Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.9 no.48 México jul./ago. 2018
https://doi.org/10.29298/rmcf.v8i48.152
Articles
Edaphic conditions, abundance, and richness of edible ectomycorrhizal fungi
1Centro Nacional de Investigación Disciplinaria en Conservación y Mejoramiento de Ecosistemas Forestales. INIFAP. México.
Ectomycorrhizal fungi (EMF) favor nutrient mobilization and the transportation of water between the substrate and the trees; therefore, the ectomycorrhizal association is essential for the adequate development of forest ecosystems. Four types of silvicultural practices were compared to establish the effects of the edaphic properties on the richness and abundance of EMF populations: regeneration felling, thinning (first and second), and release. Ten monitoring plots were delimited and georeferenced in forests under exploitation in the municipality of Zacatlán, Puebla. The richness and abundance of fungi were evaluated in 2015 and 2016; in the latter year, the soil was sampled and analyzed. Results showed significant differences in abundance between the two years with the second thinning and with the release treatment, as well as between the two thinnings between the year of the intervention and the sampling year. The percentage of organic matter and sand showed a positive correlation with the abundance, unlike with K, Ca, Mn and Mg, which were negatively correlated between treatments. No statistical differences in pH or richness of species were obtained. Nevertheless, Lactarius indigo, Boletus aestivalis and Tylopilus sp. were collected only from stands with pH values ranging between 5.76 and 5.93, in the thinning and release plots. The results show that the silvicultural practices affect the basal tree area by altering the chemical and physical properties of the soil, which in turn have an impact on the abundance of sporomes.
Key words: Ectomycorrhizal fungi; organic matter; physical properties of the soil; chemical properties of the soil; forestry systems; forest soils
Los hongos ectomicorrizógenos (HECM) favorecen la movilización nutrimental y el transporte del agua entre el sustrato y los árboles, por lo que la asociación ectomicorrícica es fundamental para el buen desarrollo de los ecosistemas forestales. Para establecer los efectos de las propiedades edáficas en la riqueza y abundancia de las poblaciones de HECM, se compararon cuatro tipos de prácticas silvícolas: corta de regeneración, de aclareo (primera y segunda) y de liberación. Se delimitaron y georreferenciaron 10 parcelas de monitoreo, en bosques bajo aprovechamiento del municipio Zacatlán, Puebla. La riqueza y la abundancia fúngica se evaluaron en 2015 y 2016; en este último, se hizo el muestreo y análisis del suelo. Se obtuvieron diferencias significativas entre años para la abundancia en la segunda corta de aclareo y en la de liberación, así como entre las cortas de aclareo que responden al periodo entre el año de intervención y el de muestreo. El porcentaje de materia orgánica y de arena registraron una correlación positiva con la abundancia, en contraste con K, Ca, Mn y Mg, que fueron negativas entre tratamientos, y no se obtuvieron diferencias para pH y la riqueza de especies; sin embargo, Lactarius indigo, Boletus aestivalis y Tylopilus sp. solo se recolectaron en rodales con valores de 5.76 a 5.93, en las parcelas de cortas de aclareo y liberación. Los resultados muestran que las prácticas silvícolas afectan el área basal arbórea alterando las propiedades químicas y físicas del suelo, lo que a su vez incide sobre la abundancia de esporomas.
Palabras clave: Hongos ectomicorrizógenos; materia orgánica; propiedades físicas del suelo; propiedades químicas del suelo; tratamientos silvícolas; suelos forestales
Introduction
Ectomycorrhizal fungi (EMF) are necessary components of the edaphic biota, which already play a prevalent role in the mobilization of nutrients between the soil and the plants, mainly of nitrogen and phosphorus, as well as in the transportation of water (Smith and Read, 1997). The establishment of the ectomycorrhizal association is essential for the adequate development of many tree species of temperate areas, notably of the Pinaceae family, such as Pinus spp. and Abies religiosa (Kunth) Schltdl. et Cham. These taxa have great economic importance, and their stands contribute 2.6687 million m3 r (cubic meters of sawtimber) (Semarnat, 2017) commercialized in Mexico and extracted from forests under forest management.
The EMF populations are sensitive to changes in abiotic factors (precipitation, altitude, exposure) and in edaphic properties, such as pH, texture, moisture retention capacity, concentrations of carbon, sodium, magnesium, calcium, and cation exchange capacity (Martínez-Peña et al., 2012; Taylor et al., 2014; Nadeau and Khasa, 2016; Smith et al., 2017). Because timber extraction and other silvicultural practices modify the physical and chemical properties of forest soil, as a consequence of the increase in the sunlight, evapotranspiration rate, and temperature, these are expected to impact the composition of the structure of ectomycorrhizal communities (Zamora-Martínez, 2010), in terms of sporome emergence and of the presence of mycorrhizae in the root systems (Perry et al., 1984; Wright et al., 1997; Pilz et al., 2003; Valdés et al., 2003; Bonet et al., 2004; Pilz et al., 2006; Valdés et al., 2009; de-Miguel et al., 2014). For example, it has been documented that soil compaction and topsoil removal, resulting from timber extraction in Pseudotsuga menziesii (Mirb.) Franco plantations, reduce the abundance and diversity of ectomycorrhizal communities (Amaranthus et al., 1996).
The influence of the properties of the soil on the taxonomic composition and the functional structure of ectomycorrhizal communities, at the level of the sporomes and the mycorrhizae, has been studied by various authors (Courty et al., 2010; Martínez-Peña et al., 2012). However, in Mexico the information related to this topic has not been sufficiently addressed, and is generally referred to as collateral or complementary in researches on the effect of the various silvicultural treatments on fungal diversity (Valdés et al., 2003; Valdés et al., 2009), as well as in papers dealing with the distribution of certain macromycetes (Zamora-Martínez et al., 2014).
