Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.10 no.51 México 2019
https://doi.org/10.29298/rmcf.v10i51.336
Articles
Fungal pretreatment of Agave lechuguilla Torr. biomass to produce ethanol
1Departamento de Biotecnología, Facultad de Ciencias Químicas, Universidad Autónoma de Coahuila. Saltillo, Coah.
2Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), Campo Experimental Saltillo. Saltillo, Coah.
3Departamento de Investigación en Alimentos, Facultad de Ciencias Químicas, Universidad Autónoma de Coahuila. Saltillo, Coah.
Recently, the biomass of Agave lechuguilla has been reported as a raw material with potential for the production of ethanol. However, the high energy expenditure in the pretreatment used, requires the search for methods that minimize this parameter and favor an improvement in the economic feasibility of the process. Compared to traditional pretreatment technologies, biological pretreatment offers an alternative in which lignin removal enzymes are able to unfold the complex structures of lignin, without the use of chemicals and with lower energy expenditure. In this work the biological pretreatment of biomass of Agave lechuguilla with Phanerochaete chrysosporium H-298 was evaluated. The two factors assessed for the optimization of the process were: the incubation time and the concentration of the nitrogen source. The results showed a maximum delignification (36.15 %), preserving cellulose without significant changes. The optimal pretreatment conditions were: 60 days of incubation and a nitrogen concentration of 1 M. The enzymatic hydrolysis of the pretreated material using the Cellic® CTec3 enzymatic complex showed a maximum glucose release of 44.9 g L-1 at 92 hours, corresponding to hydrolysis yield of 93.09 %, higher than that obtained in the hydrolysis of the biomass without pretreatment (37.92 %). The concentration of ethanol at 10 h of fermentation was 16.53 g L-1 (equivalent to an ethanol concentration >2 v/v), observing 5.7 g L-1 of glucose remainder at this incubation time.
Key words: Agave lechuguilla Torr.; biofuels ethanol; optimization; biological pretreatment; arid land
Recientemente la biomasa del cogollo de Agave lechuguilla ha sido establecida como materia prima con potencial para la producción de etanol, sin embargo, el alto gasto energético en el pretratamiento requiere la búsqueda de métodos que lo minimicen y propicie una mejora en la factibilidad económica del proceso. En comparación con las tecnologías tradicionales, el pretratamiento biológico ofrece una alternativa en la que las enzimas de remoción de la lignina son capaces de desdoblar las estructuras complejas de la misma, sin el uso de químicos y con menor gasto energético. En este trabajo se probó el uso de Phanerochaete chrysosporium H-298 en la biomasa de Agave lechuguilla. Los dos factores ensayados para la optimización del proceso fueron el tiempo de incubación y la concentración de la fuente de nitrógeno. Los resultados mostraron una máxima deslignificación (36.15 %), se preservó la celulosa sin cambios significativos. Las condiciones óptimas de pretratamiento fueron: 60 días de incubación y una concentración de nitrógeno de 1M. La hidrólisis enzimática del material pretratado con el complejo enzimático Cellic® CTec3 mostró una máxima liberación de glucosa de 44.9 g L-1 a las 92 horas, con rendimiento de hidrólisis de 93.09 %, mayor al obtenido en la hidrólisis de la muestra sin pretratar (37.92 %). La concentración de etanol a las 10 horas de fermentación fue de 16.53 g L-1 (equivalente a una concentración >2 % v/v de etanol) con 5.7 g L-1 de glucosa remanente a ese tiempo de incubación.
Palabras clave: Agave lechuguilla Torr.; biocombustibles; etanol; optimización; pretratamiento biológico; zonas áridas
Introduction
The commercialization of biofuels is currently a reality in several countries. For example, in Brazil, first generation ethanol (1G) is produced from sugar cane and in the United States of America, from corn. However, the incorporation of raw materials of edible use entails a series of undesirable consequences, in particular to the scarcity and increase of food prices (Buruiana et al., 2014). To avoid food safety problems due to the use of resources containing starch or sucrose, lignocellulosics (MLCs) are the most suitable base for the production of second generation ethanol (2G) (Althuri et al., 2017).
Huge advances have been made in the lignocellulosic industry in the last ten years. At present, five commercial plants have been inaugurated in recent years: three of these in the United States of America (one in Kansas and two in Iowa), which manufacture ethanol from corn stubble. The other two are located in Alagoas, Brazil and elaborate this product of sugarcane bagasse; and in Crecentino, Italy, with wheat straw (González et al., 2017).
Due to the complex structures of the cell wall of the plant in the MLCs, it has been demonstrated that there are several physical-chemical methods of pretreatment, among them the explosion of steam, the explosion of fiber with ammonium, dilute acid and alkali. In addition, there are pretreatments with organic solvents, which have shown their ability to reduce the recalcitrance of MLCs and improve the subsequent stage of enzymatic hydrolysis to obtain biofuels or products with high added value (Pérez-Pimienta et al., 2017).
