Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.9 no.50 México Nov./Dez. 2018
https://doi.org/10.29298/rmcf.v9i50.229
Articles
Plant-water relations in native shrubs of northeastern Mexico
1Universidad Autónoma de Nuevo León, Facultad de Ciencias Forestales. México.
2Universidad Autónoma de Nuevo León, Facultad de Ciencias Biológicas. México.
The aim of the current study was to establish how the xylem water potential (Ψ) in native shrubs such as Condalia hookeri, Cordia boissieri, Prosopis laevigata and Celtis pallida is influenced by soil water content, air temperature, relative humidity, and rainfall. Ψ was estimated at 15 days intervals between February 21 and June 30, 2017 in five different plants per species at 06:00 h and 14:00 h. During the wettest period, Ψ ranged from -0.67 (C. pallida) to -0.96 MPa (C. hookeri), in contrast, at the driest period, Ψ varied from -1.52 (P. laevigata) to -2.92 MPa (C. hookeri) at 06:00 h. At 14:00 h, C. pallida and C. hookeri achieved the highest (-1.07 MPa) and lowest (-3.10 MPa) Ψ values, respectively. Ψ at 06:00 h and rainfall were significantly and positively correlated with Ψ at 14:00 h; whereas the soil water content at different depths with Ψ values at 06:00 h was weak. P. laevigata and C. pallida maintained high values in Ψ at 06:00 h and 14:00 h under water stress conditions, so these species may be considered as drought tolerant species.
Key words: Celtis pallida Torr.; soil moisture; Tamaulipan thornscrub; xylem water potential; Prosopis laevigata (Humb. et Bonpl. ex Willd.) M.C. Johnst.; drought
El objetivo del presente estudio fue determinar la influencia del contenido de humedad del suelo, la temperatura del aire, la humedad relativa y la precipitación sobre el potencial hídrico xilemático (Ψ) de cuatro especies arbustivas nativas (Condalia hookeri, Cordia boissieri, Prosopis laevigata y Celtis pallida) del noreste de México. Las mediciones del Ψ se realizaron a intervalos de 15 días entre febrero 21 y junio 30 de 2017, en cinco plantas de cada especie a las 06:00 h y 14:00 h. En el periodo más húmedo, el Ψ a las 06:00 h varió de -0.67 (C. pallida) a -0.96 MPa (C. hookeri); en cambio, en el periodo más seco, el Ψ a las 06:00 h fluctuó de -1.52 (P. laevigata) a -2.92 MPa (C. hookeri). En tanto, a las 14:00 h, C. pallida y C. hookeri presentaron el mayor (-1.07 MPa) y menor (-3.10 MPa) valor, para los periodos húmedos y secos respectivamente. El Ψ a las 06:00 h y la precipitación mostraron correlaciones positivas con el Ψ de las 14:00 h; mientras que el contenido de agua en el suelo a diferentes profundidades con los valores de los Ψ a las 06:00 h fue débil. Dado que P. laevigata y C. pallida mantuvieron altos valores en el Ψ a las 06:00 h y 14:00 h bajo condiciones de estrés hídrico, estas especies pueden ser consideradas como especies tolerantes a la sequía.
Palabras clave: Celtis pallida Torr.; humedad del suelo; matorral espinoso tamaulipeco; potencial hídrico xilemático; Prosopis laevigata (Humb. et Bonpl. ex Willd.) M.C. Johnst.; sequía
Introduction
The study of arid and semi-arid zones is an important issue at present, because in this type of regions there are adverse conditions for the production and survival of existing forest species, as a consequence of the prolonged severe drought (García et al., 2005).
In these places, water is one of the most important factors that influence the distribution, growth and development of vegetation and is essential for all vital processes such as photosynthesis and respiration. It works as a means for the absorption of nutrients and metabolism of plants. The plant cell requires 85 to 90 % water to maintain vital dynamics and carry out enzymatic processes in a plant cell. A decrease in the water content below this level reduces the metabolic activity of the cells and the growth of the plants (Maiti et al., 2016); during this condition, the cells remain smaller and the leaves have less development which, consequently, affects the photosynthetically active leaf area (Parra et al., 1999). Furthermore, under such conditions the hydrolytic activity of the enzymes increases considerably, the transport of the ions decreases and the respiration, in general, increases (Salisbury and Ross, 2000).