Within this context, we set the objective of establishing the relationship that exists between the physical and chemical properties of the soil and the appearance of sporomes of ectomycorrhizal species in stands subjected to diverse silvicultural practices. This work was based on the premise that the measurement of variables directly related with these factors is an important element for establishing productivity zones, especially of non-timber species, as is the case of fungi, which have presence and an ecological, economic and cultural value in the temperate and temperate cold climate forest ecosystems of Mexico.
Materials and Methods
The study was carried out at ejido Rancho Nuevo Nanacamila, in the municipality of Zacatlán de las Manzanas, Puebla, Mexico, between the coordinates 20°02'54.24'' and 20°04'30.00'' N, and 98°04'42.24'' and 98°06'38.88'' W; at an altitude of 2 290 m. The predominant vegetation consists of Pinus patula Schiede ex Schltdl. et Cham. forests, with lower proportions of P. teocote Schiede ex Schltdl. et Cham., P. leiophylla Schiede ex Schltdl. et Cham., P. rudis Endl., P. pseudostrobus Lindl., Abies religiosa (Kunth) Schltdl. et Cham., Quercus spp. and Arbutus xalapensis Kunth. The physiognomic characteristics of the site vary according to the silvicultural treatment; thus, there are significant contrasts in forest density, floral associations, spacing, light input, stoniness, among others.
The area under forest management covers a surface of 283.13 ha and includes the application of three different treatments, according to the Silvicultural Development Method utilized: 1) regeneration felling (CR), which consists in leaving intact only those individuals (parent trees or seed trees) that have physical qualities of interest for exploitation; 2) release felling (CL), in which the seed trees are eliminated, and 3) thinning (CA), carried out in order to extract individual trees that do not fulfill the commercial characteristic determined by the management program. The present study assessed the first (PCA) and second (SCA) thinnings. The ejido is under a management program certified by the Rainforest Alliance (FSC certificate, RA-FM/COC-006372).
The sampling area (33 × 33 m) consisted of ten plots distributed as follows: three for the regeneration fellings and first thinning, and two for the second thinning and release treatments. The density and basal area after the intervention are shown in Table 1.
Table 1 Dasometric information of the sampling plots at ejido Rancho Nuevo Nanacamila, in the municipality of Zacatlán de Las Manzanas, Puebla.
| Silvicultural treatment | Density (Indiv. ha -1 ) |
Basal area (m -2 ha -1 ) |
|---|---|---|
| Regeneration felling | 187.50** | 11.58 |
| Release felling | 625** | 17.55 |
| First thinning | 672.22* | 37.42 |
| Second thinning | 800** | 29.67 |
*Average of three intervention years = 2006, 2007 and 2012; **Year of application = 2009.
To estimate the richness and abundance of EMF, all the sporomes of the present ectomycorrhizal species were collected during July and November 2015 and 2016. For this purpose, each sampling unit (plot) was traversed in a zig-zag pattern every week, until the total area was covered. The fungal material of each pattern was separated by species for counting purposes and placed in polyroll or wax paper bags. These materials were subsequently identified based on their macroscopic characteristics (Largent et al., 1984; Pérez-Silva y Herrera, 1991; Phillips, 1991; Rodríguez-Alcalá et al., 2002; Pérez-Moreno et al., 2010; García-Rodríguez et al., 2012). The specimens were deposited at the “Biol. Luciano Vela Gálvez” National Forest Herbarium (INIF) of the National Institute for Research on Forestry, Agriculture and Livestock (Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, INIFAP). The richness of species (S) was represented by the total number of taxa identified for each treatment, while their abundance was represented by the number of sporomes collected from each species.
The effect exerted by silvicultural practices on the production of EMF regarding the physical and chemical characteristics of the soil was determined based on a sampling of the soil carried out in 2016. The material was collected in five points per plot, at a depth of 0-30 cm to generate a compound mixture. A total of three were obtained from each plot, adding up to a total of 27, which were processed at the Soils Laboratory of the Central Regional Research Center (Centro de Investigación Regional Centro, CIR-Centro) of INIFAP. The physical and chemical properties were determined according to the Mexican Official Norm NOM-021-RECNAT-2000 (Semarnat, 2002).
Based on the field information, a comparison was made between the abundance (number of sporomes) and the richness of the EMF species between silvicultural treatments per year of collection, and between years for each practice, using the Factorial ANOVA Between-Subjects, and the Bonferroni test was utilized to adjust the p values (Zar, 2010).
The physical and chemical analysis of the soil included 18 variables, which served as a basis to assess whether or not they had a normal distribution. Otherwise, the Kruskal-Wallis non-parametric test was used to evaluate the existence of significant differences between the treatments (Zar, 2010). Whenever the comparisons showed such differences, independent samples were subjected to a paired-comparison analysis using the Mann-Whitney U test (Quinn and Keough, 2002).
Furthermore, Pearson’s correlation analysis was performed to determine the potential relationship associating the percentages and concentrations of the physical and chemical elements of the soil with the richness and abundance of EMF. Finally, correlations were carried out to link the sampled frequency of the registered plots to the edaphic data. The SPSS statistical package (IBM, 2013) was utilized for all statistical analyses.
Results and Discussion
Effect of the silvicultural treatments on the abundance and richness of EMF
The total number of sporomes collected in 2015 (1 177) was higher than that registered for 2016 (732). During the study period, the highest abundance was registered with the first thinning (Figure 1). For its part, the average richness was very similar for the various treatments: S = 16 for 2015 and S = 15 for 2016 (Figure 2).