Compared with current pretreatment technologies, the biological method offers an alternative in which oxidase-type enzymes are capable of unfolding the structures that form lignin (Arora et al., 2016) in an environmentally friendly manner, due to the absence of chemical solvents and the lower energy expenditure (Sindhu et al., 2016). This method of pretreatment is carried out by the action of white rot fungi that express this type of enzymes (lignin peroxidase, laccase, manganese peroxidase) such as: Phanerochaete chrysosporium Burds, Oxysporus sp. and Ganoderma sp. (Wan and Li, 2012). This option has shown an improvement in enzymatic hydrolysis and in the fermentation of ethanol, which makes it a process that can replace thermochemical methods (alkaline, diluted acid, ionic liquids and organo solvent), as long as it is fast enough (Yang and Savage, 2016; Huang et al., 2017).
It should be noted that thermochemical methods focused on the removal of lignin vary in pretreatment conditions, including the time of operation. Yang and Pan (2012) carried out the process in leaves of Agave americana L. with 8 % NaOH at 180 °C for 30 min, from which it turned out that the pre-treated material was enriched in glucans up to 58.8 %. Pérez et al. (2013, 2015) and Pérez-Pimienta et al. (2017) confirmed that the content of glucans increased (in the range of 41 to 66 %) after pretreatment of Agave tequilana FAC Weber bagasse with ionic liuids of 1-ethyl-3-methylimidazolium acetate [C2mim] [OAc] at 120 or 160 °C for 3 h. Finally, Caspeta et al. (2014) found the best glucan content (79.8 %) in samples of bagasse solids from Agave tequilana pretreated by an ethanol solvent at 160 °C with 0.5 % (w / w) sulfuric acid and 50 % (w / w) ethanol for 10 min.
One of the main economic activities of the inhabitants of the arid and semi-arid zones of the Altiplano Mexicano (Mexican Highlands), is the collection, stockpiling and commercialization of wild plants. These include Agave lechuguilla Torr., which is considered among the non-timber forest resources with the greatest social and economic value (Pando et al., 2004; Narcia et al., 2012). Its collection is an important factor in the economy of many families in rural areas, due to the net income it represents, where fiber is the important raw material for various national and foreign companies, which use it in the elaboration of different products, mainly in the brush industry (Castillo et al., 2008, 2013). Currently, the biomass of A. lechuguilla has been reported as a potential raw material for the production of ethanol (Carmona et al., 2017, Morales et al., 2017, Ortiz et al., 2017; Díaz et al., 2018).
However, the high energy expenditure in the pre-treatment stage by autohydrolysis (190 °C for 30 min) or diluted acid (180 °C, H2SO4 at 1.24 % w/v) has promoted the study of other methods that minimize this parameter and encourage an improvement in the economic feasibility of the process.
The mechanisms for obtaining the raw material of A. lechuguilla to obtain ethanol could have two routes. One would be the exploitation of the natural populations that occupy in the country about 20 million hectares (Castillo et al., 2011), and the second by the establishment of commercial plantations in areas abandoned to cultivation or degraded ecosystems.
If the biomass of the plantation buds is the main input and reaches a mixture of ethanol of 5.8 %, for the state of Coahuila it would require around 72 000 ha, both routes will generate the creation of new jobs, apart from the use of the lechuguilla for obtaining fiber that is currently made from wild populations. Under a commercial production system, the biomass yield per hectare would be 10 ton ha-1. Based on previous results obtained by Morales et al. (2017), the production yield of ethanol from biomass pretreated by autohydrolysis is 66 L ton-1 of dry biomass. Energy expenditure in the pretreatment stage and the cost of enzymatic complex during enzymatic hydrolysis are the factors with the greatest impact on the cost of production.
Therefore, the objective of the present work was to optimize the biological pre-treatment of biomass of Agave lechuguilla with Phanerochaete chrysosporium H-298 and to assess the production of ethanol from the enzymatic hydrolysates obtained.
Raw material
The buds are made up by a conical structure formed by the more tender leaves grouped in the center of the plant and it is regenerated after cutting, so it can be harvested from the same plant several times. The specimens of Agave lechuguilla (10 buds) were collected in Ramos Arizpe municipality, Coah.
For its conservation and storage in the laboratory of Environmental Biotechnology laboratory of the Faculty of Chemical Sciences of the Autonomous University of Coahuila (Biotecnología Ambiental de la Facultad de Ciencias Químicas de la Universidad Autónoma de Coahuila), the buds were dried in a dehydrator of Trays Koleff Mod. KL10, at 45 °C for 24 h and subsequently were discarded, milled and sieved in a Retsch SM100 cutting mill, until reaching 2 mm average particle size. Finally, the material was mixed and stored at room temperature in 28 °C in plastic containers (30 × 15 × 15 cm) until its pretreatment.