Under these conditions, the plants have developed different responses and adaptations that allow them to survive in conditions of constant water deficit (Nilsen and Orcutt, 1996). Many of these are related to a greater ability to absorb water or more efficient use of this resource. Some plants have adjusted processes such as the C4 metabolism and the acid metabolism of Crassulaceae or CAM, which allow them to adapt to more arid environments (Black and Osmond, 2003; Lüttge, 2004) and successfully face long periods of water scarcity and the colonization of adverse environments.
When the water deficit develops slowly, plants can present acclimation responses that have effects on growth, such as decreased leaf expansion and increased root growth (Potters et al., 2007; Shao et al., 2008). Another mechanism of resistance at the physiological level is the closure of stomata, structures responsible for the highest proportion of water loss in plants (Taiz and Zeiger, 2006). This response is mediated by the abscisic acid hormone (ABA) (Abe et al., 1997; Kang et al., 2002).
Since water stress is the most limiting factor in the northeastern region of Mexico, the present work focused on studying how the seasonal water potentials of native tree species are related to the availability of soil water and the components of evaporative demand (temperature, relative humidity and precipitation). The study of native species in this region provides the opportunity to investigate, from an ecophysiological perspective, the response of shrub species to changes in the availability of resources, in this case, the moisture content of the soil, to better understand how ecosystem can sustain the productivity of biomass. Therefore, as an approach to understand how water potentials of plant xylem relate to environmental conditions, an investigation was conducted on four species of native plants to describe adaptive responses to the scarcity of water resources. The application of the results can be translated into a better management of each species, with a greater probability of success in future programs of plant production and reforestation with native species tolerant to stress due to the water deficit.
Materials and Methods
Study site
This study was carried out in the Experimental Research Station of the Facultad de Ciencias Forestales de la Universidad Autónoma de Nuevo León (School of Forest Sciences of the Autonomous University of Nuevo Leon) (24°47' N; 99°32' W, 350 masl) in Linares municipality, Nuevo León state, Mexico (Figure 1). Climate is subtropical and semi-arid with a warm summer. The average monthly temperature varies from 14.7 °C in January to 22.3 °C in August. The average annual rainfall is around 800 mm (González et al., 2004). Soils are predominantly dark gray, vertisoles of clay-silt texture, with montmorillonite, which expands and contracts remarkably as the moisture content of the soil varies (González et al., 2004).
Plant material and measurement of the water potential of xylem
Four co-existing shrub species, representative of the community of native plants and of nutritional value for domestic livestock (Domínguez et al., 2014) and of multiple uses such as wood, coal and shelving, were selected: Condalia hookeri M.C. Johnst. (Rhamnaceae), Cordia boissieri A.DC. (Boraginaceae), Prosopis laevigata (Humb. et Bonpl. ex Willd.) M.C. Johnst. (Fabaceae) and Celtis pallida Torr. (Ulmaceae). Five individuals of each species were randomly chosen in a plot of the scrubland without disturbance of 20 m × 20 m, previously delimited.
Water potential measurements (Ψ) were made on terminal branches of approximately 10 cm long with leaves and <1 cm in diameter, at intervals of 15 days (between February 21 and June 30, 2017), at 06:00 a.m. and 2:00 p.m. Water condition of the species was assessed based on the tension of the xylem (Taiz and Zeiger, 1991), which is almost equivalent to the leaf water potential (Ψ) using the Scholander pressure chamber (Model 3005, Soil Moisture Equipment). The pressure supplied to the chamber was at a rate of 0.05 MPa s-1 and nitrogen gas (N2) was used as a source of pressurization (Ritchie and Hinckley, 1975). Said measurements were made between 15 and 25 seconds after collecting the samples of the plant tissue. This method consists of applying a positive pneumatic pressure to the tissue to increase its potential. This is applied to produce water exudation from the tissue on the excised surface of the branch. At the moment of getting the exudation, it is considered that the pressure in the xylem is equal to the atmospheric pressure and the applied value will be the negative of the tissue potential (Muraoka and Tzi, 2000). For safety reasons and operating instructions, the lower limit of the pressure chamber was -7.3 MPa.
