Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.9 no.49 México Set./Out. 2018
https://doi.org/10.29298/rmcf.v9i49.179
Articles
Nutrient dynamics during the decomposition process of litterfall at the Tamaluipan thorn scrub
1Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. México.
2Instituto Tecnológico de El Salto. México.
The Tamaulipan thorn scrub consists of trees and shrubs that produce large amounts of litterfall, essential for soil and nutrient cycling, which is complex to understand. The main objective of this work was to determine the contents of Ca, K, P, Mg, Cu, Fe, Mn and Zn during the process of litterfall degradation and its dynamics were compared in two seasons. Litterfall was sampled 15 days before each season and was left in the field for decomposition (10 g), using the nylon bag technique during the winter and spring seasons. The decomposition rate (k, gg-1·year-1) during the winter and spring was 0.002 and 0.003, respectively. During the winter season, Ca and P registered an accumulation trend of 34.7 and 0.32 mgg-1, respectively; K and Mg released 7.2 and 0.3 mgg-1, respectively; Cu released 1.0 μgg-1; Fe and Mn increased 402.8 and 7.9 μgg-1, respectively; Zn exhibited an accumulation of 0.04 µgg-1. During the spring season, Ca exhibited an accumulation of 10.93 mgg-1, and the P content was 0.7 mgg-1. As in the winter season, the minerals that showed a release pattern were K (6.3 mgg-1) and Mg (0.49 mgg-1). With respect to Cu, Fe, Mn and Zn, an accumulation trend was observed at a rate of 2.7, 446.3, 6.3 and 16.1 μgg-1, respectively.
Key words: Nutrient accumulation; litterfall; nutrient release; Tamaulipan thorn scrub; spring; winter
El Matorral Espinoso Tamaulipeco está constituido por árboles y arbustos que producen gran cantidad de hojarasca esencial para el suelo y el ciclo de los nutrientes, el cual es complejo de entender. El objetivo del presente trabajo fue determinar el contenido de Ca, K, P, Mg, Cu, Fe, Mn y Zn durante el proceso de degradación de la hojarasca y comparar su dinámica en dos estaciones. La hojarasca se recolectó 15 días previos a cada estación y se dejó en campo para evaluar su descomposición (10 g), mediante la técnica de bolsa de nylon durante el invierno y la primavera. La tasa de descomposición (k, g g-1 año-1) fue de 0.002 y 0.003, respectivamente. En la estación de invierno, el Ca y P registraron una acumulación de 34.7 y 0.32 mg g-1, respectivamente; el K, liberó 7.2 y el Mg, 0.3 mg g-1; el Cu 1.0 µg g-1; el Fe incrementó 402.8 y el Mn 7.9 µg g-1; el Zn presentó una acumulación de 0.04 µg g-1. En primavera, el Ca tuvo una acumulación de 10.93 mg g-1 y el contenido de P fue 0.7 mg g-1. Al igual que en la temporada invernal, los minerales que registraron liberación fueron K (6.3 mg g-1) y Mg (0.49 mg g-1). Con respecto a Cu, Fe, Mn y Zn, se observó acumulación a razón de 2.7, 446.3, 6.3 y 16.1 µg g-1, respectivamente.
Palabras Claves: Acumulación de nutrientes; hojarasca; liberación de nutrientes; Matorral Espinoso Tamaulipeco; primavera; invierno
Introduction
Plants provide most of the cover of the ecosystem, furnish the physical structure for the rest of the communities, constitute the point of departure for numerous trophic processes, and are an active element of the mineral nutrient cycles (Begon et al., 1990; Friedel et al., 2000).
Various materials are deposited on forest soils, coming from different vegetation strata, such as leaves, branches, and inflorescences, which receive the joint name of litterfall; of this, the leaves constitute the most important fraction in forest ecosystems (Santa, 1987; Barreto et al., 2018). Litterfall determines the stability and functioning of the ecosystem. It is the main source of circulation of organic matter, energy and nutrients between the plants and the soil; its content depends on the balance between the accumulation and decay processes, which are determined by the vegetal species and its chemical decomposition, among other factors (Sánchez et al., 2009).
The amount of bioelements contained in litterfall, once it has decayed, constitutes the main source of nutrients incorporated into the soil (Del Valle, 2003). For this reason, the evolution and rate of the decomposition is a key factor for the functioning of forests, as nutrients, if rapidly released, are lost through soil leaching or volatilization (Schlesinger, 2000; Castellanos and León, 2011). Conversely, if decay is too slow, the available nutrients may not be enough and they may limit the growth and development of plants (Jordan, 1985; Swift and Anderson, 1989; Bubb et al., 1998; Montagnini and Jordan, 2002; Castellanos and León, 2011).
The general pattern for the loss of litterfall weight comprises two status phases: an initial phase of quick development due to the washing of soluble compounds and the decay of labile materials (sugars, certain phenols, starches and proteins), and a second, slower phase resulting from the low decomposition of recalcitrant elements, such as cellulose, hemicelluloses, tannins and lignin (Arellano et al., 2004).