Chemical characterization of the biomass of Agave lechuguilla
The moisture content was specified with a moisture analyzer (OHAUS BL-MB23). The extractive and ash were separated by the analytical methods of the National Renewable Energy Laboratory of the United States of America (NREL): NREL / TP-510-42619 (Sluiter et al., 2005) and NREL/TP-510- 42622, respectively (Sluiter et al., 2008). The determination of cellulose (glucan), hemicellulose (xylan) and lignin was carried out according to the Laboratory Analytical Procedure (LAP): NREL /TP-510-42618) modified by Mussatto et al. (2011). 500 mg of the material was hydrolyzed with 72 % H2SO4 (w / w) for 7 min at 50 °C. Subsequently, the obtained hydrolyzate was diluted up to 4 % with distilled water.
A second hydrolysis of the reaction mixture was made by autoclaving at 121 °C for 1 h. The solution subjected to the autoclave was passed through 0.2 μm PVDF filters for analysis by high performance liquid chromatography (HPLC). The solid residues derived from the filtration were used to calculate the insoluble lignin in acid (Klason lignin). The protein content was determined by the Kjeldahl method (Nielsen, 1994).
Obtaining spores of Phanerochaete chrysosporium H-298
The culture of the strain P. chrysosporium H-298 was obtained from the Collection of Microbial Cultures (CDBB) of Center for Research and Advanced Studies of the National Polytechnic Institute (Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional) (CINVESTAV/IPN), Mexico. Its propagation was done on potato dextrose agar (PDA) for 2 days at 30 °C. The spores were collected and resuspended in sterile distilled water and stored at 4 °C until use.
Pretreatment of A. lechuguilla with P. chrysosporium H-298
For this purpose, nine Erlenmeyer flasks of 125 mL with 5 g of dried and milled core of A. lechuguilla previously sterilized with a volume of modified Kirk's medium solution (in g L-1: 2.0 de KH2PO4, 0.5 g L-1 MgSO4(5H2O, 0.1 de CaCl2(H2O, 0.03 de MnSO4(7H2O, 0.012 of yeast extract, 0.2 of ammonium tartrate and 1 mg L-1 of thiamin) en, until obtaining an initial humidity of 60 %. The medium was inoculated with 5 μl of the spore solution of P. chrysosporium H-298, under sterile conditions using a laminar flow hood. The flasks were placed in a New Brunswick ™ 124 / 24R orbital incubator at 30 °C for an incubation time of 10, 15 and 20 days. After each test, the biomass of A. lechuguilla was washed with distilled water and dried in an oven at 45 °C for 24 h. From each sample the cellulose, hemicellulose and lignin content was determined according to the protocols described.
Optimization of the biomass delignification process of A. lechuguilla with P. chrysosporium H-298
The experiments were carried out in 21 Erlenmeyer flasks of 250 mL, in which 10 g of Agave lechuguilla bud previously sterilized, 50 mL of Kirk medium solution and 10 μL of spore inoculum of P. chrysosporium H-298 were incorporated. The flasks were placed in an orbital shaker (New Brunswick™ 124/24R) at 30 °C and 150 rpm.
The optimization was done through a factorial design 32, with the statistical package Minitab™ version 17 (Minitab Inc., 2010), in which the factors of interest were 1) the incubation time (20, 40 and 60 days) and 2 ) the concentration of nitrogen (ammonium tartrate) in medium (1M, 2M and 3M) (tables 1 and 2). After each test, the biomass of A. lechuguilla was washed with distilled water and dried in an oven (Koleff Mod. KL10) at 45 °C for 24 h. At the conclusion of the procedure, each sample was determined its cellulose, hemicellulose and lignin content according to the protocol described.
Table 1 Biomass composition of Agave lechuguilla Torr. after pretreatment with Phanerochaete chrysosporium H-298 at different treatment times and nitrogen concentration.