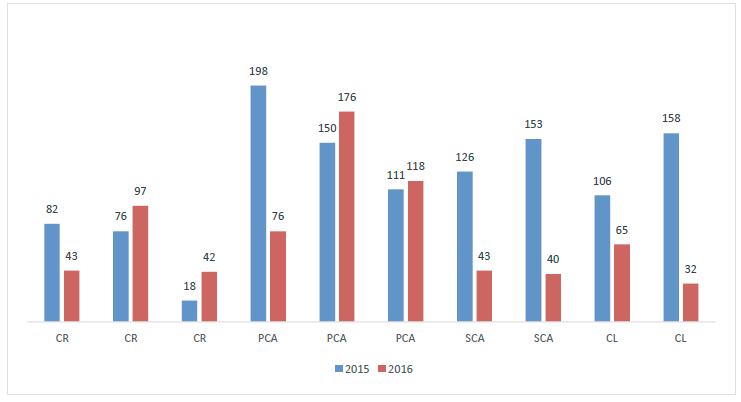
CR = Regeneration felling; PCA = First thinning; SCA = Second thinning; CL = Release felling.
Figure 1 Number of EMF sporomes collected in four silvicultural treatments during two sampling years at ejido Rancho Nuevo Nanacamila, in the municipality of Zacatlán de Las Manzanas, Puebla.

CR = Regeneration felling; PCA = First thinning; SCA = Secondthinning; CL = Release felling.
Figure 2 Richness of EMF for four silvicultural treatments during two sampling years at ejido Rancho Nuevo Nanacamila, in the municipality of Zacatlán, Puebla.
The Factorial ANOVA Between-Subjects showed that the number of sporomes collected with the regeneration felling treatment in 2015 was significantly lower (p<0.05) than that registered for other silvicultural practices, while in 2016 differences were detected between the first and the second thinnings, as well as with the release felling (Table 2). No differences were observed when the richness of species was contrasted between the various treatments.
Table 2 Results of the Factorial ANOVA Between Subjects for the comparison between the number of sporomes, the richness of species (S) and the silvicultural treatments in the same collection year.
| Treatment | Significance a | |||
|---|---|---|---|---|
| Number of sporomes | Richness (S) | |||
| 2015 | 2016 | 2015 | 2016 | |
| CR - 1CA | 0.014* | 0.172 | 0.956 | 1.000 |
| CR - 2CA | 0.030* | 1.000 | 1.000 | 1.000 |
| CR - CL | 0.037* | 1.000 | 1.000 | 1.000 |
| 1CA - 2CA | 1.000 | 0.036* | 0.956 | 0.293 |
| 1CA - CL | 1.000 | 0.043* | 1.000 | 0.833 |
| 2CA - CL | 1.000 | 1.000 | 1.000 | 1.000 |
CR = Regeneration felling; 1CA = First thinning; 2CA = Second thinning; CL = Release felling. *Statistically significant values (p<0.05). Factorial ANOVA Between Subjects: N=24; d.f. = 3. aAjustment of the p values with the Bonferroni test.
When the number of sporomes collected between 2015 and 2016 was compared by treatment, the test evidenced significant differences for the second thinning and the release felling (Table 3). There were no significant differences in the richness of species.
Table 3 Results of the Factorial ANOVA Between Subjects for the comparison between the number of sporomes and the richness (S) obtained in two years of collection for each silvicultural treatment.
| Silvicultural treatment | Significance a | |
|---|---|---|
| Num. of sporomes |
Richness (S) |
|
| Regeneration felling (2015 vs 2016) | 0.940 | 0.809 |
| First thinning (2015 vs 2016) | 0.272 | 0.936 |
| Second thinning (2015 vs 2016) | 0.001* | 0.104 |
| Release felling (2015 vs 2016) | 0.002* | 0.872 |
*Statistically significant values (p<0.05). Factorial ANOVA Between Subjects: N = 24; d.f.=3. aAdjustment of the p values using the Bonferroni test.
The results evidenced the existence of a differential distribution of the emergence of sporomes between the four assessed silvicultural practices that are associated with the felling intensity. Based on an ecosystemic perspective, the silvicultural treatment is considered as a disturbance factor that modifies the density of the forest, its spatial structure and the value of its stock (Zamora-Martínez, 2010), as well as the biotic interactions. Therefore, the magnitude of the disturbance will be in terms of the type of felling -partial (thinning) or regeneration felling (total or of the seed trees)-, since its application alters in a different way the basal area, dominant height, tree stand cover, and other dasometric variables that have significant effects on the production of EMF (Velasco et al., 2010; Bonet et al., 2012; Martínez-Peña et al., 2012). On the other hand, the actions performed during timber extraction, whether manual or mechanized, have an impact on fungal populations since they cause soil compaction and reduce evapotranspiration (de-Miguel et al., 2014).
The regeneration felling was the silvicultural treatment that yielded the lowest number of sporomes in the two assessment periods (2015 and 2016), as can be seen in Figure 1. This treatment causes a high degree of disturbance affecting the associated fungal communities. Also, it implies the extraction of virtually all the trees (except for parent or seed trees); favors increased solar radiation, edaphic temperature and the temperature of the strata of residual herbal and shrub vegetation, as well as of the vegetation that develops during the years following the intervention (six years for the present study).
In this regard, Luoma et al. (2004) point out that EMF production diminishes as the felling intensity increases, although in Pinus pinaster Aiton. forests subjected to intensive management, it has been documented to favor the development of specific taxa, such as the Lactarius deliciosus group (Bonet et al., 2012). However, it should be noted that other environmental factors that impact the emergence of sporomes are the distribution and intensity of precipitations (Martínez de Aragón et al., 2007; Velasco et al., 2010).