In montane tropical forests, the nutrients released from the topsoil are the main source of supply and maintenance of soil fertility (Vitousek et al., 1995; Parzych and Trojanowski, 2006), which meet the needs of the plants (García-Oliva et al., 2003; Barreto and León, 2005). The decomposition of the litterfall releases N, P, K, Ca, Mg and other nutrients at various rates, depending on their quality (Montagnini and Muñiz-Miret, 1999; Berg, 2000).
Kuruvilla et al. (2016) examined the production dynamics of the litterfall, the decomposition and the release of nutrients of Munrochloa ritchei (Munro) M. Kumar & Remesh, a rare species of bamboo, endemic in the Western Ghats, India. The reduction of the N and K contents was continuous, while P, Ca and Mg exhibited temporary accumulation phases before the final release. The release of nutrients from decaying litterfall followed this order: N = Mg > K = Ca > P.
Research on the decomposition of litterfall is helpful for determining the amount of nutrients that pass from the litterfall reservoir to the mineral soil and how they influence the latter’s fertility. López et al. (2014) and González-Rodríguez et al. (2013) have carried out studies on this topic and on the contribution of nutrients from litterfall to the Tamaulipan thorn scrub. Nevertheless, few studies on the ecosystems of the state of Nuevo León address the release of macro- and micronutrients from litterfall decomposition and the influence on the behavior of these nutrients in each season of the year during the process of their decomposition. For this reason, the aim of the present work was to determine the release of macro- and micronutrients during the decomposition of litterfall and to compare the content of these in two different seasons of the year (winter and spring) in the Tamaulipan thorn scrub of southeastern Nuevo León.
Materials and Methods
Study site
The study was carried out at the Experimental Campus of the Forest School of the Universidad Autónoma de Nuevo León (Autonomous University of Nuevo León), located 8 km south of Linares municipality, at 24°47' N and 99°32' W, and 350 masl.
The climate of the region is subtropical and semiarid, with a warm summer, rains from April to November, and an intraestival drought period. The mean monthly temperature ranges between 14.7 °C and 22.3 °C, with maximum temperatures of 45 °C during the summer. The mean annual precipitation is 805 mm, with a bimodal distribution (González et al., 2004). The dominant soils are Vertisols with montmorillonite, with a depth of 0.80 to 1.50 m, a dark gray color, and a silty clayey texture; they contract and expand visibly in response to the changes in soil moisture content (López, 2014).
Vegetation
The main vegetation type of the studied ecosystem corresponds to the Tamaulipan thorn scrub, a shrub/subarboreal formation with dominant floristic elements measuring 4 to 6 m in height, which are perennial, mostly thorny, with small, deciduous leaves. The most representative species are: Acacia amentacea DC., Acacia farnesiana (L.) Willd., Acacia schaffneri (S. Watson) F. J. Herm., Castela erecta Turpin, Celtis pallida Torr., Condalia hookeri M. C. Johnst., Cordia boissieri A. D C., Diospyros texana Scheele, Eysenhardtia texana Scheele, Forestiera angustifolia Torr., Havardia pallens (Benth.) Britton & Rose, Lantana macropoda Torr., Leucophyllum frutescens (Berland.) I. M. Johnst. and Zanthoxylum fagara (L.) Sarg. The floristic characterization of this type of community has been documented before, by Domínguez et al. (2013).
Litterfall decomposition and collection frequency
The so-called litter bag method (Bocock and Gilbert, 1957) was used to collect newly fallen dead leaves, branches and inflorescences in bags. Each 10 g bag reflected a relative proportion of the structural components of the original material: leaves, branches, reproductive structures and other components of litterfall. A total of 80 bags were placed at random, in winter and spring, in an undisturbed plot in the Tamaulipan thorn scrub. 10 samples were retained in the laboratory as controls. Subsequently, 10 bags were collected at random at 5, 10, 30, 45, 60, 75 and 90 days, after having been placed on the soil in each season. The samples were dried in a 292AD FelisaTM convection oven at 70 °C during 72 hours. This value was used to calculate the loss of the baseline biomass equivalent to decay. The remaining mass values were adjusted to the simple exponential regression model (Olson, 1963) as follows:
Where:
X t = Litterfall mass at a given time (years)
X 0 = Baseline litterfall mass
β 0 = Value at time zero
e = Napier’s constant
k = Decomposition constant
t = Time expressed as days
Also, the theoretical time (years) required for 50 %, 95 % and 99 % of the litterfall to decay was determined according to the following formulas:
Once the residual dry weight of each sample was estimated, the litterfall was ground in a 3383 Thomas Willey mini mill with a No. 20 (0.85 mm2) mesh. The ground litterfall was subsequently stored in ZiplocTM type bags labeled by date, repetition and sampling season for purposes of chemical analysis.
Nutrients content
By way of a sample, 1.0 g were used to determine the concentration of minerals (Ca, K, P, Mg, Cu, Fe, Mn and Zn); they were incinerated in a F-48010 Thermo Scientific muffle furnace at 550 °C, during 4 h; the ashes were digested in a hydrochloric acid solution (HCl) and nitric acid (HNO3), by humid digestion (Cherney, 2000). The Ca, K, Mg, Cu, Fe, Mn and Zn contents were analyzed using atomic absorption spectrophotometry, with a PinAAcle 900F Perkin-Elmer TM spectrophotometer. The calcium concentrations were quantified with nitrous oxide-acetylene flame, and potassium, magnesium, copper, manganese, iron and zinc, with air-acetylene flame. Phosphorus (P) was quantified by colorimetry, using a Lambda 25 Perkin-Elmer TM spectrometer.
