Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.9 no.50 México Nov./Dez. 2018
https://doi.org/10.29298/rmcf.v9i50.256
Articles
Drought effect over the distribution and density of Dendroctonus mexicanus Hopkins, 1905 in temperate forest
1Tundra, Ciencia Ciudadana. México.
2Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. Universidad Autónoma de Nuevo León.México.
The temperate forests of Nuevo León are susceptible to the attack of Dendroctonus bark beetles, which are considered the most destructive insects in Mexican pine forests. These insects play an important role in forests; however at high densities they can alter some ecological processes. In this study, the infestations of Dendroctonus mexicanus are described according to the altitude at which the outbreaks occur, to the infested area, and to the temporary changes of these variables from 2008 to 2012. In addition, the relationship between the number of infested hectares and the standardized precipitation index is analyzed. From 2008 to 2012, 1 435.13 hectares were found to be infested. The infestations were located at an altitude between 1 176 and 3 010 masl, covering practically the entire distribution of the genus Pinus in the area. In the study period there was no evidence of an increase in the altitude at which the infestations occurred; however, an increase in the altitudinal range is observed. The high mortality caused by D. mexicanus to Pinus, regardless of the altitude at which they are found, is an indicator of the expansion of the regional altitudinal range of D. mexicanus after a dry year.
Key words: Climate change; Dendroctonus mexicanus Hopkins; 1905; population dynamics; bark beetles; altitudinal range; forest pest
Los bosques templados de Nuevo León son susceptibles al ataque de insectos descortezadores del género Dendroctonus, los cuales son considerados como los más destructivos en bosques de pino en México; donde tienen un papel importante, ya que a densidades altas pueden alterar los procesos ecológicos. En este estudio se caracterizaron las infestaciones por Dendroctonus mexicanus con base en la altitud a la que se presentan y la superficie infestada; así como, los cambios temporales de estas variables de 2008 a 2012; además, se analizó la relación entre el número de hectáreas afectadas y el índice de precipitación estandarizada. Se contabilizaron 1 435.13 ha dañadas de 2008 a 2012. Las infestaciones se registraron desde los 1 176 hasta 3 010 msnm, que cubren, prácticamente, toda la distribución del género Pinus en la zona. En el periodo estudiado no se observó evidencia de un incremento en la altitud a la que se localizaron los insectos; pero sí, un aumento en el intervalo altitudinal. La alta mortalidad causada por D. mexicanus en hospederos del género Pinus, independientemente de la altitud a la que se ubiquen, es un indicador de la expansión del intervalo altitudinal regional de D. mexicanus después de un año seco.
Palabras clave: Cambio climático; Dendroctonus mexicanus Hopkins; 1905; dinámica poblacional; insectos descortezadores; intervalo altitudinal; plaga forestal
Introduction
The temperate forests of northeastern Mexico are located in the mountain systems of the Sierra Madre Oriental. In the state of Nuevo León, 451 300 ha are covered with pine or pine-oak forests (Palacio-Prieto et al., 2000). These are susceptible to attack by bark beetles of the Curculionidae family, specifically to those of the genera Dendroctonus, Ips and Pseudips, which are considered the most destructive in the pine forests of Mexico (Cibrián et al., 1995) and of the United States of America (Wood, 1963; Paine et al., 1997).
The genera Dendroctonus and Pinus are related to each other in terms of evolution (Zúñiga et al., 2006). Therefore, at low densities, they are necessary for the functioning of the ecosystems (Wood, 1982); however, at high densities they can alter the ecological processes, as well as the makeup, structure or environmental services of the conifer forests (Malmström and Raffa, 2000; Hawkes et al., 2003; Kurz et al., 2008; McFarlane and Witson, 2008; Jenkins et al., 2008).
The causes of the growth of the bark beetle population can be explained by hypotheses related to both intrinsic (Coulson et al., 1989; Williams and Liebhold, 2002; Edmonds et al., 2005; Raffa et al., 2008; Westfall and Ebata, 2009; Evangelista et al., 2011) and extrinsic factors (Safranyik and Linton, 1998; Turchin et al., 1999; Lombardero et al., 2000; Turchin et al., 2003; Trzcinski and Reid, 2009). Furthermore, the behavior of an infestation outbreak can be linked to the variability of the climate (Logan et al., 1999), the altitude (Rubin-Aguirre et al., 2015), and, indirectly, to the effects of the host trees on the climate (Bentz et al., 2010).