| Time (days) |
Lignin (%) |
Cellulose (%) |
Hemicellulose (%) |
Lignin degradation (%) |
|---|---|---|---|---|
| 1M | ||||
| 0 | 19.07 ± 0.85 | 17.95 ± 0.94 | 11.31 ± 0.42 | - |
| 20 | 16.02 ± 0.61 | 17.97 ± 0.91 | 11.29 ± 0.21 | 16.0 ± 0.61 |
| 40 | 12.91 ± 0.53 | 17.88 ± 0.84 | 11.16 ± 0.48 | 32.3 ± 0.53 |
| 60 | 12.18 ± 0.68 | 17.54 ± 0.89 | 11.12 ± 0.40 | 36.15 ± 0.68 |
| 2M | ||||
| 0 | 19.07 ± 0.85 | 17.95 ± 0.94 | 11.31 ± 0.42 | - |
| 20 | 17.85 ± 0.55 | 17.98 ± 0.13 | 11.30 ± 0.18 | 6.4 ± 0.55 |
| 40 | 17.92 ± 0.48 | 17.97 ± 0.28 | 11.27 ± 0.23 | 6.0 ± 0.48 |
| 60 | 18.00 ± 0.62 | 17.92 ± 0.41 | 11.14 ± 0.52 | 5.6 ± 0.62 |
| 3M | ||||
| 0 | 19.07 ± 0.85 | 17.95 ± 0.94 | 11.31 ± 0.42 | - |
| 20 | 17.83 ± 0.15 | 17.97 ± 0.35 | 11.12 ± 0.32 | 6.5 ± 0.15 |
| 40 | 18.26 ± 0.24 | 17.96 ± 0.39 | 11.29 ± 0.31 | 4.2 ± 0.24 |
| 60 | 18.39 ± 0.28 | 17.88 ± 0.28 | 11.27 ± 0.36 | 3.6 ± 0.28 |
Table 2 Enzymatic hydrolysis of biomass from Agave lechuguilla Torr. pretreated with Phanerochaete chrysosporium H-298 under optimal conditions and biomass without pretreatment.
| Time (hours) |
Biomass without pretreatment | Pretreated biomass | ||
|---|---|---|---|---|
| Glucose (g L-1) |
Hydrolysis yield (%)a |
Glucose (g L-1) |
Hydrolysis yield (%)b |
|
| 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 12 | 8.93 ± 0.16 | 18.09 ± 0.16 | 34.07 ± 0.47 | 70.64 ± 0.47 |
| 24 | 11.59 ± 0.31 | 23.48 ± 0.31 | 36.19 ± 0.52 | 75.03 ± 0.52 |
| 48 | 17.85 ± 0.25 | 36.16 ±0.25 | 43.09 ± 0.91 | 89.34 ± 0.91 |
| 72 | 18.10 ± 0.64 | 36.66 ± 0.64 | 43.74 ± 0.73 | 90.69 ± 0.73 |
| 92 | 18.72 ± 0.32 | 37.92 ± 0.32 | 44.90 ± 1.14 | 93.09 ± 1.14 |
aInitial glucans in sample without pretreatment = 17.95 %; bInitial glucans in pretreated sample = 17.54 %
Enzymatic hydrolysis of the biomass of Agave lechuguilla pretreated with P. chrysosporium H-298
As in the previous tests, three Erlenmeyer flasks of 125 mL were used, in which 10 g (dry basis) of biomass of A. lechuguilla pretreated under optimal conditions with P. chrysosporium H-298 were placed. Subsequently, 40 g of a citrate buffer at a pH of 4.8 was added, which corresponds to a solids loading of 25 % (w/w). The commercial enzymatic complex Cellic™ CTec3 was incorporated into the flasks at an enzyme load of 25 Units of Filter Paper (UPF) g-1 of glucans. The hydrolysis was carried out at 50 °C and a stirring speed of 200 rpm for 92 h. At hydrolysis times of 12, 24, 48, 72 and 92 h, samples were taken from the hydrolyzate and centrifuged at 10 000 rpm for 10 min (HarausTM MegafugeTM 16 R). The liquid fraction was filtered through 0.22 μm PVDF filters. Samples were analyzed on an Agilent 1260 Infinity liquid chromatograph (HPLC) equipped with a refractive index detector at 45 °C using an Agilent Hi-Plex H column at 35 °C (7.7 × 300 mm), 5 mM H2SO4 cone as phase mobile, at a flow rate of 5.0 mL min-1. The yield of enzymatic hydrolysis (saccharification) was expressed as g of glucose released g-1 from initial glucans in the pretreated material.
Fermentation of enzymatic hydrolyzate of biomass of A. lechuguilla
The fermentation of the hydrolysates was carried out in three Erlenmeyer flasks of 125 mL, with 30 mL of enzymatic hydrolyzate. The hydrolysates were supplemented with the following nutrients (in g L-1): yeast extract 10, KH2PO4 1.17, CaCl2 0.09, MgSO4(7H2O 0.36, (NH4)2SO4 4.14. The medium was supplemented with 15 mL L-1 of a saline solution (stock saline solution) containing NaCl 1.26, CuSO4(5H2O 0.26, FeSO4(5H2O 0.22, MnCl2(4H2O 0.12 y ZnCl2(7H2O 0.32.The fermentation conditions were: 5.5 of medium pH, 30 °C, 100 rpm agitation speed, 10 % (v/v) inoculum (Saccharomyces cerevisiae ATCC 4126), during 10 h of incubation (Table 3). took a sample of the fermentation broth at 0, 2, 4, 6, 8 and 10 h, which were centrifuged at 10 000 rpm for 10 min, and the liquid fraction was filtered through PVDF filters for the quantification of ethanol and glucose by HPLC.
