Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.8 no.42 México jul./ago. 2017
Articles
Chemical, structural and functional properties of lechuguilla (Agave lechuguilla Torr.)
1Departamento de Biotecnología, Facultad de Ciencias Químicas, Universidad Autónoma de Coahuila. México.
2Novo Nordisk Foundation Center for Biosustainability, Technical University of Denmark, Kemitorvet, Building 220, 2800, Kongens Lyngby, Denmark.
3Campo Experimental Saltillo, CIRNE, INIFAP. México.
Agave lechuguilla Torr. Is a non-timber forest resource in the arid and semi-arid zones of northeastern Mexico, due to its range of distribution, it is interesting to evaluate the potential of this raw material for biotechnological applications such as the production of second generation biofuels and chemical products with high value added for the benefit of the people area rural. The objective of the present work was to evaluate the chemical, structural and functional properties of the biomass of A. lechuguilla, to determine its potential for obtaining antioxidants as products of high added value. The study of the material by Fourier Transform Infrared Spectrometry (FTIR) and Scanning Electron Microscopy (SEM) confirmed the common characteristics of the lignocellulosic compounds of this type of materials. According to the chemical characterization, the main component were extractive compounds (45.34 %), followed by structural sugars such as cellulose and hemicellulose (34.87 %) and total lignin (7.32 %). The effect of different solvents (methanol, ethanol and acetone) and with NaOH in the preparation of antioxidant extracts was evaluated. All extracts showed antioxidant activity (DPPH, FRAP and CAT assays) with a better affinity for extraction and preservation with the use of acetone (89.75 % by DPPH, 1.60 mM Fe (II) g-1 biomass by FRAP and 1.75 mg α-tocopherol g-1 biomass by CAT). The results reveal the potential of A. lechuguilla for the extraction of antioxidants as products of interest in the pharmaceutical and food industry.
Key words: Agave lechuguilla Torr.; chemical composition; structural characteristics; functional properties; antioxidants
Agave lechuguilla es un recurso forestal no maderable de las zonas áridas y semiáridas del noreste de México; por el área de distribución que ocupa, resulta interesante evaluar el potencial de esta materia prima para aplicaciones biotecnológicas como la producción de biocombustibles de segunda generación y productos químicos con alto valor agregado en beneficio de los productores del área rural. El objetivo del presente trabajo fue evaluar las propiedades químicas, estructurales y funcionales de la biomasa de A. lechuguilla, que permitan determinar su potencial para la obtención de antioxidantes como productos de alto valor agregado. El estudio del material por Espectrometría Infrarroja por Transformadas de Fourier (FTIR) y por Microscopía Electrónica de Barrido (SEM) confirmaron las características comunes de los compuestos lignocelulósicos de este tipo de materiales. De acuerdo a la caracterización química, los principales componentes fueron los compuestos extractivos (45.34 %), seguidos de los azúcares estructurales: celulosa y hemicelulosa (34.87 %) y lignina total de 7.32 %. Se evaluó el efecto de diferentes solventes (metanol, etanol y acetona) con NaOH en la obtención de extractos antioxidantes. Todos los extractos mostraron actividad antioxidante (ensayos DPPH, FRAP y CAT), con una mejor afinidad para su extracción y preservación con la acetona (89.75 % por DPPH; 1.60 mM Fe(II) g-1 de biomasa por FRAP y 1.75 mg α-tocoferol g-1 de biomasa por CAT). Los resultados revelan el potencial de A. lechuguilla para la extracción de antioxidantes como productos de interés en la industria farmacéutica y alimentaria.
Palabras clave: Agave lechuguilla Torr.; antioxidantes; características estructurales; composición química; propiedades funcionales
Introduction
Various agave species have shown --both in vitro and in vivo-- antimicrobial and antifungal (García et al., 1999; Sánchez et al., 2005; Verástegui et al., 2008; De Rodríguez et al., 2011), antiinflammatory (Peana et al., 1997; García et al., 2000), antioxidant (Singh et al., 2003; Ben Hamissa et al., 2012), molluscicide (Brackenbury and Appleton, 1997; Abdel-Gawad et al.,1999), antidiabetic (Andrade-Cetto and Heinrich, 2005) and cytotoxic activity against certain cancer cell lines (Yokosuka and Mimaki, 2009; Man et al., 2010; Podolak et al., 2010; Chen et al., 2011). These properties are attributed to certain phytochemicals such as saponins, sapogenins, phenolic compounds and fructans. In addition to the beneficial properties mentioned above, the saponins of various plants are antiparasitic, antiviral, antioxidant, antiulcerogenic, inmunomodulatory, hepatoprotective, neuroprotective, antimutagenic, antispasmodic, with lipid-lowering effects, healing action on wounds and hypocholesterolemic activities (Sparg et al., 2004).