Moreover, there is evidence that indicates that the basal area (BA) of the stand significantly affects EMF production (Martínez-Peña et al., 2012). The BA of the plots located in the areas subjected to regeneration felling, six years after the intervention, was 11.58 m2 ha-1; this value was lower than the values estimated for other treatments (Table 1, Figure 1). For example, for the first and second thinnings, the respective estimated basal areas were 37.42 m2 ha-1 and 29.67 m2 ha-1, both of which are above the 15-20 m2 ha-1 interval documented for this variable as a value associated to maximum EMF productions in pine forests (Bonet et al., 2008; Bonet et al., 2010).
As for the differences in the number of sporomes observed between the first felling and the second, they are biased by the difference in the number of plots considered, although a higher abundance was expected as the forest mass grew in age and vigor. A possible explanation lies in the length of the period between the intervention and the sampling year, which was ten years for the first felling, and six, for the second felling. After any felling, whether selective or for regeneration purposes, the physical and chemical properties of the soils are modified; this is reflected in the loss of nutrients, the alteration of the apparent density and the porosity, as well as of the capacity to retain water, among others (Kishchuk et al., 2014).
The recovery period can exceed ten years, depending on the intensity of the felling (Zhou et al., 2015), and the negative impacts at soil level affect the abundance of EMF. Given the nutritional dependence of these organisms on their arboreal hosts, as a result of the lower availability of nutrients, there is a reduction of their photosynthetic activity and the translocation of carbohydrates toward the EMF (Nehls, 2008).
It is worth noting that we still lack the research required to accurately explain the effect of silvicultural practices on the fungal populations present in stands subjected to forest management programs, particularly on those species of mushrooms that are collected for commercial purposes. In this regard, Pilz et al. (2006) point out that the production of sporomes of Chantarellus spp. diminishes during the first year of timber harvesting, but recovers almost entirely after six years.
The release felling -which consists in eliminating the tree species that are in the process of growing, old trees, and herbaceous and shrub taxa (Daniel, 1983)- causes soil compaction, with the resulting loss of ectomycorrhizal inoculum at soil level and in particular “refuge plants” (Wiensczyk et al., 2002; Kennedy et al., 2003; Martínez, 2008), reflected in a reduced abundance of sporomes.
Effect of the edaphic conditions on the abundance and richness of EMF
One of the challenges of the forest sector is the application of silvicultural practices for the sustainable management of the resources. Maintaining the productivity of the soil is critical for this purpose, as it has an impact not only on the conservation potential of the long-term timber production, but also in other functions of the forest ecosystem, such as the presence of the associated biota and, particularly, of the EMF. When the assessed silvicultural treatments were compared, significant differences (p>0.05) resulted for 11 of the edaphic variables considered: the percentage of organic matter, the percentage of sand, and available phosphorus (P), potassium(K), calcium (Ca), magnesium (Mg), sodium (Na), iron (Fe), zinc (Zn), copper (Cu) and pH (Tables 4 and 5).
Table 4 Mean values of the physical and chemical properties of the soils with significant differences in the Mann-Whitney U paired analysis.
| Edaphic variable / Silvicultural treatments |
CR | CL | 1CA | 2CA |
|---|---|---|---|---|
| Organic matter (%) | 38.25 | 8.15 | 46.46 | 8.04 |
| Sand (%) | 81.59 | 84.01 | 86.29 | 82.26 |
| P bray (mg L-1) | 1.11 | 0.77 | 2.11 | 1.36 |
| K (mg L-1) | 306.18 | 352.80 | 196.6 | 373.79 |
| Mg (mg L-1) | 197.47 | 225.46 | 162.8 | 235.39 |
| Na (mg L-1) | 49.15 | 13.86 | 28.64 | 17.56 |
| Fe (mg L-1) | 84.99 | 41.20 | 39.26 | 29.22 |
| Zn (mg L-1) | 1.89 | 0.37 | 0.65 | 0.72 |
| Cu (mg L-1) | 0.25 | 0.26 | 0.39 | 0.35 |
| pH | 5.45 | 5.76 | 5.65 | 5.93 |
| Ca (mg L-1) | 1 133.08 | 1 236.70 | 1 093.18 | 1 594.20 |
CR = Regeneration felling; CL = Release felling; 1CA = First thinning; 2CA = Second thinning.
Table 5 Results of Kruskal-Wallis test comparing the differences in the physical and chemical variables of the soil between silvicultural treatments.
| Physical-chemical soil variables | Significance |
|---|---|
| Organic matter (%) | <0.001* |
| Inorganic N (mg L-1) | 0.857 |
| P bray (mg L-1) | 0.027* |
| K (mg L-1) | 0.001* |
| Ca (mg L-1) | 0.032** |
| Mg (mg L-1) | 0.017* |
| Na (mg L-1) | 0.007* |
| Fe (mg L-1) | <0.001* |
| Zn (mg L-1) | 0.001* |
| Mn (mg L-1) | 0.171 |
| Cu (mg L-1) | 0.004* |
| pH | 0.007* |
| Saturation point (%) | 0.071 |
| Field capacity (%) | 0.071 |
| Permanent withering point (%) | 0.071 |
| Sand (%) | 0.05* |
| Clay (%) | 0.585 |
| Silt (%) | 0.060 |
*Results that had a statistically significant difference (p>0.05);**Element with a significant difference with the Kruskal-Wallis test, but with no significant paired comparison (Mann-Whitney U); N=28; d.f. = 54 for each case.
The comparison between treatment pairs for the edaphic properties evidenced the same correlations; most relevant among these was those the correlation between the organic matter and the percentage of sand (Table 6).