Analysis
Glucose and ethanol were determined by HPLC (Agilent 1260) equipped with a refractive index detector at 45 °C, using an Agilent Hi-Plex H column at 35 °C (7.7 × 300 mm) and as a mobile phase as a mobile solution. of 5 mM H2SO4 at a flow of 0.5 mL min-1. All experiments were done in triplicate and the average values were recorded. The analysis of variance (ANOVA) applied together with a Fisher F test with a value of p <0.05 with the Minitab® version 17 statistical package (Minitab Inc., 2010).
Results and Discussion
Pretreatment of A. lechuguilla with P. chrysosporium H-298
The kinetics of degradation of lignin during exploratory studies of the biomass pre-treatment of A. lechuguilla with P. chrysosporium H-298 is illustrated in Figure 1. The results obtained indicate a maximum degradation of lignin (15.9 %) after 15 days of treatment at a temperature of 30 °C, which remains without significant changes after 20 days. Cellulose and hemicellulose remain unchanged through this process.
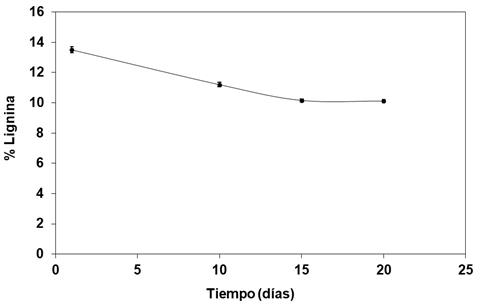
Lignina = Lignin; Tiempo (días)= Time (days)
Figure 1 Kinetic degradation of lignin of Agave lechuguilla Torr. with P. chrysosporium H-298 at 30 °C.
Zeng et al. (2013) performed a purification and biochemical characterization study of two extracellular enzymes of P. chrysosporium (lignin peroxidase-LiP and manganese peroxidase-MnP) responsible for the degradation of lignin, which resulted in both peroxidases showing a maximum activity at 30 °C.
Optimization of the biomass delignification process of A. lechuguilla with P. chrysosporium H-298
Table 1 describes the degradation of biomass lignin from A. lechuguilla with P. chrysosporium H-298 at different treatment times and at different concentrations of ammonium tartrate as a source of nitrogen. A maximum removal of lignin (36.15 %) can be observed after 60 days of treatment at a concentration of 1M of ammonium tartrate; those that exceeded 1M caused a low degradation of lignin (maximum 6 % in the 2M and 3M tests), behavior that was also observed in the study carried out by Haddadin et al. (2009) who concluded that high concentrations of nitrogen manage to inhibit the production of lignin peroxidases and lacquers, as well as low yields of lignin removal; however, small concentrations of nitrogen are necessary for the optimal growth of P. chrysosporium.
It was observed that the cellulose and hemicellulose content remains without major changes during pretreatment, with a loss of only 2 % of cellulose, which demonstrates the efficiency of the pretreatment, which, in addition to increasing the digestibility through the degradation of lignin, allows to preserve the structural sugars for its hydrolysis in the saccharification stage. Some studies by Zhang et al. (2012) proved that the pretreatment time is the most important factor for the degradation of lignin with P. chrysosporium in rice straw.
Figure 2 illustrates the increase in lignin removal from 16 % to 36.15 % in treatments at 20 and 60 days respectively, which corroborates that the treatment time was the most important factor for this process (Zhang et al., 2012).
Enzymatic hydrolysis of biomass of A. lechuguilla pretreated with P. chrysosporium H-298
Table 2 shows the data indicating a maximum glucose release of 44.90 g L-1 corresponding to 93.09 % hydrolysis yield of the pretreated material at 92 h. This yield was more than 2 times higher than that obtained with the biomass without pretreatment (37.92 %, with a glucose release of 18.72 g L-1). This increase was not significant after 48 h of incubation for both cases. It is likely that in this period, the cellulases began to be inhibited. This result can be attributed to an increase in the diffusional limitation of cellulases due to a high load of solids, or lignin affected the action of enzymes, blocking access to cellulose (Ríos et al., 2017).