On the other hand, the dependence of the beetles on the temperature (Raffa et al., 2008) and the reduction of the precipitation and moisture are directly related to the defense capability of the trees (Wermelinger, 2004; Raffa et al., 2005; Six et al., 2014) and to the survival of the beetles in winter (Safranyik and Linton, 1998).
The present study analyzes the drought effect, expressed as the standardized precipitation index (SPI) on the surface area affected by D. mexicanus Hopkins 1905, and the changes in altitude exhibited by the population between 2008 and 2012. The objectives were: i) to characterize the infestations with Dendroctonus mexicanus Hopkins, 1905, based on the altitude at which the outbreaks occur, on the infested surface area, and on the temporary changes in these variables during a five-year period; and ii) to correlate the number of infested hectares with the standardized precipitation index.
Materials and Methods
Description of the study area
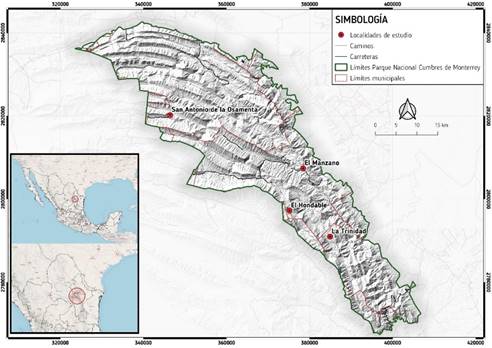
This study was carried out in the temperate pine and pine-oak forests located within the Cumbres de Monterrey National Park (PNCM, by its Spanish acronym), in the western-central area of the state of Nuevo León; in the physiographic province of the Sierra Madre Oriental. The types of climate are: (Cw1), temperate with summer rains, and (ACw), semiwarm subhumid with summer rains.
Field and laboratory work
The areas where bark beetles were present were identified based on the information generated from the infestation records delivered to the management of the Cumbres de Monterrey National Park. The data of location, host tree, insect species, and infested surface area were verified in field for each of the years from 2008 to 2012. The surface areas and the altitudes were registered with Garmin eTrex Legend GPS, and the insects were identified by means of taxonomy codes. The areas where the location and surface area data were positioned in a geographical information system (GIS) (Arc Gis 10.1), with the coordinates in WGS84 Datum format. The altitude was verified with the topographic charts of the Instituto Nacional de Estadística y Geografía, INEGI (National Institute of Statistics and Geography) (Rayones: G14C43; Allende: G14C36 and San Antonio de las Alazanas: G14C35). All the surface areas were quantified for the five study years. Figure 1 shows the analyzed localities.
The precipitation and temperature data utilized to estimate the SPI corresponded to stations 19069, 19048 and 19002 of the Comisión Nacional del Agua, Conagua (National Water Commission). This index made it possible to quantify the precipitation deficit in the year, which reflects the impact of the drought on the ecosystem. Its values range between 2 and -2; they are representative of the variability of the precipitation in terms of its history. The negative values (≤-1) are associated with periods of drought, while the positive values indicate a surplus (McKee et al., 1993). The period of drought ended when the SPI reached positive values that correspond to precipitations above the median (Conagua, 2015).
Results and Discussion
The four selected localities were: Hondable, Manzano, (Santiago, N.L.) Trinidad (Montemorelos, N.L.) and San Antonio (Santa Catarina, N.L.). The host species were: Pinus pseudostrobus Lindl. and P. teocote Schiede ex Schltdl. & Cham. at Hondable, Manzano and Trinidad; P. teocote, P. greggii Engelm. ex Parl. and P. cembroides Zucc. at San Antonio, which coincide with those cited by Salinas-Moreno (2010) for D. mexicanus.
During the study period, 1 435.13 hectares were found to be infested with Dendroctonus mexicanus (Table 1). The number of hectares was estimated based on a variance analysis. The year 2012 had the largest number of affected hectares, with differences in relation to all the previous years (2008-2011: F=0.003, d.f. =3; 2009-2012: F=0.05 d.f.=3; 2010-2012: F= 0.05, d.f.= 3; 2011-2012: F=0.004, d.f.= 3). Although the year 2010 seemingly had more affected hectares than 2008, 2009 and 2011, there were no statistical differences (F= 2.35, d.f.= 15).