Environmental variables
The environmental variables such as air temperature (ºC) and relative humidity (%) were recorded daily with HOBO Pro type automated sensors (HOBO Pro Temp / RH Series). The daily amount of precipitation (mm) was obtained with a "tipping bucket" rain gauge (Forestry Suppliers, Inc.). Additionally, at each sampling date, the gravimetric moisture content of the soil (kg H2Okg-1 soil, dry base) was estimated from four samples taken at different depths of 0-10, 10-20, 20-30, 30-40 and 40-50 cm using a Veihmeyer design auger (Model 215, Soil Moisture Equipment Corp.). Each sample was placed in metal crucibles. For the determination of this content, samples were weighed immediately to obtain the wet weight (Psh), later they were placed in a forced air oven at 105 °C for 24 hours until reaching constant dry weight (Pss). The gravimetric moisture content of the soil was determined with the following equation (Gardner, 1986):
Where:
W = Gravimetric moisture content expressed in kg·H2O·kg-1 soil
Psh = Humid soil weight (g)
Pss = Dry soil weight (g), dried at 105 °C in a kiln
Statistical Analysis
Since the water potential data of the xylem (06:00 and 14:00 h) and the water content in the soil were not normally distributed (Kolmogorov-Smirnov test) and the analysis of the variance did not show homogeneity for the majority of the sampling dates (Levene test), the data were subjected to the non-parametric Kruskal-Wallis test (Ott, 1993). The relationships between water potentials (at 06:00 h and 14:00 h) and environmental variables were determined by means of the Spearman correlation analysis or by ranges, since the null hypothesis of normality was rejected at P<0.05. All the statistical methods applied were carried out in accordance with the SPSS program (Statistical Package for the Social Sciences), standard version 13.0 for Windows (SPSS, 2000).
Results and Disscusion
Air temperature, relative humidity and precipitation recorded 15 days before each sampling date during the experimental period are shown in Table 1.
Table 1 Air temperature (°C), relative humidity (%) and rainfall (mm) recorded 15 days before each sampling date.
| Sampling date | 06:00 h | 14:00 h | Rainfall (mm) | ||
|---|---|---|---|---|---|
|
Temp. (°C) |
HR (%) |
Temp. (°C) |
HR (%) |
||
| February 21st | 8.7 | 78.3 | 31.3 | 11.2 | 1.5 |
| March 7th | 19.6 | 92.2 | 31.1 | 48.3 | 17.0 |
| March 22nd | 19.2 | 91.5 | 31.1 | 45.7 | 44.0 |
| April 4th | 19.3 | 49.5 | 35.2 | 25.7 | 0.4 |
| April 18th | 16.6 | 96.4 | 30.5 | 45.0 | 38.2 |
| May 2nd | 16.0 | 79.6 | 36.5 | 28.8 | 2.0 |
| May 17th | 25.2 | 86.5 | 38.2 | 36.2 | 10.8 |
| June 2nd | 23.4 | 89.9 | 32.5 | 57.0 | 152.4 |
| June 14th | 23.3 | 92.7 | 35.6 | 51.4 | 10.0 |
| June 30th | 24.9 | 91.4 | 35.5 | 53.9 | 63.6 |
Temp. = Temperature; HR = Relative humidity
The cumulative rainfall recorded during the study period was 340 mm. The maximum precipitation peak occurred on June 2nd with 152 mm and the minimum precipitation was recorded on April 4th with 0.4 mm. The seasonal moisture content of the soil, at different depths of the soil layer, showed a typical response with rainfall events. Significant differences were detected (P<0.022 and P<0.026) in soil moisture content between the soil layers for the sampling dates of March 22nd and April 18th, respectively (Figure 2). During the driest period, May 2, the soil moisture content was around 0.10 kg kg-1, while, during the wettest period, June 2, the soil moisture content varied from 0.21 kg kg -1 (depth 0-10 cm) to 0.16 kg kg-1 (40-50 cm depth) and coincides with the peak of highest rainfall. The gravimetric moisture content of the soil at the depth of 0-10 cm was more sensitive to rainfall events than the deeper layers. However, since there was great variability in rainfall and air temperature, the water content of the soil could not be controlled and maintained and, therefore, soil moisture may not be available for absorption in deep roots due to at the low rate of infiltration.