When comparing the maximum yield of hydrolysis obtained with previous results by Ortíz et al. (2017), using biomass of A. lechuguilla pretreated by autohydrolysis and the Celluclast 1.5 L enzyme complex (60.85 %, with a glucose release of 59 g L-1), it can be proved that the effectiveness of biological pretreatment with P. chrysosporium H -298. However, contrasting this same parameter with the same enzyme complex (Cellic® CTec3), the previous study by Morales et al. (2017) achieved a similar performance (96.5 %, with a glucose release of 108.0 g L-1). In both cases the material was pretreated by autohydrolysis, the release of glucose was higher than that resulted in the present work. This is due to the fact that during the hydrothermal pretreatment, all the extractives are removed and more than 90 % of the hemicellulose is hydrolyzed, which allows the material to be enriched in cellulose and duplicates its composition with respect to the non-raw material (> 40 %).
Fermentation of enzymatic hydrolyzate of biomass of A. lechuguilla pretreated with P. chrysosporium H-298
Table 3 shows the results of the fermentation process of the enzymatic hydrolyzate obtained by Saccharomyces cerevisiae ATCC 4126. The maximum ethanol production was 16.53 g L-1, in a fermentation time of 10 h, equivalent to an ethanol concentration of 2 % v/v. The maximum conversion efficiency according to the theoretical value (EC) was 90.03 % at 6 h of incubation; after this period, the EC decreased to 82.69 % at 10 o'clock. This behavior is normal due to the reduction of the concentration of glucose in the fermentation medium.
Table 3 Kinetic parameters of fermentation study of biomass enzymatic hydrolysates of Agave lechuguilla Torr. pretreated with P. chrysosporium H-298.
| Time (h) | Glucose (g L-1) | Ethanol (g L-1) | YE/G a | %ECb |
|---|---|---|---|---|
| 0 | 45.2 ± 0.84 | 0.0 ± 0.0 | 0.0 | 0.0 |
| 2 | 25.1 ± 0.25 | 8.49 ± 0.17 | 0.42 | 82.82 |
| 4 | 20.4 ± 0.43 | 10.34 ± 0.22 | 0.39 | 77.17 |
| 6 | 15.9 ± 0.19 | 12.59 ± 0.28 | 0.50 | 98.03 |
| 8 | 10.3 ± 0.11 | 14.59 ± 0.16 | 0.35 | 70.02 |
| 10 | 5.7 ± 0.15 | 16.53 ± 0.29 | 0.42 | 82.69 |
aYE/G = Ethanol yield (g ethanol/g consumed glucose); EC = Conversion efficiency (Ethanol yield/ Maximum theoretical ethanol yield * 100); Maximum theoretical ethanol yield = 0.51 g ethanol/g glucose.
The concentration of ethanol obtained is attributed to the fact that it may be higher to increase the fermentation time, since a constant consumption of glucose was noticed until 10 h. However, this period was not sufficient to detect a total glucose consumption (glucose remaining of 5.7 g L-1). In the fermentation of enzymatic hydrolysates of biomass from Agave lechuguilla treated by autohydrolysis, Ortiz et al. (2017) recorded a maximum conversion efficiency of 91 %, lower than that obtained in this study. This difference in CD can be attributed to the fact that biological pretreatment with P. chrysosporium H-298 does not generate inhibitory byproducts of the fermentation stage, unlike the autohydrolysis process, in which inhibitors such as acetic acid, furfural and hydroxymethylfurfural are produced (HMF ) during pretreatment.
Conclusions
Increasing the biomass delignification of A. lechuguilla with P. chrysosporium from 15.9 % up to 36.15 % is possible by extending the pretreatment time and the addition of a nitrogen source (ammonium tartrate) at low concentration (1 M). The delignification of the material by this method lead to an increase in enzymatic digestibility of almost 100% (hydrolysis yield) compared to the untreated sample (40%), with the production of hydrolysates with a high glucose content without the presence of inhibitors; this favors the high ethanol yields.
Future studies should focus on evaluating the degradation of lignin in the fibrous fraction of the material, with the separation of the fraction rich in extracts by means of sieves; with this it will be possible to have a higher content of initial cellulose and potential to obtain sugars and ethanol.
Acknowledgements
The authors thank the Fondo Sectorial SAGARPA-CONACyT (Sector Fund SAGARPA-CONACyT) for the financial support granted to project No. 175404 entitled “Desarrollo de tecnologías de producción de etanol a partir de biomasa de plantaciones de lechuguilla (Agave lechuguilla) existentes y nuevas plantaciones de la región semidesértica del norte de México” ("Development of technologies for the production of ethanol from biomass of existing lechuguilla (Agave lechuguilla) plantations and new plantations in the north semi-desert region from Mexico").