Table 1 Surface area infested with Dendroctonus mexicanus Hopkins, 1905 in the four studied localities (2008 through 2012).
| Locality | Surface area infested with D. mexicanus (ha) | |||||
|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | Total | |
| Hondable | 53 | 103.05 | 99.73 | 60.78 | 117.8 | 434.35 |
| Manzano | 4.96 | 7.54 | 66.47 | 65.12 | 15.96 | 160.5 |
| Trinidad | 17.38 | 5.18 | 210.29 | 25.62 | 448.38 | 706.85 |
| San Antonio | 26.76 | 0 | 0 | 29.33 | 77.79 | 133.88 |
| Total | 102.1 | 115.77 | 376.49 | 180.84 | 659.93 | 1435.13 |
The genus Pinus is host to D. mexicanus, whose preferred altitude interval is 2 100 to 2 500 m, with variations of 800 to 3 400 m (Salinas-Moreno, 2004). In the study area, the host trees are distributed at altitudes ranging between 1 100 and 3 200 masl. The lowest altitude at which infestations with D. mexicanus occurred was that of Trinidad, at 1 176 masl, in the year 2012, and the highest, that of San Antonio, at 3 010 masl, in 2011.
In general, no increase was observed in the altitude at which the infestations occurred; however, the altitude interval broadened in three localities (Hondable, Trinidad and San Antonio) in the years 2011 and 2012. Likewise, the number of outbreaks increased in the same period (Table 2).
Table 2 Altitude interval and number of outbreaks of infestation with Dendroctonus mexicanus Hopkins, 1905 in the four studied localities (2008 through 2012).
| Locality | Year | Number of outbreaks year-1 | Altitude interval (m) |
|---|---|---|---|
| Hondable | 2008 | 1 | 200 |
| 2009 | 2 | 381 | |
| 2010 | 6 | 281 | |
| 2011 | 1 | 561* | |
| 2012 | 18 | 684* | |
| Manzano | 2008 | 1 | 140 |
| 2009 | 1 | 50 | |
| 2010 | 5 | 319* | |
| 2011 | 10 | 186 | |
| 2012 | 6 | 149 | |
| Trinidad | 2008 | 3 | 730 |
| 2009 | 2 | 78 | |
| 2010 | 3 | 162 | |
| 2011 | 1 | 461 | |
| 2012 | 22 | 1139* | |
| San Antonio | 2008 | 2 | 260 |
| 2009 | 0 | 0 | |
| 2010 | 0 | 0 | |
| 2011 | 6 | 891* | |
| 2012 | 12 | 778* |
* = Years in which the altitude interval increased.
A general increase in the mortality of the genus Pinus was observed, regardless of the altitude; this may be an indicator of the expansion of its regional distribution. In P. ponderosa forests in the southern United States of America, various species of Scolytidae were observed to exhibit high mortality rates at sites located at lower altitudes and with higher degrees of drought (Negrón and Popp, 2004; Negrón et al., 2009). The same tendency was documented in forests of Michoacán, with a great abundance of Scolytidae in low altitude areas (Rubin-Aguirre et al., 2015).
In all localities, the driest year was 2011 (Table 3), this may have led to hydric stress and weakening of the trees (Allen et al., 2010; Allen et al., 2015); furthermore, it led to an increase in the population density of bark beetles (Bentz et al., 1991; Safranyk and Linton, 1998; Ungerer et al., 1999; Raffa et al., 2008).