Table 6 (Mann Whitney U) paired comparisons between treatments concerning the physical and chemical soil elements that registered significant differences with the Kruskal-Wallis test.
| Edaphic properties/Comparison between treatments |
CR - 1CA | CR - 2CA | CR - CL | 1CA - 2CA | 1CA - 2CA | 2CA - CL |
|---|---|---|---|---|---|---|
| Organic matter (%) | 1.000 | 0.120 | 0.039* | 0.004* | 0.012* | 1.000 |
| Sand (%) | 0.049 | 1.000 | 1.000 | 0.372 | 1.000 | 1.000 |
| P bray (mg L-1) | 0.380 | 1.000 | 1.000 | 1.000 | 0.046* | 0.176 |
| K (mg L-1) | 0.589 | 0.504 | 0.467 | 0.008* | 0.005* | 1.000 |
| Ca*+(mg L-1) | 1.000 | 0.083 | 1.000 | 0.057 | 0.823 | 1.000 |
| Mg (mg L-1) | 1.000 | 0.571 | 0.372 | 0.094 | 0.046* | 1.000 |
| Na (mg L-1) | 1.000 | 0.346 | 0.014* | 0.673 | 0.037* | 1.000 |
| Fe (mg L-1) | 0.007* | <0.001* | 0.072 | 0.992 | 1.000 | 0.615 |
| Zn (mg L-1) | 0.056 | 0.526 | 0.001* | 1.000 | 0.628 | 0.351 |
| Cu (mg L-1) | 0.006* | 1.000 | 1.000 | 0.467 | 0.022* | 1.000 |
| pH | 1.000 | 0.024* | 0.029* | 0.379 | 0.488 | 1.000 |
CR = Regeneration felling; 1CA = First thinning; 2CA = Second thinning; CL = Release felling. *Results that exhibited a significant difference (p<0.05) with the Mann-Whitney U paired analysis. N=6 and d.f. = 2 for each case. **Element are exhibiting a significant difference with the Kruskal-Wallis test but with no difference in the significant correlation with the Mann-Whitney U test.
The effect of the quantity and quality of organic matter on forest soils is a result of the fact that it is a quantitative, functional component of the ecosystem that plays a vital role in the physical and chemical environment. Its decay integrates elements of fertility and toxicity into the substrate, intercepts the light and regulates the thermal gradients between the soil and the atmosphere; furthermore, it increases the uptake of rainwater but reduces its evaporation. For this reason, it serves as a physical barrier for many chemical processes and elements (Facelli and Pickett, 1991).
In general, organic matter has been documented to diminish according to the intensity of the felling (Zhou et al., 2015). This agrees with the significant differences between the first thinning and the release (Table 5), but not with those between the release and the regeneration felling, probably because the waste material of the timber extraction (branches, stumps, bark, needles) is left at the exploitation site, while it is removed from the plots where the release was applied.
When the applied silvicultural treatments were contrasted, the significant differences in pH were registered between the regeneration and release fellings and the second thinning, which exhibited higher values, although the records turned out to be very similar (Tables 4 and 6).
The significant differences in K and P concentrations between the thinnings agreed with the values cited in the literature in the sense that these elements reduce as the canopy opens (Table 1) (Kishchuk et al., 2014; Zhou et al., 2015). However, K concentration was higher with the release than with the first thinning (Table 4), even though the BA was smaller (Table 1). A possible cause is the presence of abundant shrubs and herbs that contribute dead leaves to the forest floor, which has a higher pH and a higher concentration of basic cations, such as K (Kishchuk et al., 2014).
The main edaphic factors that were related to the abundance of sporomes were the percentages of organic matter, sand, and silt. The texture of the soil, determined by the percentage of silt and sand, affects the richness of species and the emergence of EMF sporomes (Martínez-Peña et al., 2012; Taylor et al., 2014). Soils with high sand percentages reduce water retention, while silty soils increase it, thereby favoring sporome production. The results documented herein differ from those cited above, as there was a negative correlation between the percentage of silt and the abundance of EMF, and a positive correlation for sand and for the content of organic matter (Table 7), which serves as an evapotranspiration buffer and contributes to maintaining the soil temperature. These two actions are likely to have contributed to reducing the loss of water, favoring the emergence of sporomes.
Table 7 Pearson’s analysis of the correlation between the richness and abundance of EMF and the results of the physical and chemical analysis of the soil.
| Soil fertility | Richness (S) | Abundance | ||
|---|---|---|---|---|
| Pearson’s Coefficient of Correlation |
p <0.05 | Pearson’s Coefficient of Correlation |
p <0.05 | |
| Dead Organic Matter (%) | 0.351 | 0.067 | 0.634* | <0.001* |
| Inorganic N (mg L-1) | -0.142 | 0.471 | -0.083 | 0.674 |
| P bray (mg L-1) | -0.158 | 0.421 | 0.020 | 0.918 |
| K (mg L-1) | -0.244 | 0.211 | -0.640* | <0.001* |
| Ca (mg L-1) | -0.408* | 0.031* | -0.564* | 0.002* |
| Mg (mg L-1) | -0.231 | 0.237 | -0.647* | <0.001* |
| Na (mg L-1) | 0.057 | 0.775 | 0.127 | 0.521 |
| Fe (mg L-1) | -0.007 | 0.971 | -0.138 | 0.484 |
| Zn (mg L-1) | -0.214 | 0.274 | -0.190 | 0.332 |
| Mn (mg L-1) | -0.475* | 0.011* | -0.397* | 0.036* |
| Cu (mg L-1) | 0.089 | 0.653 | 0.275 | 0.157 |
| pH | -0.111 | 0.572 | -0.197 | 0.315 |
| Saturation point (%) | 0.248 | 0.203 | 0.292 | 0.132 |
| Field capacity (%) | 0.248 | 0.204 | 0.292 | 0.132 |
| Permanent withering point (%) | 0.248 | 0.204 | 0.294 | 0.130 |
| Sand (%) | 0.277 | 0.154 | 0.404* | 0.033* |
| Clay (%) | 0.138 | 0.485 | 0.097 | 0.625 |
| Silt (%) | -0.335 | 0.082 | -0.453* | 0.015* |
*Results that had a statiscally significant difference (p<0.05).