Statistical analyses
When the data corresponding to each season and to each nutrient were tested, they were shown not to meet the normality assumptions (Kolmogorov-Smirnov and Shapiro Wilk tests) or the variance homogeneity assumptions (Levene’s test); therefore, they were subjected to the Kruskal-Wallis nonparametric test (Ott, 1993) in order to detect differences between the seasons for each of the analyzed nutrients. The statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 22 for Windows (SPSS, 2013).
Environmental variables
The temperature (°C) and relative moisture (%) at the study site were registered (on an hourly basis) using automated HOBO sensors (H8 Family, Forestry Suppliers, Inc.) Precipitation (mm) was quantified at the site with a HOBOTM automated pluviometer. Table 1 shows the mean monthly temperature and relative moisture and the monthly precipitation registered during the period at the study site.
Table 1 Air temperature (°C) and relative moisture (%) monthly mean and monthly accumulated precipitation (mm).
| Month | Environmental variable | ||
|---|---|---|---|
| Temperature | Relative moisture | Precipitation | |
| December | 13.18 | 70.3 | 4.0 |
| January | 13.25 | 67.7 | 34.0 |
| February | 17.50 | 63.6 | 1.2 |
| March | 21.40 | 71.6 | 18.4 |
| April | 23.15 | 73.7 | 31.2 |
| May | 24.60 | 81.8 | 133.2 |
| June | 25.50 | 78.7 | 83.0 |
Results and Discussion
Decomposition of the litterfall
The litterfall decomposition rate is shown in Table 2, which illustrates the value of the decomposition constant (k, gg-1 year-1) for winter (0.002) and spring (0.003); this is a low decomposition rate. A study carried out in the Tamaulipan thorn scrub estimated the k values at an interval of 0.42 and 0.50 (Marmolejo et al., 2013). On the other hand, the k values for a different type of vegetation -Coffea arabica L., Pinus oocarpa Schiede ex Schltdl. and Eucalyptus grandis Hill ex Maiden plantations- were 0.87, 1.08 and 0.72, respectively (Farfán and Urrego, 2007). Rivera et al. (2013) cite a value of k=0.00913 in fallowed areas aged over 20 years, and a time of 75.9 days for the decomposition of 50 % of the matter, and 504.3 days for 99 %. In the present study, the litterfall decomposition was shown to require 0.8 years in winter, and 0.6 years in spring. The time required for the decomposition of 99 % ranges between 5.2 years in winter and 3.7 years in spring, which means that the litterfall will take longer to decay in winter.
Table 2 Litterfall decomposition rate (k) in winter and in spring, and estimated decomposition time for the disintegration of 50 %, 95 % and 99 % of the baseline dry weight.
| Seasons |
k (gg-1year-1) |
Decomposition (years) | R2 | ||
|---|---|---|---|---|---|
| t.50 | t.95 | t.99 | |||
| Winter | 0.002 | 0.8 | 3.4 | 5.2 | 0.819 |
| Spring | 0.003 | 0.6 | 2.4 | 3.7 | 0.462 |
The coefficient of determination (R2) of the model is included
Table 3 shows the results of the Kruskal-Wallis test for detecting significant differences between the macro- and micro-nutrient contents of the litterfall in the two seasons of the year (winter and spring) during the decomposition process, at different incubation times. Macronutrients like Ca and Mg showed significant differences at seven of the nine considered incubation times; the element P exhibited significant differences only at one incubation time. As for micronutrients, Mn had significant differences at six times; micronutrient Zn exhibited differences only at two times.
Table 3 Results of the Kruskal-Wallis test for the detection of differences, between winter and spring, in the content of nutrients in litterfall subjected to different incubation times.