Table 3 Standardized precipitation index (SPI) and the infected area per in template forests in the four studied localities.
| Locality | Year | Surface Area | SPI | Correlation coefficient |
|---|---|---|---|---|
| Hondable | 2008 | 53 | -1.62 * | 0.97 |
| 2009 | 103.05 | -1.55* | ||
| 2010 | 99.73 | 1.84** | ||
| 2011 | 60.77 | -1.82* | ||
| 2012 | 117.8 | 1.95** | ||
| Manzano | 2008 | 4.96 | 0.138** | 0.60 |
| 2009 | 7.54 | -1.55* | ||
| 2010 | 66.47 | 1.84** | ||
| 2011 | 65.12 | -2.00* | ||
| 2012 | 16.15 | 1.95** | ||
| Trinidad | 2008 | 17.38 | -1.16*** | 0.77 |
| 2009 | 5.18 | -1.33*** | ||
| 2010 | 210.29 | 1.86** | ||
| 2011 | 25.62 | -1.33*** | ||
| 2012 | 448.38 | -1.22*** | ||
| San Antonio | 2008 | 26.76 | 0.95** | 0.93 |
| 2009 | 0 | 0.68** | ||
| 2010 | 0 | 0.73** | ||
| 2011 | 29.33 | -2.00* | ||
| 2012 | 77.79 | -1.50* |
* = Extremely dry year; ** = Humid year, Normal year; *** =Moderately dry year.
In the present study, only the locality Hondable registered a high correlation between the affected surface area and the standardized precipitation index for the same year (R2=-0.72). Manzano (R2= 0.08), Trinidad (R2= 0.05) and San Antonio (R2= -0.16) exhibited no correlations. The result for Hondable may be due to the fact that three out of the five years of the study had SPIs with extreme drought. However, their effects on the populations of bark beetles cannot not always be observed in the same year but become evident in a later year.
In Oregon, infestations with D. ponderosa were correlated with the precipitation of the year in which the population growth was registered, as well as with that of the previous year (Preisler et al., 2012).
In the four studied localities, a high negative correlation was determined between the standardized precipitation index and the surface area infested in the following year (Hondable: R2=-0.97; Manzano: R2=-0.60; Trinidad: R2=-0.77; San Antonio: R2=-0.93); the data are shown in Table 3. This correlation can be accounted for by the fact that some extreme climate events bring abundant resources for certain species of bark beetles (Gandhi and Herms, 2010).
Larger infested surface areas and broader altitude intervals (1 176-2 936 masl) were registered in the year 2012; furthermore, the SPI was moderate to extremely dry in 2011 and 2012; both situations may represent an accumulated drought effect in the trees, particularly in the locality of Trinidad, where 2008 and 2009 were dry years, and the infested surface area increased significantly in 2010. The years 2011 and 2012 were also dry in the same locality, and the infested surface area increased in 2012.
Some research shows an expansion in the distribution of the insects because the increase in temperature creates new niches for this growth (Nealis and Peter, 2009). Although the response of the bark beetles to climate change does not appear to be linear but rather more complex, there already are direct effects on their population and on their hosts, which renders the analysis difficult (Bentz et al., 2010). For various species of Scolytidae, increases in the altitude interval of the infestations have been shown to be related to dry summers (Marini et al., 2012). Therefore, the climate is regarded as a more important limiting factor than the availability of hosts (Bentz et al., 2010). Likewise, according to other studies, warmer temperatures are associated to the reproduction and survival of Scolytidae species (Bentz et al., 1991; Tykarski, 2006).
There is no evidence of an increase in the altitude at which infestations occurred between 2008 and 2012; however, an increase in the altitude interval can be observed. In 2008, the infestations appeared between 1 471 and 2 300 m (interval of 829 m) while in 2012 they were between 1 176 and 2 936 m (range of 1 760 m).
Conclusions
1 435.13 ha were found to be infested with D. mexicanus in the 2008-2012 period, and an increase was observed in 2012.
Infestations with D. mexicanus occurred at an altitude between 1 176 and 3 010 masl, almost equal to the entire altitude interval at which its hosts are distributed: 1 100 to 3 200 m.
The high mortality caused by D. mexicanus in the genus Pinus, regardless of the altitude at which these trees are distributed, is an indicator of the expansion of the regional altitude interval of D. mexicanus after a dry year.
The standardized precipitation index is negatively correlated with the surface affected by D. mexicanus in the year following that in which the drought occurred, or as an accumulated effect of two years of drought; thus, negative SPI values result in an increase of the affected surface area.
Acknowledgements
To the Consejo Nacional de Ciencia y Tecnología (Conacyt) (National Council of Science and Technology) (Conacyt) for the master's scholarship granted to the first author.