REFERENCES
Althuri, A., L. K. S. Gujjala and R. Banerjee. 2017. Partially consolidated bioprocessing of mixed lignocellulosic feedstocks for ethanol production. Bioresource Technology. 245: 530-539. https://doi.org/10.1016/j.biortech.2017.08.140. [ Links ]
Arora, A., S. Priya, P. Sharma, S. Sharma and L. Nain. 2016. Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocatalysis and Agricultural Biotechnology 8: 66-72. https://doi.org/10.1016/j.bcab.2016.08.00. [ Links ]
Buruiana, C. T., C. Vizireanu, G. Garrote and J. C. Parajó. 2014. Optimization of corn stover biorefinery for coproduction of oligomers and second-generation bioethanol using non-isothermal autohydrolysis. Industrial Crops and Products 54: 32-39. https://doi.org/10.1016/j.indcrop.2014.01.003. [ Links ]
Carmona, J. M., T. K. Morales M., S. I. Mussatto, D. Castillo Q. y L. J. Ríos G. 2017. Propiedades químicas, estructurales y funcionales de la lechuguilla (Agave lechuguilla Torr.) Revista Mexicana de Ciencias Forestales 8 (42): 100- 122. https://doi.org/10.29298/rmcf.v8i42.21. [ Links ]
Caspeta, L., M. A. Caro B., T. Ponce N. and A. Martinez. 2014. Enzymatic hydrolysis at high-solids loadings for the conversion of agave bagasse to fuel ethanol. Applied Energy. 113: 277-286. https://doi.org/10.1016/j.apenergy.2013.07.036. [ Links ]
Castillo, Q. D., C. A. Berlanga R., M. Pando M., y A. Cano P. 2008. Regeneración del cogollo de Agave lechuguilla de cinco procedencias bajo cultivo. Revista Ciencia Forestal en México 3(103): 27-40. [ Links ]
Castillo Q., D, O. Mares A. y E. E. Villavicencio G. 2011. Lechuguilla (Agave lechuguilla Torr.) planta suculenta de importancia económica y social de las zonas áridas y semiáridas de México. Boletín de la Sociedad Latinoamericana y del Caribe de Cactáceas y otras Suculentas. 8(2):6-9. [ Links ]
Castillo Q., D., J. T. Sáenz R., M. Narcia V. y J. A. Vázquez R. 2013. Propiedades físico-mecánicas de la fibra de Agave lechuguilla Torr. de cinco procedencias bajo plantaciones. Revista Mexicana de Ciencias Forestales 4(19): 78-91. https://doi.org/10.29298/rmcf.v4i19.380. [ Links ]
Díaz B., D. I., J. R. de La Cruz, J. C. López L., T K. Morales M., E. Ruiz, L J. Ríos G., I. Romero and E. Castro. 2018. Optimization of dilute acid pretreatment of Agave lechuguilla and ethanol production by co-fermentation with Escherichia coli MM160. Industrial Crops Products 114: 154-163. https://doi.org/10.1016/j.indcrop.2018.01.074. [ Links ]
González B., E., J. C. Santana M., F. J. Ríos F., H. M Poggi V., A. C. Ramos V., E. Cristiani U. and T. Ponce N. 2017. Phenolic compounds inhibit cellulase and xylanase activities of Cellulomonas flavigena PR-22 during saccharification of sugarcane bagasse. Fuel 196: 32-35. https://doi.org/10.1016/j.fuel.2017.01.080. [ Links ]
Haddadin, M. S. Y., J. Haddadin, O. I. Arabiyat and B. Hattar. 2009. Biological conversion of olive pomace into compost by using Trichoderma harzianum and Phanerochaete chrysosporium. Bioresource Technology 100(20): 4773-4782. https://doi.org/10.1016/j.biortech.2009.04.047. [ Links ]
Huang, S., D. Huang, Q. Wu, M. Hou, X. Tang and J. Zhou. 2017. The effects of environmental C/N on the activities of lignin-degrading enzymes produced by Phanerochaete chrysosporium. Pedosphere 160. https://doi.org/10.1016/S1002-0160(17)60391-6. [ Links ]
Minitab, Inc. 2010. Minitab 17 Statistical Software. Ver. 17. Minitab, INC. State College, PA USA n/p. [ Links ]
Morales M., T. K., D. I Díaz B., J. A. Rodríguez de la G., J. Morlett Ch., A. J. Castro M., J. Quintero and L.J. Ríos González. 2017. Assessment of different saccharification and fermentation configurations for ethanol production from Agave lechuguilla. BioResources 12(4): 8093-8105. [ Links ]
Mussatto, S. I., L. M. Carneiro, J. P. A. Silva, I. C. Roberto and J. A. Teixeira. 2011. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydrate Polymers 83(2): 368-374. [ Links ]