| Incubation time (days) | Statistic | Nutrients in litterfall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | K | P | Mg | Cu | Fe | Mn | Zn | ||
| 0 | χ2 | 13.166 | 1.463 | 13.166 | 8.251 | 12.091 | 0.571 | 5.143 | 14.286 |
| P value | <0.001* | 0.226 | <0.001* | 0.004* | 0.001* | 0.450 | 0.023* | <0.001* | |
| 5 | χ2 | 5.851 | 13.166 | 0.143 | 3.863 | 7.406 | 0.091 | 3.571 | 1.851 |
| P value | 0.016* | <0.001* | 0.705 | 0.049* | 0.007* | 0.762 | 0.059 | 0.174 | |
| 10 | χ2 | 9.606 | 0.091 | 3.023 | 7.823 | 5.491 | 2.520 | 2.520 | 0.966 |
| P value | 0.002* | 0.762 | 0.082 | 0.005* | 0.019* | 0.112 | 0.112 | 0.326 | |
| 15 | χ2 | 4.860 | 10.667 | 0.427 | 6.827 | 2.160 | 4.167 | 7.260 | 3.527 |
| P value | 0.027* | 0.001* | 0.514 | 0.009* | 0.142 | 0.041* | 0.007* | 0.060 | |
| 30 | χ2 | 2.063 | 0.206 | 0.023 | 12.623 | 11.063 | 0.463 | 3.863 | 1.286 |
| P value | 0.151 | 0.650 | 0.880 | <0.001* | 0.001* | 0.496 | 0.049* | 0.257 | |
| 45 | χ2 | 6.606 | 0.463 | 0.006 | 3.291 | 0.823 | 0.823 | 7.823 | 0.463 |
| P value | 0.010* | 0.496 | 0.940 | 0.070 | 0.364 | 0.364 | 0.005* | 0.496 | |
| 60 | χ2 | 7.823 | 5.851 | 2.286 | 1.651 | 0.206 | 9.606 | 9.606 | 5.143 |
| P value | 0.005* | 0.016* | 0.131 | 0.199 | 0.650 | 0.002* | 0.002* | 0.023* | |
| 75 | χ2 | 10.566 | 2.766 | 0.966 | 5.143 | 3.291 | 12.623 | 13.720 | 0.571 |
| P value | 0.001* | 0.096 | 0.326 | 0.023* | 0.070 | <0.001* | <0.001* | 0.450 | |
| 90 | χ2 | 1.851 | 0.006 | 0.143 | 4.806 | 10.566 | 1.120 | 0.006 | 3.291 |
| P value | 0.174 | 0.940 | 0.705 | 0.028* | 0.001* | 0.290 | 0.940 | 0.070 | |
Statistically significant probabilities (p<0.05) are shown in bold characters.
*P values indicating statistical differences (p<0.05).
The results indicate that a greater retention, and therefore a lesser release, of nutrients occurred in the spring, when the precipitation was more abundant. This agrees with the data cited by Tripathi and Singh (1992), who suggested that most nutrients become immobilized during the rainy season.
Macronutrients release
In winter and spring, both macro- (Ca>P) and micronutrients (Fe>Mn>Zn>) tend to exhibit immobilization at the beginning, and are subsequently accumulated during the incubation time, as shown in figures 1a, 1c, 2a, 2c and 2d. The nutrients K>Mg were released in both seasons. The order of release registered in the present study differs from the data cited by Kuruvilla et al. (2016) in Munrochloa ritchei plants, for which the release pattern was Mg>K. Cu was the only element released in winter, while the release pattern of Ca was greater than the P in spring. For an incubation period ranging between 0 and 90 days, the accumulation of Ca amounted from 45.7 to 80.5 mg g-1 in winter, and 76.9 to 87.8 mg g-1 in the spring. There was no release in either season; however, an accumulation of 34.7 mg g-1 occurred in the winter, and of 10.9 mg g-1 in the spring (Figure 1a), and there was an increase of 76 % and 14 %, respectively, in relation to the baseline value. These results are contrary to those observed by Farfán and Urrego (2007) in Coffea arabica L. and Cordia alliodora (Ruiz & Pav.) Oken plantations, which exhibited a release of 34 % and 54 %, respectively, during an experimental period of 365 days. Thus, the incubation time, the environmental conditions and the quality of the litterfall were limiting factors for Ca release.
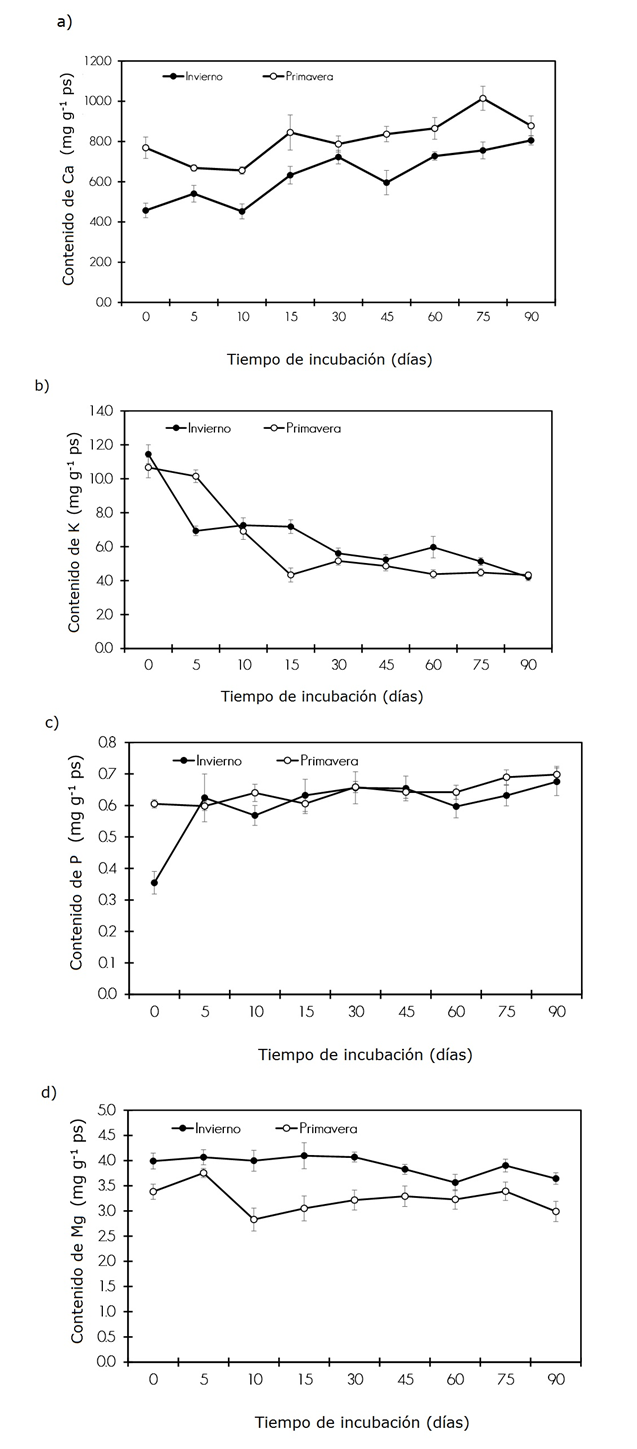
Figure 1 Litterfall content of Ca (a), K (b), P (c) and Mg (d) in the Tamaulipan thorn scrub during winter and spring at different incubation times during the decomposition process.