REFERENCES
Allen, C. D., A. K. Macalady, H. Chenchouni, D. Bachelet, N. McDowell, M. Vennetier, T. Kitzberger, A. Rigling, D. D. Breshears, E. H. T. Hogg, P. González, R. Fensham, Z. Zhang, J. Castro, N. Deminova, J. H. Lim, G. Allard, S. W. Running, A. Semerci and N. Cobb. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259(4): 660-684. DOI: 10.1016/j.foreco.2009.09.001. [ Links ]
Allen, D. C., D. D. Breshears and N. McDowell . 2015. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene- Ecosphere 6:1-54. DOI: https://doi.org/10.1890/ES15-00203.1. [ Links ]
Bentz, B. J., J. A. Logan and G. D. Amman. 1991. Temperature-dependent development of the mountain pine beettle (Coleoptera: Scolytidae) and simulation of its phenology. The Canadian Entomologist 123: 1083-1094. DOI: https://doi.org/10.4039/Ent1231083-5. [ Links ]
Bentz, B. J. , J. Regniere, C. J. Fettig, E. M. Hansen, J. L. Hayes, J. A. Hicke, R. G. Kelsey, J. F. Negron and S. J. Seybold. 2010. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 60(8): 602-613. [ Links ]
Cibrián, T. D., J. T. Méndez M., R. Campos B., H. O. Yates III y J. F. Lara, 1995. Insectos Forestales de México. Universidad Autónoma Chapingo. Texcoco, Edo. de Méx., México. 453 p. [ Links ]
Comisión Nacional del Agua (Conagua). 2015. Índice estandarizado de precipitación. Datos del índice Estandarizado de Precipitación históricos desde 1951 hasta el último mes de los diferentes periodos. http://smn1.conagua.gob.mx/climatologia/sequia/SPI/spi.html (2 de enero de 2018). [ Links ]
Coulson, R., R. M. Feldman, P. J. H. Sharpe, P. E. Pulley, T. L. Wagner and T. L. Payne. 1989. An overview of the tambeetle model of Dendroctonus frontalis population dynamics. Holarctic Ecology, (12): 445-450. https://doi.org/10.1111/j.1600-0587.1989.tb00921.x [ Links ]
Edmonds, R., J. K. Agee and R. I. Gara. 2005. Forest Health and Protection. Waveland Press Inc. Long Grove, IL USA. 648 p. [ Links ]
Evangelista, P. H., S. Kumar, T. J. Stohlgren and N. E. Young. 2011. Assessing forest vulnerability and the potential distribution of pine beetles under current and future climate scenarios in the Interior West of the US. Forest Ecology and Management 262: 307-316. DOI: https://doi.org/10.1016/j.foreco.2011.03.036. [ Links ]
Gandhi, K. J. K. and D. A. Herms. 2010. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biological Invasions 12(2):389-405. DOI: https://doi.org/10.1007/s10530-009-9627-9. [ Links ]
Jenkins, M. J., E. G. Hebertson, P. Wesley and A. Jorgensen. 2008. Bark beetles, fuels, fires and implications for forest management in the Intermountain West. Forest Ecology and Management : 254(1):16-34. DOI: https://doi.org/10.1016/j.foreco.2007.09.045. [ Links ]
Kurz, W., C. Dymond, G. Stinson, G. Rampley, E. Neilson, A. Carroll, T. Ebata and L. Safranyik. 2008. Mountain pine beetle and forest carbon feedback to climate change. Nature, 452:987-990. DOI: http://doi.org/10.1038/nature06777. [ Links ]
Hawkes, B. C., S. Taylor, C. Stockdale, T. Shore, R. Alfaro, R. Campbell and P. Vera. 2003. Impact of mountain pine beetle on stand dynamics in British Columbia. In: Shore, T. L., J. E. Brooks and J. E. Stone (eds.). Mountain Pine Beetle Symposium: Challenges and Solutions, Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre. Inf. Rep. BC-X-399. Kelowna, British Columbia, Canada. pp. 177-199. [ Links ]
Logan, J. A. and B. J. Bentz. 1999. Model analysis of mountain pine beetle seasonality. Environmental Entomology 28: 924-934. [ Links ]