Narcia, V. M., D. Castillo Q., J. A. Vázquez R. y C.A. Berlanga R. 2012. Turno Técnico de la lechuguilla (Agave lechuguilla Torr.) en el noreste de México. Revista Mexicana de Ciencias Forestales 3(9): 81-88. [ Links ]
Nielsen, S. S. 1994. Introduction to the chemical analysis of foods. Jones and Bartlett publishers. New York, NY USA. pp. 209-212. [ Links ]
Ortiz M., O. H., T. K. Morales M., L. J. Ríos G., J. A. Rodríguez de la G. J. Quintero y G. E. Aroca. 2017. Bioethanol production from Agave lechuguilla biomass pretreated by autohydrolysis. Revista Mexicana de Ingeniería Química 16(2): 467-476. [ Links ]
Pando M., M., O. Eufracio, E. Jurado and E. Estrada. 2004. Post-harvest growth of lechuguilla (Agave lecheguilla Torr. Agavaceae) in northeastern Mexico. Economic Botany 58(1): 78-82. https://doi.org/10.1663/00130001(2004)058[0078:PGOLAL]2.0.CO;2. [ Links ]
Pérez P., J. A., M. G. Lopez O., P. Varanasi, V. Stavila, G. Cheng, S. Singh and B. A. Simmons. 2013. Comparison of the impact of ionic liquid pretreatment on recalcitrance of agave bagasse and switchgrass. Bioresource Technology 127: 18-24. https://doi.org/10.1016/j.biortech.2012.09.124. [ Links ]
Pérez P., J. A., M. G. Lopez O., J. A. Chávez C., P. Varanasi, V. Stavila, G. Cheng, S. Singh and B. A. Simmons. 2015. Characterization of agave bagasse as a function of ionic liquid pretreatment. Biomass & Bioenergy. 75: 180-188. https://doi.org/10.1016/j.biombioe.2015.02.026. [ Links ]
Pérez-Pimienta, J. A., A. Vargas-Tah, K. M. López-Ortega, Y. N. Medina-López, J. A. Mendoza-Pérez, S. Avila, S. Singh, B. A. Simmons, I. Loaces y A. Martínez. 2017. enzymatic saccharification and fermentation of ionic liquid and organosolv pretreated Agave bagasse for ethanol production. Bioresource Technology 225:191-198. [ Links ]
Rios-González, L. J., T. K. Morales-Martínez, M. F. Rodríguez-Flores, J. A. Rodríguez-De la Garza, D. Castillo-Quiroz, A. J. Castro-Montoya y A. Martinez. 2017. Autohydrolysis pretreatment assessment in ethanol production from agave bagasse. Bioresource Technology 242:184-190. [ Links ]
Sindhu, R., P. Binod and A. Pandey. 2016. Biological pretreatment of lignocellulosic biomass - an overview. Bioresource Technology 199:76-82. http://dx.doi.org/10.1016/j.biortech.2015.08.030. [ Links ]
Sluiter, A., R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton. 2005. Determination of extractives in biomass. Report No. TP-510-42619. Golden, CO USA. pp. 1-9. [ Links ]
Sluiter, A., B. Hames, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton. 2008. Determination of ash in biomass. National Renewable Energy Laboratory. Report No. TP-510-42622. Golden, CO USA. pp. 1-5. [ Links ]
Wan, C. and Y. Li. 2012. Fungal pretreatment of lignocellulosic biomass. Biotechnology Advances 30: 1447-1457. ttps://doi.org/10.1016/j.biotechadv.2012.03.003. [ Links ]
Yang, L., Y. Land P. E. Savage. 2016. Near and supercritical ethanol treatment of biocrude from hydrothermal liquefaction of microalgae. Bioresource Technology 211: 779-782. https://doi.org/10.1016/j.biortech.2016.03.151. [ Links ]
Yang, Q. and X. Pan. 2012. Pretreatment of Agave americana stalk for enzymatic saccharification. Bioresource Technology 126: 336-340. https://doi-org.proxy.infosal.uadec.mx/10.1016/j.biortech.2012.10.018. [ Links ]
Zeng, G. M., M. H. Zhao, D. L. Huang, C. Lai, C. Huang, Z. Wei and M. Cheng. 2013. Purification and biochemical characterization of two extracellular peroxidases from Phanerochaete chrysosporium responsible for lignin biodegradation. International Biodeterioration and Biodegradation 85: 166-172. https://doi.org/10.1016/j.ibiod.2013.07.005. [ Links ]
Zhang, S., M. Jiang, Z. Zhou, M. Zhao and Y. Li. 2012. Selective removal of lignin in steam-exploded rice straw by Phanerochaete chrysosporium. International Biodeterioration and Biodegradation 75: 89-95 [ Links ]
Received: May 06, 2018; Accepted: November 23, 2018











 texto en
texto en 