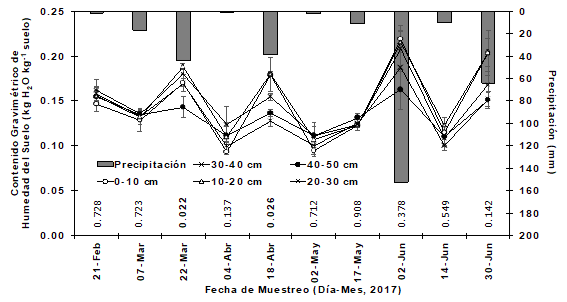
Contenido gravimétrico de humedad del suelo = Gravimetric moisture content of the soil; Fecha de muestreo = Samplig date; Precipitación = Precipitation
The P value is shown on each sampling date within the graph. Each value represents the mean ± standard error (n = 4). The accumulate precipitation (mm) is included for 15 days before each sampling date.
Figure 2 Seasonal pattern in the gravimetric moisture content of the soil at five depths.
The responses of the water content of the soil in the present work agrees with other studies that have demonstrated the seasonal variability of substrate moisture in arid and Mediterranean grassland ecosystems (Anderson et al., 2001; Bussotti et al., 2002; González et al., 2000; López-Hernández et al., 2010; González-Rodríguez et al., 2016). The erratic and dry environmental conditions that were observed during most of the experimental period could be related to frequent seasons of extreme environmental conditions such as droughts, typical of the northeastern region of Mexico (González et al., 2000, 2004).
The seasonal variation of the water potential of the xylem at 06:00 h and 14:00 h in the four plant species is shown in Figure 3. At 06:00 h, the values were significantly different (P<0.05) among the four species on all sampling dates (Figure 3a). In the wettest period (June 2nd), the highest in the water potential at the same time (-0.67 MPa and -0.76 MPa) were registered in C. pallida and C. boissieri, respectively, which are higher than -0.96 MPa observed in P. laevigata and -0.93 MPa in C. hookeri.
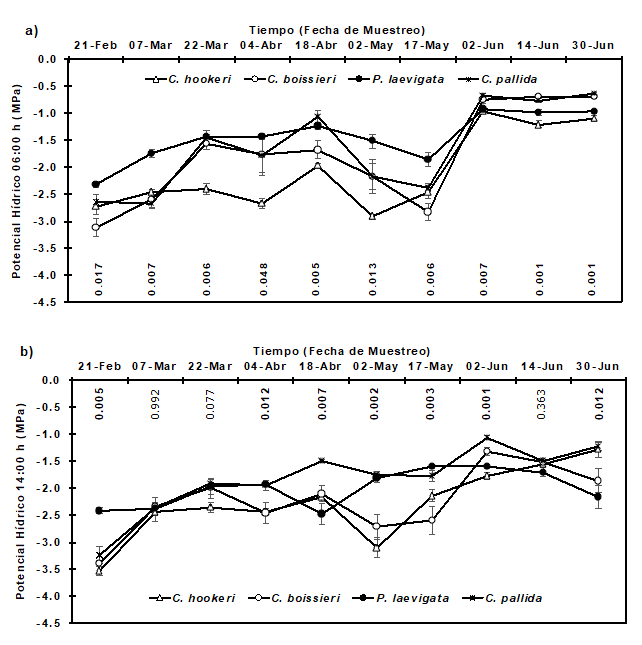
Potencial hídrico = Water potential; Tiempo (fecha de muestreo) = Time (sampling date).
The value of P is shown on each sampling date. Each value represents the mean ± standard error (n = 5).
Figure 3 Water potential of xylem at 06:00 h and 14:00 h in four species of native plants.
On the contrary, in the driest period (May 2nd), when the shrub species faced severe water stress, the most outstanding value (-1.52 MPa) in the potential at 06:00 h was registered in P. laevigata, while the lowest one (-2.92 MPa) was detected in C. hookeri, which suggests that the latter species is more sensitive to the lack of water.
It can be inferred that the recovery of the values of initiation of response in the water potential at 06:00 h in the four shrub species for the sampling dates from June 2 to June 30 is mainly related to the events of precipitation (Table 1).