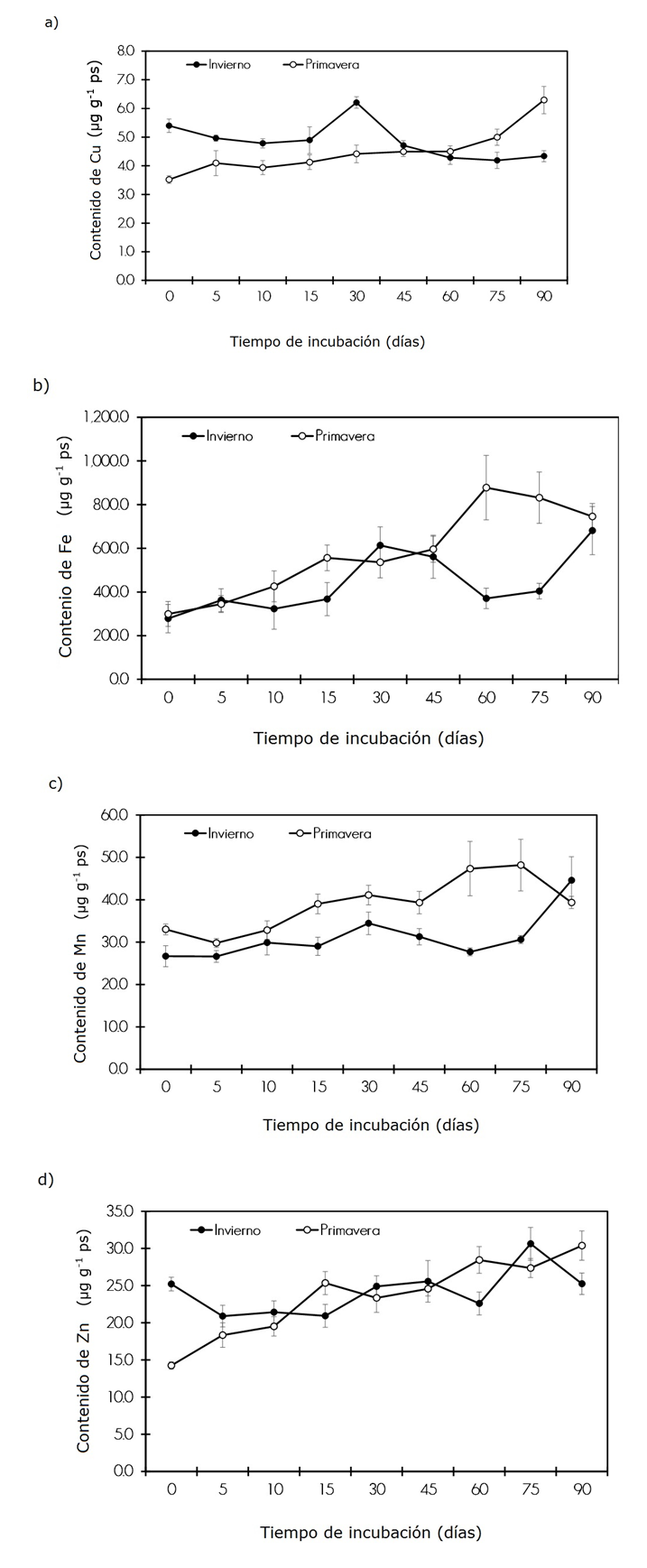
Contenido de Cu, Fe, Mn, Zn = Content of Cu, Fe, Mn, Zn; Tiempo de incubación (días) = Incubation time (days); Invierno = Winter; Primavera = Spring.
Each charted value represents the mean (n = 10) ± standard error.
Figure 2 Litterfall Cu (a), Fe (b), Mn (c) and Zn (d) content in the Tamaulipan thorn scrub at different incubation times during the decomposition process.