As for the mineral nutrients, the elements that exhibited significant differences were K, Ca, Mg and Mn. Ca and Mn were negatively correlated with the richness of species (Table 7). This correlation is due to the affinity that EMF generally have for acidic environments, predominant in conifer forests. Thus, when the Ca content increases, the substrate becomes alkaline and, thus, less propitious for the development of fungi; consequently, the number of fungal species diminishes.
Pearson’s analysis showed a positive correlation between the abundance of sporomes, percentage of organic matter and the percentage of sand. Conversely, the correlation for K, Mg and the percentage of silt was negative. On the other hand, both the richness of species and the abundance of sporomes had a negative correlation with Ca and Mg (Table 7).
In particular, Taylor et al. (2014) highlight the existence of a fine-scale variation in the mushroom niches which is mainly related to the evolution of the soil, as the texture is closely linked to the content of assimilable microelements. Furthermore, the value of the quotient between the soluble and the totals decreases as the content of coarse elements in the texture of the soil increases.
The pH is an essential factor for the assimilation of macro and microelements. An increase in pH reduces the solubility and absorption of elements, such as copper, iron, zinc (Tables 4 and 7) and, notably, manganese; however, it increases the solubility and absorption of molybdenum (Mo); although the concentration of this element was not found to be significant for the abundance and richness of EMF (Table 7).
About EMF production, there are records of the significant effect of the pH on the yield of individual fungal species, e.g., Lactarius deliciosus group, which shows a marked preference for acidic soils (Bonet et al., 2012). In the research carried out at ejido Rancho Nuevo Nanacamila, Lactarius indigo (Schwein.) Fr. and Boletus aestivalis (Paul) Fr. were collected only from plots subjected to release felling, which had a pH of 5.76; the presence of Tylopus sp. was registered in sites with less acidic values (5.93), in stands subjected to a second thinning.
Recent research points out that Boletus loyo Phil. ex Speg. develops in soils with high contents of organic matter, low to medium concentrations of N, P and Ca and high concentrations of K, Mg, Fe, Mn, Zn, and Cu, which favor the production of their sporomes (Pereira et al., 2016). Sun et al. (2017) highlight the importance of various elements, including K and Mg in the emergence of EMF sporomes in an association of Cedrus deodara (Roxb.) G. Don, which agrees with the information documented herein. We should note that in the present study, both K and Mg generally had negative correlations with the abundance of EMF (Table 7), but positive correlations between treatments (Table 6).
The results agreed with those reported above, for there are significant differences in the decrease of the mineral elements of the soil, according to which of the assessed silvicultural practices is applied. For instance, with the first thinning, K, Ca and Mg diminish considerably, and the release felling results in the lowest concentrations of P, Na, and Zn (Table 4). We should point out that the cited nutrients had negative correlations with the abundance of sporomes, except the available phosphorus and sodium (Table 7).
Based on the above, particularly on the percentage of organic matter as an element indicating the quality of the soil, and on the statistically significant differences obtained for this variable, it is evident that any of these silvicultural practices alter the annual production rate of EMF and has an impact on the rest of the physical and chemical properties of the soil, as well as on the abundance and richness of EMF taxa (Martínez-Peña et al., 2012).
Conclusions
The silvicultural practices of regeneration felling, release felling and thinning generate changes in the composition and structure of the tree mass, which have an impact on the abundance and richness of the ectomycorrhizal mushroom taxa. This is particularly noticeable in the plots subjected to regeneration felling, i.e., in those sites where the extraction of tree mass is more intensive. The differences registered between the thinnings are due to the time elapsed between the year of the first intervention and the sampling year, which was longer for the first thinning and corresponded to a greater abundance of sporomes.
The physical and chemical properties of the soil, particularly the percentages of organic matter and sand, registered a positive correlation with the abundance of sporomes, unlike with K, Ca, Mn and Mg concentrations. Conversely, significant differences between silvicultural treatments were negative and had a direct effect on the abundance and richness of EMF.
The species Lactarius indigo, Boletus aestivalis and Tylopilus sp. were registered only in stands with less acidic pH values (5.76 to 5.93), which correspond to thinning and release fellings.
More detailed studies, at the species level, need to be carried out to establish the specific fungal responses to edaphic factors, as the nutritional requirements of the EMF differ between taxa.