In regard to the same potential at 14:00 h., significant differences (P<0.05) were identified among the native species in seven of ten sampling dates (Figure 3b). In the wettest (June 2nd), when the water potential at the time indicated was the highest and when the water content of the soil in the layer 0-50 cm was higher than 0.20 kg kg-1 (20 %) (Figure 2), C. pallida recorded the highest value (-1.07 MPa), while C. hookeri the lowest (-1.78 MPa); on the other hand, on the driest sampling date (May 2nd), when the average water content in the soil was 0.10 kg kg-1 (10 %), the values in the water potential at 2:00 p.m. varied from -1.76 MPa (C. pallida) to -3.10 MPa (C. hookeri). Similar results have been documented by González and Cantú (2001); López-Hernández et al. (2010); González et al., 2011; González-Rodríguez et al. (2011); and González et al. (2016); Adhikari and White (2014) who studied the adaptation of native shrubs to drought stress.
For the sampling dates between March 2 and May 17th, when the average moisture content in the soil was approximately 0.12 kg kg-1, an unusual response was found in the water potential at 14:00 h in particular, in C. hookeri and C. pallida, for which the water potential registered in the afternoon was higher than the morning one. In fact, the imbalance between the moisture content of the soil (or water potential of the soil) before sunrise and the water potential of the plant tissue (leaf and xylem) has been recorded in various mesophytic and xerophytic plants (Donovan, 2003).
Among the possible factors responsible for this imbalance is the hydraulic resistance to the continuous soil-plant (Myers and Neales, 1984), the smallest size of the plant (Brown and Archer, 1990); the heterogeneity of soil moisture (Ourcival et al., 1994); the reduction of the water potential of the cells due to nocturnal growth (Boyer, 1995); the low osmotic potential (Berger et al., 1996), open stomata and transpiration at night (Donovan et al., 2003, Bucci et al., 2004, Howard et al., 2009). In addition, it is assumed that night transpiration and hydraulic lifting benefit the absorption of plant nutrients under drought conditions (Matzner and Richards, 1996; Sellin, 1999; Donovan et al., 2003).
These observations are consistent with the results obtained in the present work, but in the context of the determinations of the water potential of the plant since the aforementioned processes were not carried out in this work.
With the exception of C. hookeri, in the depths 0-10 cm (0.705) and 10-20 cm (0.717), the water potential at 06:00 h of all the shrub species studied did not show a significant correlation (P>0.05) with the moisture content of the soil for each depth of the soil. On the other hand, the water potential values at 06:00 h correlated significantly and positively with those at 2:00 p.m. the values fluctuated from 0.867 in C. pallida, to 0.900 in C. boissieri. Regarding precipitation, all species showed a positive and significant relationship (Table 2); the values ranged from 0.697 (C. pallida) to 0.894 (C. hookeri).
Table 2 Spearman correlation coefficients (n = 10) and P value between the water potential at 06:00 h with the water potential at 14:00 h, gravimetric moisture content at different depths and precipitation received 15 days before of sampling during the study period in four shrub species.
| Species | Water potential (14:00 h) | Soil gravimetric moisture content (kg H2O·kg-1 at different depths (cm) |
Rainfall | ||||
|---|---|---|---|---|---|---|---|
| 0-10 | 10-20 | 20-30 | 30-40 | 40-50 | |||
| C. hookeri | 0.893** | 0.705* | 0.717* | 0.474ns | 0.395ns | 0.377ns | 0.894** |
| C. boissieri | 0.900** | 0.371ns | 0.395ns | 0.182ns | 0.085ns | 0.036ns | 0.711* |
| P. laevigata | 0.220ns | 0.480ns | 0.480ns | 0.286ns | 0.152ns | 0.176ns | 0.766** |
| C. pallida | 0.867** | 0.503ns | 0.503ns | 0.309ns | 0.164ns | 0.212ns | 0.697* |
Highly significant difference (**P≤0.01); Significant difference (*P≤0.05); ns = Non-significant difference (P>0.05).