Lombardero, M., M. Ayres, B. Ayres and J. Reeve. 2000. Cold tolerance of four species of bark beetle (Coleoptera: Scolytidae) in North America. Environmental Entomology 29: 421-432. [ Links ]
Malmström, C. M. and K. F. Raffa. 2000. Biotic disturbance agents in the boreal forest: considerations for vegetation change models. Global Change Biology 6: 35-48. DOI: 10.1046/j.1365-2486.2000.06012.x. [ Links ]
Marini, L., M. P. Ayres, A. Battisti and M. Faccoli. 2012. Climate affects severity and altitudinal distribution of outbreaks in an eruptive bark beetle. Climatic Change. 115(2):327-341. DOI: 10.1007/s10584-012-0463-z. [ Links ]
Mcfarlane, B. L. and D. O. Witson. 2008. Perceptions of ecological risk associated with mountain pine beetle (Dendroctonus ponderosae) infestations in Banff and Kootenay National Parks of Canada. Risk Analysis, 28: 203-212. DOI: https://doi.org/10.1111/j.1539-6924.2008.01013.x. [ Links ]
Nealis, V. and B. Peter. 2009. Risk Assessment of the Threat of Mountain Pine Beetle to Canada's Boreal and Eastern Pine Forests. Natural Resources Canada. Canadian Forest Service. Information Report BC-X-417. Otawa, Ontario, Canada. 31p. [ Links ]
Negrón, J. F. and J. B. Popp. 2004. Probability of ponderosa pine infestation by mountains pine beetle in the Colorado Front Range. Forest Ecology and Management 191:17-27. DOI. https://doi.org/10.1016/j.foreco.2003.10.026. [ Links ]
Negrón, J., J. McMillin, J. Anhold and D. Coulson. 2009. Bark Beetle-Caused Mortality in a Drought-Affected Ponderosa Pine Landscape in Arizona, USA. Forest Ecology and Management 257 (4): 1353-62. DOI: doi:10.1016/j.foreco.2008.12.002. [ Links ]
Palacio-Prieto, J. L., G. Bocco, A. Velázquez, J. F. Mas, A. Victoria, L. Luna-González, G. Gómez-Rodríguez, J. López-García, M. Palma, I. Trejo-Vázquez, A. Peralta, J. Prado-Molina, A. Rodríguez-Aguilar, R. Mayorga-Saucedo y F. González. 2000. La condición actual de los recursos forestales en México: resultados del Inventario Forestal Nacional 2000. Universidad Nacional Autónoma de México. Investigaciones Geográficas 43:183-203. [ Links ]
Paine, T. D., K. F. Raffa and T. C. Harrington. 1997. Interactions among Scolytid bark beetles, their associated fungi, and live host conifers. Annual Review Entomology 42: 179-206. [ Links ]
Preisler, H. K., J. A. Hicke , A. A. Ager and J. L. Hayes . 2012. Climate and weather influences on spatial temporal patterns of mountain pine beetle populations in Washington and Oregon. Ecology 93(11):2421-2434. DOI: https://doi.org/10.1890/11-1412.1. [ Links ]
Raffa, K. F., B. H. Aukema, N. Erbilgin, K. D. Klepzig and K. F. Wallin. 2005. Interactions among conifer terpenoids and bark beetles across multiple levels of scale: An attempt to understand links between population patterns and physiological processes. Recent Advances in Phytochemistry 39: 79-118. [ Links ]
Raffa, K. F. , H. Aukema, J. Bentz, L. Carroll, A. Hicke, G. Turner and H. Romme. 2008. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58: 501-517. DOI: https://doi.org/10.1641/B580607. [ Links ]
Rubin-Aguirre, R., C. Sáenz, R. Lindig, A. del Rio, C. Tena, R. Campos and E. del-Val. 2015. Bark Beetle Pests in an Altitudinal Gradient of a Mexican Managed Forest. Forest Ecology and Management 343: 73-79. DOI: 10.1016/j.foreco.2015.01.028. [ Links ]