Referencias
Amaranthus, M. P., D. Page D., A. Harvey, E. Cazares and L. F. Bednar. 1996. Soil compaction and organic matter affect conifer seedling Nonmycorrhizal and ectomycorrhizal root tip abundance and diversity. USDA Forest Service. Pacific Northwest Research Station. Research Paper PNW-494. Portland, OR USA. 12 p. [ Links ]
Bonet, J. A., C. R. Fischer and C. Colinas. 2004. The relationship between forest age and aspect on the production of sporocarps of ectomycorrhizal fungi in Pinus sylvestris forests of the central Pyrenees. Forest Ecology and Management 203(1-3): 157‐175. [ Links ]
Bonet, J. A., T. Pukkala, C. R.Fischer, M. Palahí, J. Martínez de Aragón and C. Colinas. 2008. Empirical models for predicting the production of wild mushrooms in Scots pine (Pinus sylvestris L) forests in the Central Pyrenees. Annals of Forest Science 65 (2): 206-214. DOI:10.1051/forest:2007089. [ Links ]
Bonet, J. A., M. Palahí, C. Colinas, T. Pukkala, C. R. Fischer, J. Miina and J. Martínez de Aragón. 2010. Modelling the production and species richness of wild mushrooms in pine forests of Central Pyrenees in north-eastern Spain. Canadian Journal of Forest Research 40(2): 347-356. http://dx.doi.org/10.1139/X09-198. [ Links ]
Bonet, J. A., S. de-Miguel, J. Martínez de Aragón, T. Pukkala and M. Palahí. 2012. Immediate effect of thinning on the yield of Lactarius group deliciosus in Pinus pinaster forests in Northeastern Spain. Forest Ecology and Management 265: 211-217. [ Links ]
Courty, P. E., M. Buée, A. Diedhiou, P. Frey-K., F. Le Tacon, F. Rineau, M. P. Turpault, S. Uroz and J. Garbaye. 2010. The role of ectomycorrhizal communities in forest ecosystems: new perspectives and emerging concepts. Soil Biology Biochemistry 42:679-698. doi:10.1016/j.soilbio.2009.12.006. [ Links ]
Daniel, T. W., J. A. Helms and F. S. Baker. 1983. Principios de silvicultura. 1a Edición en Español. McGraw-Hill. México, D. F., México. 486 p. [ Links ]
De-Miguel, S., J. A. Bonet, T. Pukkala and M. Martínez de A. 2014. Impact of forest management intensity on landscape-level mushroom productivity: a regional model-based scenario analysis. Forest Ecology and Management 330: 218-227. [ Links ]
Facelli, J. M. and S. T. A. Pickett. 1991. Plant litter: its dynamics and effects on plant community structure. The Botanical Review 57(1): 1-32. [ Links ]
García-Rodríguez, J. L., H. Sarmiento L., D. Amador S. y J. M. Mejía B. 2012. Hongos silvestres ectomicorrícicos de bosques templados fríos de los municipios de Pueblo Nuevo, San Dimas y Durango, Durango. Libro Técnico Núm.8. CIR Norte Centro, Campo Experimental Valle del Guadiana. Durango, Dgo. México. 127 p. [ Links ]
IBM Corporation. 2013. IBM SPSS Statistics for Windows Version 22.0. Armonk, New York, NY USA. n/p. [ Links ]
Kennedy, P. G., A. D. Izzo and T. D. Bruns. 2003. There is high potential for the formation of common mycorrhizal networks between understory and canopy trees in a mixed evergreen forest. Journal of Ecology 91:1070-1080. [ Links ]
Kishchuk, B. E., S. Quideau, Y. Wang and C. Prescott. 2014. Long-term soil response to variable-retention harvesting in the EMEND (Ecosystem Management Emulating Natural Disturbance) experiment, northwestern Alberta. Canadian Journal of Soil Science 94(3): 263-279. [ Links ]
Largent, D. 1984. How to identify mushrooms to genus I: Macroscopic features. Mad River Press Inc. Eureka, CA USA. 166 p. [ Links ]
Luoma, D. L., J. L. Eberharta, R. Molina and M. Amaranthus. 2004. Response of ectomycorrhizal fungus sporocarp production to varying levels and patterns of green-tree retention. Forest Ecology and Management 202(1-3): 337-354. [ Links ]
Martínez de Aragón, J., J. A. Bonet, C. R. Fisher and C. Colinas. 2007. Productivity of ectomicirrhizal and selected ediblesaprothrofic fungi in pine forest of the pre-Pyrenees mountains, Spain: predictive equations for forest management of mycological resources. Forest Ecology and Management 252: 239-256. [ Links ]
Martínez P., F. 2008. Producción de carpóforos de macromicetes epígeos en masas ordenadas de Pinus sylvestris L. Tesis doctoral. Escuela Técnica Superior de Ingenieros de Montes. Universidad Politécnica de Madrid. Madrid, España. 291 p. [ Links ]
Martínez-Peña, S. de Miguel, T. Pukkala, J. A. Bonet, P. Ortega M., J. Aldea and J. Martínez de A. 2012. Yield models for ectomycorrhizal mushrooms in Pinus sylvestris forests with special focus on Boletus edulis and Lactarious deliciousus group. Forest Ecology and Management 282: 63-69. [ Links ]
Nadeau, M. B. and D. P. Khasa. 2017. Edaphic Selection Pressures as Drivers of Contrasting White Spruce Ectomycorrhizal Fungal Community Structure and Diversity in the Canadian Boreal Forest of Abitibi-TeÂmiscamingue Region. PLoSONE 11(11): e0166420. doi:10.1371/journal.pone.0166420. [ Links ]
Nehls, U. 2008. Mastering ectomycorrhizal symbiosis: the impact of carbohydrates. Journal of Experimental Botany 59:1097-1108. [ Links ]
Pereira, G., J. Sepúlveda, P. Novoa, A. Pozo y C. Atala. 2016. Caracterización de un relicto de bosque nativo con fructificación anual del hongo comestible Boletus loyo Phil. ex Speg. Quebracho - Revista de Ciencias Forestales 24 (1-2): 18-25. [ Links ]