During the course of the study and according to the water potential data, the four plant species faced mild to severe drought periods; P. laevigata and C. pallida showed higher water potential values at 06:00 h as well as at 2:00 p.m. compared with the other species, indicating an isohydric water regulation and probably a better adaptation to severe conditions. of drought (Breshears et al., 2009). It seems that P. laevigata and C. pallida are examples of shrub species that have adapted to the low water availability of the soil, since they tend to maintain a humidification of the tissues, while the adaptation of C. hookeri and C. boissieri in dry periods it seems to depend on the strategies that allow them to cope with internal desiccation and, consequently, have lower values in water potentials (Gebrekirstos et al., 2006).
The study suggests that the first two species can serve as a model to evaluate drought adaptation strategies in plant species with high water potential, while the latter would function as an adequate model to study the adaptation of the plant to drought with a low potential. In addition, P. laevigata is the species that under conditions of stress due to drought tends to achieve and maintain significantly greater water potential.
Despite their different capacities to withstand a wider range of water availability, all species investigated are considered suitable candidates for the restoration and continued conservation of drought-prone sites. Therefore, a combination of P. laevigata and C. pallida with other drought-tolerant native species can be recommended for reforestation, in order to prevent soil erosion and maintain, in a sustainable manner, the vegetation cover of the scrub ecosystems.
Based on the uncertain development of climatic variables and the range of responses to water stress of the species, maintaining the current diversity of Tamaulipan thorn scrub may be difficult in the future. In fact, the growth of the plant and the survival in the field depend, to a large degree, of a complex interaction of numerous environmental factors and internal mechanisms of the plant. Therefore, the assessment of the variability of the water status of the xylem in native shrubs of the northeastern scrublands of Mexico adapted to drought conditions represents an important step in the evaluation of some ecophysiological characteristics of plants for water stress studies.
Conclusions
The implications of this study showed that the species P. laevigata and C. pallida recorded the highest values of the water potential of the xylem at 06:00 a.m. and at 2:00 p.m., under conditions of water stress, so these species considered as drought tolerant; on the other hand, C. hookeri and C. boissieri, which recorded lower, lower values, would be susceptible to water deficit, and therefore, will be in a situation of physiological disadvantage in conditions of water scarcity.
The gradual decrease in the xylem water potential at the hours defined for the four shrub species gave a remarkable response to trends in soil water content and precipitation. The temperature of the air negatively influenced the values of the water potential of the xylem in all species. In contrast, the water potential values of the xylem increased as the water content of the soil and the relative humidity increased.
With this kind of studies it is possible to identify and know the patterns of absorption and water requirements among shrub species and understand the processes of plant succession. Based on this, drought tolerant species can be selected, which can be used in the productive recovery of arid and semi-arid ecosystems, in such a way that reforestation with them generates favorable conditions for the natural recovery of the ecosystems of this kind.
Acknowledgements
The authors thank the Facultad de Ciencias Forestales de la Universidad Autónoma de Nuevo León (School of Forest Sciences of the Autonomous University of Nuevo León) and to the Consejo Nacional de Ciencia y Tecnología (National Council of Science and Technology) (Conacyt) for the funding granted through the Fondo Sectorial de Investigación para la Educación clave 250732. (Conacyt) (Research Sectorial Fund for Education code 250732). Also, to the anonymous reviewers for making critical observations to this study.
To Ing. Joel Bravo Garza and to Manuel Hernández, as well, for their having helped in field work and laboratory tasks.