As for K, this nutrient exhibited a fluctuation of 11.4 to 4.2 mg g-1 in winter, and 10.6 to 4.3 mg g-1 in the spring. Therefore, according to Figure 1b, 63 % (7.2 mg g-1) of this element was released in the winter, and 59 % (6.3 mg g-1), in the spring. These observations suggest that K is a rapidly released element, as previously documented by Arce (2007) and by Castellanos and León (2011) for Ormosia coccinea (Aubl.) Jacks. and Vochysia lomatophylla Standl. plantations. This mobility of K has been cited; thus, we infer that it is not linked to the organic structure of leaf tissues but is freely located and therefore is easily washed or removed (Attiwill, 1968; Tukey, 1970; Parker, 1983).
The concentration of P ranged between 0.3 and 0.6 mg g-1 in winter and between 0.6 and 0.7mg g-1 in spring. In either season, little mobility of this element was observed during the litterfall decomposition process, as the values remained relatively stable from day 5 to day 60 of incubation, when it began to accumulate, up to day 90. At the end of the study, 91 % P was accumulated in winter, and 15 % in spring, with respect to the baseline amount (Figure 1c). These results agree with those cited for Eucalyptus grandis litterfall, which exhibited P immobilization (Farfán and Urrego, 2007). A similar tendency was observed by Castellanos and León (2011) in Acacia mangium Willd. litterfall; these authors point out P as the most restrictive element for the decomposition and mineralization of organic matter, with a tendency to immobilization, due to its scarce availability in the soil. According to Will (1967), during the litterfall decomposition process, P is retained by microorganisms or by organic compounds.
Mg exhibited a fluctuation of 3.9 to 3.6 mg g-1 in winter and of 3.3 to 2.9 mg g-1 in the spring. The release of this element was slow in both seasons, amounting to 0.34 mg g-1, equivalent to 9 % of the baseline quantity, in winter, and to 0.39 mg g-1, or 12 % of the baseline quantity, in the spring (Figure 1d). These results indicate a low release of Mg compared to that of K. Moro and Domingo (1996) documented a similar pattern for Adenocarpus decorticans Boiss. litterfall in the Filabres mountain range in Spain, which exhibited a release of 0.96 % of Mg and 1.69 % of K. However, Kuruvilla et al. (2016) observed a greater release of Mg than of K in Munrochloa ritchei.
Micronutrient release
The Cu content ranged between 5.4 to 4.3 μg g-1 in winter and 3.5 to 6.3 μg g-1 in the spring. In winter, Cu increased to 0.8 μg g-1 at 30 days; subsequently, at the end of the season, 1.0 μg g-1 was released. Conversely, in the spring the accumulation was 2.7 μg g-1 at 90 days of incubation. Therefore, release was only around 7 %, only in the winter (Figure 2a).
The Fe element ranged between 278.4 and 681.3 µg g-1 in winter, and between 299.5 and 745.9 µg g-1 in the spring. In either season, there was no release of Fe during the experimental period. Figure 2b illustrates how, at the end of the incubation, Fe had a similar average increase (424.55 µg g-1) in winter and in the spring.
The content of Mn was 26.6 to 44.5 µg g-1 in winter, and 33.0 to 39.3 µg g-1 in the spring. Figure 2c shows a similar average increase in Mn, of 12.1 µg g-1, at the end of the incubation period.
No significant variation was registered in winter in the Zn content, which remained virtually constant (25.2±5.22 μg g-1) at 0, 30, 45 and 90 days, while it varied between 14.2 and 30.3 µg g-1 in the spring. There was no release of this element during the incubation period. Figure 2d shows that Zn remained relatively stable in winter; however, in spring, at the end of the incubation period, it increased by 13 % (6.1 µg g-1) with respect to the initial time.
Conclusions
The behavior of the nutrients at the first stage of the litterfall decomposition process evidences similarities in winter and in spring. In most cases, there is accumulation and immobilization of nutrients (Ca, P, Cu, Mn, Fe, and Zn). Only K and Mg -in this order of importance- exhibit a tendency to be released. There is a slight release of K (0.42 mg g-1) in winter, compared to the spring. There is a slow release of Mg in both seasons. Ca and P are accumulated in both winter and spring, reaching its highest values in the latter season, and its highest accumulation in the former, with regard to the mean. The micronutrients (Cu, Mn, Zn, and Fe) tend to accumulate more in the spring than in winter.
The results for the 50 % litterfall decomposition rates show that the decomposition will be 0.2 years (73 days) slower in winter than in spring. It is inferred that environmental factors, the chemical composition of the litterfall, biological decomposing agents and soil factors determine the mobility or immobility of the nutrients. Greater precipitations occurred during the spring, and the immobility of the nutrients was then more evident. The rapid release of K observed in both seasons suggests that it is a mobile nutrient, freely located and not associated to the litterfall structures.
Acknowledgments
The authors wish to express their gratitude to the Consejo Nacional de Ciencia y Tecnología, Conacyt (National Council on Science and Technology) for the funding of the Conacyt 250732 project. They are equally grateful to three anonymous reviewers who enriched this paper through their constructive critical comments.