Safranyik, L. and D. A. Linton. 1998. Mortality of mountain pine beetle larvae, Dendroctonus ponderosae (Coleoptera: Scolytidae) in logs of lodgepole pine ( Pinus contorta var. latifolia ) at constant low temperatures. Journal of the Entomological Society of British Columbia 95: 81-87. [ Links ]
Salinas-Moreno, Y., M. G. Mendoza, M. A. Barrios, R. Cisneros, J. Macías-Sámano and G. Zúñiga. 2004. Areography of the genus Dendroctonus (Coleoptera: Curculionidae: Scolytinae) in Mexico. Journal of Biogeography, 31: 1163-1177, doi. 10.1111/j.1365-2699.2004.01110.x [ Links ]
Salinas-Moreno, Y. , A. Ager, C. Vargas, J. Hayes and G. Zúñiga . 2010. Determining the Vulnerability of Mexican Pine Forests to Bark Beetles of the Genus Dendroctonus Erichson (Coleoptera: Curculionidae: Scolytinae). Forest Ecology and Management 260 (1): 52-61. DOI: doi:10.1016/j.foreco.2010.03.029. [ Links ]
Six, D., E. Biber and E. Long. 2014. Management for Mountain Pine Beetle Outbreak Suppression: Does Relevant Science Support Current Policy? Forests 5 (1). Multidisciplinary Digital Publishing Institute: 103-33. DOI: doi:10.3390/f5010103. [ Links ]
Trzcinski, M. and M. Reid. 2009. Intrinsic and extrinsic determinants of mountain pine beetle population growth. Agricultural and Forest Entomology 11: 185-196. DOI: https://doi.org/10.1111/j.1461-9563.2008.00408.x. [ Links ]
Turchin, P., A. D. Taylor and J. D. Reeve. 1999. Dynamical Role of Predators in Population Cycles of a Forest Insect: An Experimental Test. Science 285: 1068- 1070. [ Links ]
Turchin, P. 2003. Complex population dynamics: a theoretical/empirical synthesis. Princeton: Princeton University Press. Princeton, NJ, USA. 456 p. [ Links ]
Tykarski, P. 2006. Beetles associated with scolytids (Coleoptera, Scolytidae) and the elevational gradient: Diversity and dynamics of the community in the Tatra National Park, Poland. Forest EcologyManagement 225(1-3): 146-159. DOI: https://doi.org/10.1016/j.foreco.2005.12.034. [ Links ]
Ungerer, M. J., M. P. Ayres and M. J. Lombardero. 1999. Climate and the northern distribution limits of Dendroctonus frontalis Zimmerman (Coleoptera: Scolytidae). Journal of Biogeography 26:1133-1145. DOI: https://doi.org/10.1046/j.1365-2699.1999.00363.x. [ Links ]
Wermelinger, B. 2004. Ecology and Management of the spruce bark beetle Ips typographus a review of recent research. Forest Ecology and Management 202 (1-3): 67-82. DOI: doi:10.1016/j.foreco.2004.07.018. [ Links ]
Westfall, J. and T. Ebata . 2009. Summary of forest health conditions in British Columbia. British Columbia Ministry of Forests and Range. Pest Management Report Num. 15. University of Minesota. Mineapolis, MN, USA. 76 p. [ Links ]
Williams, D. and A. Liebhold. 2002. Climate Change and outbreak ranges of two Northamerican beetles. Agricultural and Agroforestry Entomology 4: 87-99. DOI: https://doi.org/10.1046/j.1461-9563.2002.00124.x. [ Links ]
Wood, S. L. 1963. A revision of the bark beetles genus Dendroctonus Erichson (Coleoptera: Scolytidae). Great Basin Naturalist 23: 1-117. [ Links ]
Wood, S. L. 1982. The Bark Ambrosia Beetles of North and Central America (Coleoptera: Scolytidae): a taxonomic monograph. Great Basin Naturalist 6: 1-1359. [ Links ]
Zúñiga, G., R. Cisneros, Y. Salinas-Moreno, J. L. Hayes and J. E. Rinehart. 2006. Genetic structure of Dendroctonus mexicanus (Coleoptera: Curculionidae:Scolytinae)in the trans-Mexican volcanic belt. Annals of the Entomological Society 99: 945−958. DOI 10.1603/0013-8746(2006)99[945:GSODMC]2.0.CO;2. [ Links ]
Received: March 21, 2018; Accepted: September 17, 2018











 texto em
texto em