REFERENCES
Abe, H., K. Yamaguchi-Shinosaki, T. Urao, T. Iwasaki, D. Hosokawa and K. Shinosaki. 1997. Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 9:1859-1868. [ Links ]
Adhikari, A. and J. D. White. 2014. Plant water use characteristics of five dominant shrub species of the Lower Rio Grande Valley, Texas, USA: Implications for shrubland restoration and conservation. Conservation Physiology 2(1):cou005. [ Links ]
Anderson, L. J., M. S. Brumbaugh and R. B. Jackson. 2001. Water and tree-understory interactions: a natural experiment in a savanna with oak wilt. Ecology 82:33-49. [ Links ]
Berger, A., M. Grouzis and C. Fournier. 1996. The water status of six woody species coexisting in the Sahel (Ferlo, Senegal). Journal of Tropical Ecology 12:607-627. [ Links ]
Black, C. C. and B. Osmond. 2003. Crassulacean acid metabolism photosynthesis: “working the night shift”. Photosynthesis Research 76:329-341. [ Links ]
Boyer, J. S. 1995. Measuring the water status of plants and soils. Academic Press. San Diego, CA USA. 178 p. [ Links ]
Breshears, D. D., O. B. Myers, C. W. Meyer, F. J. Barnes, C. B. Zou, C. D. Allen, N. G. McDowell and W. T. Pockman. 2009. Tree die-off in response to global change-type drought: Mortality insights from a decade of plant water potential measurements. Frontiers in Ecology and the Environment 7:185-189. [ Links ]
Brown, J. R. and S. Archer. 1990. Water relation of perennial grass and seedling vs adult woody plants in a subtropical savanna, Texas, USA. Oikos 57:366-374. [ Links ]
Bucci, S. J., F. G. Scholz, G. Goldstein, F. C. Meinzer, J. A. Hinojosa, W. A. Hoffmann and A. C. Franco 2004. Processes preventing nocturnal equilibration between leaf and soil water potential in tropical savanna woody species. Tree Physiology 24:1119-1127. [ Links ]
Bussotti, F., D. Bettini, P. Grossoni, S. Mansuino, R. Nibbi, C. Soda and C. Tani. 2002. Structural and functional traits of Quercus ilex in response to water availability. Environmental and Experimental Botany 47:11-23. [ Links ]
Domínguez G., T. G., R. G. Ramírez L., H. González R., I. Cantú S., M. V. Gómez M. and M. del S. Alvarado. 2014. Mineral content in four browse species from Northeastern Mexico. Pakistan Journal of Botany 46(4):1421-1429. [ Links ]
Donovan, L. A., J. H. Richards and M. J. Linton. 2003. Magnitude and mechanism of disequilibrium between predawn plant and soil water potentials. Ecology 84:463-470. [ Links ]
García O., N. C., R. Trejo C., A. Pedroza S., F. Gómez L., J. H. Esparza M. y M. Sepulveda B. 2005. Bases moleculares de la resistencia a sequía en plantas. Revista Chapingo Serie de Zonas Áridas 4(2):65-74. [ Links ]
Gardner, W. H. 1986. Water content. In: Klute, A., G. S. Campbell., R. D. Jacson., M. M. Mortland and D. R. Nielsen (eds.). Methods of Soil Analysis. Part I. ASA and SSSA. Madison, WI USA. pp. 493-544. [ Links ]
Gebrekirstos, A., D. Teketay, M. Fetene and R. Mitlöhner. 2006. Adaptation of five co-occurring tree and shrub species to water stress and its implication in restoration of degraded lands. Forest Ecology and Management 229:259-267. [ Links ]
González R., H., I. Cantú S., M. V. Gómez M. and W. R. Jordan. 2000. Seasonal plant water relationships in Acacia berlandieri. Arid Soil Research and Rehabilitation 14:343-57. [ Links ]
González R., H. y I. Cantú S. 2001. Adaptación a la sequía de plantas arbustivas del matorral espinoso Tamaulipeco. Ciencia UANL 4(4):454-461. [ Links ]
González R., H., I. Cantú S., M. V. Gómez M. and R. G. Ramírez L. 2004. Plant water relations of thornscrub shrub species, northeastern Mexico. Journal of Arid Environments 58(4):483-503. [ Links ]
González R., H., I. Cantú S., R. G. Ramírez L., M. V. Gómez M., M. Pando M. y J. M. López H. 2011. Potencial hídrico xilemático de cuatro especies arbustivas nativas del noreste de México. Revista Chapingo Serie de Ciencias Forestales y del Ambiente 17:97-109. [ Links ]