REFERENCES
Arce, U. C. 2007. Dinámica de descomposición y mineralización de macro nutrimentos en hojarasca de plantaciones de Ormosia coccinea (Aubl.) Jackson," Huayruro" y Vochysia lomatophylla Standl, "Quillosisa'', Iquitos, Loreto, Perú. Folia Amazónica 16(2): 101-106. [ Links ]
Arellano, R., J. Paolini, L. Vásquez y E. Mora. 2004. Producción y descomposición de hojarasca en tres agroecosistemas de café en el estado de Trujillo, Venezuela. Revista Forestal Venezolana 48(1): 7-14. [ Links ]
Attiwill, P. M. 1968. The loss of elements from decomposing litter. Ecology 49(1): 142-145. [ Links ]
Barreto S., L. H. y J. D. León P. 2005. Masa total y contenido de nutrientes en raíces finas de ecosistemas forestales (Pinus patula Schltdl y Cham., Cupressus lusitanica Mill. y Quercus humboldtii Bonpl.) de Piedras Blancas, Antioquia-Colombia. Revista Facultad Nacional de Agronomía 58(2): 2907-2929. [ Links ]
Barreto S., W., E. Périco, M. Schmidt D., M. Santos and R. L. Cajaiba. 2018. Are litterfall and litter decomposition processes indicators of forest regeneration in the neotropics? Insights from a case study in the Brazilian Amazon. Forest Ecology and Management 429: 189-197. [ Links ]
Begon, M., J. L. Harper and C. R. Townsend. 1990. Ecology Individuals, Populations and Communities. Blackwell Scientific Publications. Malden, MA USA. 945 p. [ Links ]
Berg, B. 2000. Litter decomposition and organic matter turnover in northern forest soils. Forest Ecology and Management 133(2): 13-22. [ Links ]
Bocock, K. L. and J. W. Gilbert. 1957. The disappearance of leaf litter under different woodland conditions. Plant and Soil 9(1): 179-185. [ Links ]
Bubb, K. A., Z. H. Xu, J. A. Simpson and P. G. Safigna. 1998. Some nutrient dynamics associated with litterfall and litter decomposition in hoop pine plantations of southeast Queensland, Australia. Forest Ecology and Management 110(1): 343-352. [ Links ]
Castellanos, B. J y J. D. León. 2011. Descomposición de hojarasca y liberación de nutrimentos en plantaciones de Acacia mangium (Mimosaceae) establecidas en suelos degradados de Colombia. Revista de Biología Tropical 59(1): 113-128. [ Links ]
Cherney, D. J. R. 2000. Characterization of forages by chemical analysis. In: Givens, D. I., E. Owen, R. F. E. Axford and H. M. Omed (eds.). Forage evaluation in ruminant nutrition. CAB International. Wallingford, UK. pp. 281-300. [ Links ]
Del Valle A., J. I. 2003. Cantidad, calidad y nutrimentos reciclados por la hojarasca fina de bosques pantanosos del Pacífico Sur Colombiano. Interciencia 28(8): 443-449. [ Links ]
Domínguez G., T. G., H. González R., R. G. Ramírez L., A. E. Estrada C., I. Cantú S., M. V. Gómez M., J. A. Villarreal Q., M. S. Alvarado y G. Alanís F. 2013. Diversidad estructural del Matorral Espinoso Tamaulipeco durante las épocas seca y húmeda. Revista Mexicana de Ciencias Forestales 4(17): 106-123. [ Links ]
Farfán, V. F. y J. B. Urrego. 2007. Descomposición de la hojarasca y liberación de nutrimentos de Coffea arabica, Cordia alliodora, Pinus oocarpa y Eucalyptus grandis, en sistemas agroforestales con café. Cenicafé 58(1): 20-39. [ Links ]
Friedel, M. H., W. A. Laycock and G. N. Bastin. 2000. Assessing rangeland condition and trend. In: Mannetje, L. T. and R. M. Jones (eds.). Field and Laboratory Methods for Grassland and Animal Production Research. CABI Publishing. Wallingford, UK. pp. 227-262. [ Links ]
García-Oliva, F., B. Sveshtarova and M. Oliva. 2003. Seasonal effects on soil organic carbon dynamic in a tropical deciduous forest ecosystem in western Mexico. Journal of Tropical Ecology 19(2): 179-188. [ Links ]
González, R. H., I. Cantú, M. V. Gómez and R. G. Ramírez. 2004. Plant water relations of thornscrub shrub species, northeastern Mexico. Journal of Arid Environments 58(4): 483-503. [ Links ]
González-Rodríguez, H., R. G. Ramírez-Lozano, I. Cantú-Silva, M. V. Gómez-Meza, M. Cotera-Correa, A. Carrillo-Parra y J. J. Marroquín-Castillo. 2013. Producción de hojarasca y retorno de nutrientes vía foliar en un matorral desértico micrófilo en el noreste de México. Revista Chapingo Serie Ciencias Forestales y del Ambiente 19(2): 249-262. [ Links ]
Jordan, C. F. 1985. Nutrient Cycling in Tropical Forest Ecosystems. Principles and Their Application in Management and Conservation. John Wiley and Sons. Chichester, New York, Brisbane, Toronto, Singapore. 190 p. [ Links ]