Pérez-Moreno, J., A. Lorenzana F, V. Carrasco H. y A. Yescas P. 2010. Los hongos comestibles silvestres del Parque Nacional Izta-Popo, Zoquiapan y Anexos. Colegio de Postgraduados, Semanart, Conacyt. Estado de México, México. 167 p. [ Links ]
Pérez-Silva, E. y T. Herrera S. 1991. Iconografía de macromicetos de México I Amanita. Publicación especial del Instituto de Biología 6, UNAM. México, D.F., México. 136 p. [ Links ]
Perry, D. A, S. L. Rose, D. Pilz and M. M. Schoenberger. 1984. Reduction of natural ferric iron chelators in disturbed forest soils. Soil Science Society of America Journal 48: 379-382. [ Links ]
Phillips, R. 1991. Mushrooms of North America. Little Brown and Company. Toronto, Canada. 319 p. [ Links ]
Quinn, G. P. and M. J. Keough. 2002. Experimental desing and data analysis for Biologists. Cambridge University Press. New York, NY USA. 537 p. [ Links ]
Pilz, D., L. Norvell, E. Danell and R. Molina. 2003. Ecology and management of commercially harvested chanterelle mushrooms. United States Department of Agriculture. Forest Service, Portland, OR USA. 90 p [ Links ]
Pilz, D. , R. Molina and J. Mayo. 2006. Effect of thinning Young forests on Chanterelle mushroom production. Journal of Forest Ecology 104: 9-14. [ Links ]
Rodríguez-Alcalá, O., L. Villaseñor I., M. Cenado M. y A. Arias G. 2002. Guía ilustrada de los hongos del Bosque La Primavera. Universidad de Guadalajara. Guadalajara, Jal., México. 109 p. [ Links ]
Secretaría de Medio Ambiente y Recursos Naturales (Semarnat). 2002. Norma Oficial Mexicana NOM-021-RECNAT-2000, que establece las especificaciones de fertilidad, salinidad y clasificación de suelos, estudios, muestreo y análisis. Diario Oficial de la Federación. 31 de diciembre de 2002. México, D.F., México. 85 p. [ Links ]
Secretaría de Medio Ambiente y Recursos Naturales (Semarnat). 2017. Anuario Estadístico de la Producción Forestal 2016. https://www.gob.mx/cms/uploads/attachment/file/282951/2016.pdf (20 de enero de 2018). [ Links ]
Smith, S. E. and D. J. Read. 1997. Mycorrhizal symbiosis. 2nd Edition. Academic Press. London, UK. 605 p [ Links ]
Smith, M. E., T. W. Henkel, G. C. Williams, M. C. Aime, A. K. Fremier and R. Vilgalys. 2017. Investigating niche partitioning of ectomycorrhizal fungi in specialized rooting zones of the monodominant leguminous tree Dicymbe corymbosa. New Phytologist 215(1): 443-453. doi: 10.1111/nph.14570. [ Links ]
Sun, Q., Y. Liu, H. Yuan and B. Lian.2016. The effect of environmental contamination on the community structure and fructification of ectomycorrhizal fungi. MicrobiologyOpen 6(1). doi: 10.1002/mbo3.396. [ Links ]
Taylor, D. L., T. N. Hollingsworth, J. W. McFarland, N. J. Lennon, C. Nushbaum and R. W. Ruess. 2014. A first comprehensive census of fungi in soil reveals both gyperdiversity and fine-scale niche partitioning. Ecological Monographs 84: 3-20. [ Links ]
Valdés, M., J. Córdova, M. Gómez and A. Fierros M. 2003. Understory vegetation and ectomycorrhizal sporocarp diversity response to pine regeneration methods in Oaxaca, Mexico. Western Journal of Applied Forestry 18: 101-108. [ Links ]
Valdés, M. , V. Pereda, P. Ramírez, R. Valenzuela and R. M. Pineda. 2009. The ectomycorrhizal community in a Pinus oaxacana forest under different silvicultural treatments. Journal of Tropical Forest Science 21(2): 88-97. [ Links ]
Velasco B., E., M. C. Zamora-Martínez, C. Nieto de Pascual P., J. I. Martínez-Valdez y A. Montoya. 2010. Modelos predictivos de la producción de hongos silvestres comestibles en bosques de coníferas, Tlax. Revista Mexicana de Ciencias Forestales. 1(1): 128-145. [ Links ]
Wiensczyk, A. M., G. Sharmin, D. M. Durall, M. D. Jones and S. W. Simard. 2002. Ectomycorrhizae and forestry in British Columbia: A summary of current research and conservation strategies. B.C. Journal of Ecosystems and Management 2(1): 1-20. [ Links ]
Wrigth, E., M. Jones, D. Durall, M. Kranabetter, T. Wile and P. Kroeger. 1997. The effects of timber harvesting of mushrooms and mycorrhizae of the Date Creek Research Forest. Forest Service British Columbia. Extension Enote Num. 25. Prince Rupert, BC Canada. 5 p. [ Links ]
Zamora-Martínez, M. C. 2010. El monitoreo de las poblaciones de macromicetos ectomicorrícicos y su relevancia en estudios ecológicos y el manejo sustentable. Tesis de Maestría en Ciencias. Facultad de Ciencias, Universidad Nacional Autónoma de México (UNAM). México, D. F., México. 84 p. [ Links ]
Zamora-Martínez, M. C., A. González H., F. Islas G., E. N. Cortés B. y L. I. López V. 2014. Distribución geográfica y ecológica de 13 especies de hongos silvestres comestibles en Oaxaca. Revista Mexicana de Ciencias Forestales 5 (21): 76-93. [ Links ]
Zar, J. H. 2010. Biostatistical analysis. Pearson College. Victoria, BC, Canada. 944 p. [ Links ]
Zhou, Y., Ch. Zhou, Z. Wu, L. Zheng, X. Hu, H. Chen and J. Gan. 2015. Effects of Cutting Intensity on Soil Physical and Chemical Properties in a Mixed Natural Forest in Southeastern China. Forests (6): 4495-4509. doi:10.3390/f6124383. [ Links ]
Received: January 01, 2018; Accepted: April 13, 2018











 texto en
texto en 