González-Rodríguez, H., I. Cantú-Silva, R. G. Ramírez-Lozano, M. V. Gómez-Meza, J. Sarquis-Ramírez, N. Coria-Gil, J. R. Cervantes-Montoya and R. K. Maiti. 2011a. Xylem water potentials of native shrubs from northeastern Mexico. Acta Agriculturae Scandinavica, Section B-Plant Soil Science 61:214-219. [ Links ]
González-Rodríguez, H., W. Himmelsbacha, J. I. Sarquís-Ramírez, I. Cantú-Silva, R. G. Ramírez-Lozano and J. M. López-Hernández. 2016. Seasonal water relations in four co-existing native shrub species from Northeastern Mexico. Arid Land Research and Management 30(4):375-388. [ Links ]
Howard, A. R., M. W. Van Iersel, J. H. Richards and L. A. Donovan. 2009. Night-time transpiration can decrease hydraulic redistribution. Plant, Cell & Environment 32:1060-1070. [ Links ]
Kang, J., H. Choi, M. Im and S. Y. Kim. 2002. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 14(2):343-357. [ Links ]
López-Hernández, J. M., H. González-Rodríguez, I. Cantú-Silva, R. G. Ramírez-Lozano, M. V. Gómez-Meza, M. Pando-Moreno, J. I. Sarquís-Ramírez, N. Coria-Gil, R. K. Maiti and N. C. Sarkar. 2010. Adaptation of native shrubs to drought stress in north-eastern México. International Journal of Bio-resource and Stress Management 1(1):30-37. [ Links ]
Lüttge, U. 2004. Ecophysiology of crassulacean acid metabolism. Annals of Botany 93:629-652. [ Links ]
Maiti, R., H. González-Rodríguez and N. S. Ivanova. 2016. Autoecology and ecophysiology of woody shrubs and trees: Concepts and Applications. John Willey & Sons Ltd.Oxford, UK. 333 p. [ Links ]
Matzner, S. L. and J. H. Richards. 1996. Sagebrush (Artemisia tridentata Nutt.) roots maintain nutrient uptake capacity under water stress. Journal of Experimental Botany 47:1045-1056. [ Links ]
Muraoka, T. y E. A. Tzi. 2000. Mejoramiento del uso del agua en la agricultura, el papel de las técnicas nucleares. CENA. Piracicaba, Brasil. 131 p. [ Links ]
Myers, B. A. and T. F. Neales. 1984. Seasonal changes in the water relations of Eucalyptus behriana (F. Muell) and E. microcarpa (Maiden) in the field. Australian Journal of Plant Botany 32:495-510. [ Links ]
Nilsen, E. T. and D. M. Orcutt. 1996. Physiology of plants under stress. Abiotic factors. John Wiley and Sons. New York, NY USA. 351 p. [ Links ]
Ott, L. 1993. An introduction to statistical methods and data analysis, 2nd ed. Duxbury Press. Boston, MA USA. 769 p. [ Links ]
Ourcival, J. M., C. Floret, E. Le Floch and R. Pontanier. 1994. Water relations between two perennial species in the steppes of southern Tunisia. Journal of Arid Environments 28:333-350. [ Links ]
Parra Q., R. A., J. L Rodríguez O. and V. A. González H. 1999. Transpiración, potencial hídrico y prolina en zarzamora bajo déficit hídrico. TERRA Latinoamericana 17:125-130. [ Links ]
Potters, G., T. P. Pasternak, Y. Guisez, K. J. Palme and M. A. K. Jansen. 2007. Stress-induced morphogenic responses: growing out of trouble? Trends in Plant Science 12(3):99-105. [ Links ]
Ritchie, G. A. and T. M. Hinckley. 1975. The pressure chamber as an instrument for ecological research. Advances in Ecological Research 9:165-254. [ Links ]
Salisbury, F. B. y C. W. Ross. 2000. Fisiología de las Plantas. Ed. Paraninfo Thomson Learning. Madrid, España. 758 p. [ Links ]
Sellin, A. 1999. Does pre-dawn water potential reflect conditions of equilibrium in plant and soil water status? Acta Oecologica 20:51-59. [ Links ]
Shao, H. B., L. Y. Chu, C. A. Jaleel and C. X. Zhao. 2008. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biologies 331(3):215-225. [ Links ]
Statistical Package for the Social Sciences (SPPS). 2000. SPSS. Standard released version 13 for Windows, SPPS Inc. Chicago, IL USA. n/p. [ Links ]
Taiz, L. and E. Zeiger. 1991. Plant Physiology. The Benjamin/Cummings Public Company Inc. Redwood City, CA USA. 565 p. [ Links ]
Taiz, L. and E. Zeiger. 2006. Plant Physiology. 4th ed. Sinauer Associates. Sunderland, MA USA. 690 p. [ Links ]
Received: March 13, 2018; Accepted: October 31, 2018











 texto em
texto em