintenance pruning in two common urban trees species of North America. Urban Forestry and Urban Greening 30:24-31. [ Links ]
Saavedra R, L. L., D. Alvarado R., P. Hernández D., T. Martínez T., G. Mora A. y J. Villa C. 2016. Condición de copa, indicadores de salud en árboles urbanos del Bosque San Juan de Aragón, Ciudad de México. Madera y Bosques 22(2):15-27. [ Links ]
Scholz, T., A. Hof and T. Schmitt. 2018. Cooling effects and regulating ecosystem services provided by urban trees-novel analysis approaches using tree cadaster data. Sustainability 10(3):2-18. [ Links ]
Schomaker, M. E., S. J. Zarnoch, W. A. Bechtold, D. J. Latelle, W. G. Burkman and S. M. Cox. 2007. Crown-condition classification: a guide to data collection and analysis. USDA Forest Service. Southern Research Station. General Technical Report SRS-102. Asheville, NC USA. 78 p. [ Links ]
Sharma, D., M. I. Sabela, S. Kanchi, P. S. Mdluli, G. Singh, T. A. Stenstrom and K. Bisetty. 2016. Biosynthesis of ZnO nanoparticles using Jacaranda mimosifolia flowers extract: Synergistic antibacterial activity and molecular simulated facet specific adsorption studies. Journal of Photochemistry and Photobiology, B: Biology 162:199-207. [ Links ]
Somogyi, M. 1952. Notes on sugar determination. The Journal of Biological Chemistry 195:19-23. [ Links ]
Steinman, J. 1998. Tracking the health of trees over time on forest health monitoring plots. In: Hansen, M and T. Burk (eds.). Proceedings held at Boise Centre on the Grove. Integrated tools for natural resources inventories in the 21st century. USDA Forest Service. 16-20 August. Boise, ID USA. pp. 334-339. [ Links ]
Stojnic, S., S. Pekec, M. Kebert, A. Pilipovic, D. Stojanovic, M. Stojanovic and S. Orlovic. 2016. Drought effects on physiology and biochemistry of Pedunculate Oak (Quercus robur L.) and Hornbeam (Carpinus betulus L.). Saplings grown in urban area of Novi Sad, Serbia. South-East European Forestry 7(1):1-8. [ Links ]
Suryanto, H., S. Supriyanto and N. F. Haneda. 2018. Molasses injection to improve growth and vitality of Kesambi (Schleicera oleosa Merr) as lac insect host plant. Journal Penelitian Kehutanan Wallacea 7(2):173-181. [ Links ]
Uhrin, P. and J. Supuka. 2016. Quality assessment of urban trees using growth visual and chlorophyll fluorescence indicators. Ekológia (Bratislava) 35(2):160-172. [ Links ]
Valenzuela N., L. M. , P. Maillard, J. L. González B. y G. González C. 2013. Balance de carbohidratos en diferentes compartimentos vegetales de encino (Quercus pétrea y haya (Fagus sylvatica), sometidos a defoliación y sombra. Revista Chapingo, Serie Zonas Áridas 8(1):33-38. [ Links ]
Westfall, J. A., A. W. Bechtold and K. C. Randolph. 2009. Tree crown indicator. In: Westfall, J. A. (ed.). FIA National Assessment of data quality for forest health indicators. U.S. Department of Agriculture, Forest Service. North-eastern research service. General Technical Report NSR-53. Newtown Square, PA USA. pp. 3-15. [ Links ]
Wise, J. C, A. H. VanWoerkom, S. G. Acimovic, G. W. Sundin, B. M. Cregg and C. Vandervoort. 2014. Trunk injection: Discriminating delivering system for horticulture crop IPM. Entomology, Ornithology and Herpetology 3(2):2-7. [ Links ]
Witham, F. H., D. F. Blaydes and R. M. Devlin. 1971. Experiments in plant physiology. Van Nostrand Reinhold Company. New York, NY USA. 245 p. [ Links ]
Zaouchi, Y., S. Rezgui and T. Bettaieb. 2015. Influence of mycorrhization an adaptation capacity of Jacaranda mimosifolia D. Don grow in urban conditions. Journal of New Sciences 18(5):679-688. [ Links ]
Zaragoza H, A. Y., V. M. Cetina A., M. A. López L., A. Chacalo H., M. L. de la Isla B. y H. González R. 2014. Indicador condición de copa y su aplicación en tres parques del Distrito Federal. Revista Mexicana de Ciencias Forestales 5(25):1-16. [ Links ]
Zhang, C. J., S. H. Lim, J. W. Kim, G. Nah, A. Fischer and D. S. Kim. 2016. Leaf chlorophyll fluorescence discriminates herbicide resistance in Echinochloa species. Weed Research 56(6):424-433. [ Links ]
Received: September 14, 2018; Accepted: February 22, 2019











 texto em
texto em 