Kuruvilla, T., C. M. Jijeesh and K. K. Seethalakshmi. 2016. Litter production and decomposition dynamics of a rare and endemic bamboo species Munrochloa ritcheyi of Western Ghats, India. Tropical Ecology 57(3): 601-606. [ Links ]
López H., J. M. 2014. Producción de hojarasca y retorno potencial de nutrimentos en tres sitios del estado de Nuevo León, México. Tesis Doctoral, Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. Linares, N.L., México. 83p. [ Links ]
López H., J. M., H. González R., R. G. Ramírez L., J. I. del Valle A., I. Cantú S., M. Pando M., A. E. Estrada C. y M. V. Gómez M. 2014. Producción de hojarasca y depósito potencial de nutrientes de las hojas en el Matorral Espinoso Tamaulipeco. Revista Mexicana de Ciencias Forestales 6(30): 74-89. [ Links ]
Marmolejo M., J. G., C. M. Cantú A. y M. A. Gutiérrez S. 2013. Degradación de la hojarasca en sitios con vegetación primaria y secundaria del Matorral Espinoso Tamaulipeco. Revista Mexicana de Ciencias Forestales 4(17): 175-181. [ Links ]
Montagnini, F. and N. Muñiz-Miret. 1999. Vegetation and soils of tidal floodplains of the Amazon estuary: A comparison of várzea and terra firme forests in Pará, Brazil. Journal of Tropical Forest Science 11(2): 420-437. [ Links ]
Montagnini, F. y C. Jordan. 2002. Reciclaje de nutrimentos. In: Guariguata, M.R. y G. H. Kattan. (eds.), Ecología y conservación de bosques lluviosos neotropicales. Cartago, Costa Rica. pp. 167-190. [ Links ]
Moro, M. J y F. Domingo. 1996. Descomposición de hojarasca en la leguminosa Adenocarpus decorticans: pérdida de peso y dinámica de los nutrientes en la Sierra de los Filabres (Almería). Revista Mediterránea Serie de Estudios Biológicos 2(15): 13-19. [ Links ]
Olson J., S. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44(2): 322-331. [ Links ]
Ott, L. 1993. An introduction to statistical methods and data analysis. 2nd ed. Duxbury Press. Boston, MA USA. 775 p. [ Links ]
Parker, G. G. 1983. Throughfall and stemflow in the forest nutrient cycle. Advances in Ecological Research 13(1): 57-133. [ Links ]
Parzych, A. and J. Trojanowski. 2006. Precipitation and duff fall as natural sources of nitrogen and phosphorus for forest soils in the Słowiński National Park. Baltic Coastal Zone 10: 47-59. [ Links ]
Rivera V., R., L. Soto P., C. A. Núñez C., B. De Jung, M. G. Hernández R. y J. A. B. Ordóñez D. 2013. Producción y tasa de descomposición de hojarasca en Acahuales de selva caducifolia en Chiapas. Revista Mexicana de Ciencias Forestales 4(20): 20-30. [ Links ]
Sánchez, S., G. Crespo y M. Hernández. 2009. Descomposición de la hojarasca en un sistema silvopastoril de Panicum maximum y Leucaena leucocephala (Lam) de Wit cv. Cunningham. I. Influencia de su composición química. Pastos y Forrajes 32(4): 2-8. [ Links ]
Santa, R. I. 1987. Contribución al estudio de la dinámica de la materia orgánica y bioelementos en bosques en la Sierra de Béjar. Tesis Doctoral. Universidad de Salamanca. Salamanca, España. 464 p. [ Links ]
Schlesinger, W. H. 2000. Biogeoquímica: un análisis del cambio global. Colección Ariel Ciencia. Barcelona, España. 592 p. [ Links ]
Statistical Package for the Social Sciences (SPSS). 2013. Statistical Package for the Social Sciences. Ver. 22. SPSS Inc. Armonk, NY USA. n/p. [ Links ]
Swift, M. J and J. M. Anderson. 1989. Decomposition. In: Lieth, H. and M. J. A. Werger (eds.). Tropical rain forest ecosystems: Biogeographical and ecological studies. Ecosystems of the world 14A. Elsevier Science. New York, NY USA. pp. 547-569. [ Links ]
Tripathi, K. P. and K. P. Singh. 1992. Nutrient immobilization and release patterns during plant decomposition in a dry tropical bamboo savanna, India. Biology and Fertility of Soils 14(1): 191-199. [ Links ]
Tukey, H. B. 1970. The leaching of substances from plants. Annual Review of Plant Physiology 21(1): 305-324. [ Links ]
Vitousek, P. M., G. Gerrish, D. R. Turner, L. R. Walker and D. Mueller-Dombois. 1995. Litterfall and nutrient cycling in four Hawaiian montane rainforests. Journal of Tropical Ecology 11(2): 189-203. [ Links ]
Will, G. M. 1967. Decomposition of Pinus radiata litter on the forest floor. Part I. Changes in dry matter and nutrient content. New Zealand Journal of Science 10(4): 1030-1044. [ Links ]
Received: December 14, 2017; Accepted: August 15, 2018











 texto em
texto em 