In addition, the hydrolysis of fructans may be a source of prebiotic oligossacharides (Ávila-Fernández et al., 2011) and monossacharides such as glucose and fructose. Because fructans are the main reserve of water-soluble carbohydrates of agaves, they are the main component of several species (35 % to 70 % on a dry weight basis), and because their hydrolysis releases 80 % to 86 % of fructose and 10 % to 15 % of glucose, they are used for the production of syrups -one of the most recent applications of these plants (Escamilla-Treviño, 2012).
Although fresh leaves represent approximately 25 % of the plant (on a wet basis), such materials are not utilized (Iñiguez-Covarrubias et al., 2001), as their non-structural sugar content is lower than that of the central part of the individual, traditionally known as the head or pine; however, the leaves can be used for the extraction of fiber, or in the production paper or of biofuels like ethanol (De Paula et al., 2012; Idarraga et al., 1999; and Narváez-Zapata and Sánchez-Teyer, 2009).
Lechuguilla (Agave lechuguilla Torr., Asparagaceae) (Tropicos, 2017) is a non-timber forest resource; it is a native taxon of the arid and semi-arid areas in the south of the United States of America and the northeast of Mexico (Castillo et al., 2011). Its national distribution area covers a surface area of approximately 20 million hectares and comprises the states of Coahuila, Chihuahua, Nuevo León, Durango, San Luis Potosi, Tamaulipas and Zacatecas (Castillo et al., 2011).
A. lechuguilla provides significant socioeconomic benefits to the inhabitants of rural areas, since the extraction of its fiber has been a family subsistence activity for more than 70 years and generates direct employment (Castillo et al., 2013), in addition to the advantages brought by the commercialization of the obtained raw material (Castillo et al., 2011), whose transformation results in various products, including brushes for industrial and domestic use; its main market is international: 93 % of its production is exported, mainly to the United States of America, the Netherlands, Switzerland and Honduras (Castillo et al., 2011), with Mexico as the sole exporter.
Traditionally, the fiber of lechuguilla is obtained from the bud, which is composed of the tenderest leaves of the plant, grouped at its center (Castillo et al., 2005; Narcia et al., 2012); this fiber has less woodiness than that of the lateral leaves. At present, its use in the field is related to high levels of marginalization. It is therefore necessary to look for new alternatives to the extraction of fiber, such as the obtainment of chemical products with added value, which would provide an opportunity to improve the quality of life of the inhabitants of the arid and semi-arid areas, where this species thrives.
Given the limited information on the use of Agave lechuguilla for biotechnological applications (Hernández et al., 2005; Almaraz-Abarca et al., 2013), the aim of the present study was to assess the chemical, structural, and functional properties of its biomass in order to determine its potential for the extraction of antioxidants as chemical products of high added value.
Materials and Methods
Harvesting of the raw material and preparation of samples
According to the Official Mexican Standard NOM-008-SEMARNAT-1996, which limits the use of the bud of Agave lechuguilla Torr., samples from natural populations were collected during the month of March at the Paredón ejido in the municipality of Ramos Arizpe, Coahuila, Mexico (25°55'47 "N 101°55'47” W), located at an altitude of 728 m, with a mean annual temperature of 33 °C, and a mean annual precipitation of 269 mm; its climate is very dry or semi-warm desert, with a cool winter (García, 1973), and its vegetation type is rosetophilous desert shrub (Marroquín et al., 1981).
The collected samples were washed with tap water in order to remove dirt and other impurities. Once dry, they were cut by hand to a size of approximately 1- 2 cm2, and placed in a Koleff-KL10 convection oven until a moisture level of <10 % (p/p) was reached. The dehydrated samples were ground in a Retsch cutting -SM100 mill to a mean particle size of 2 mm. The sample thus obtained was homogenized and stored in plastic containers at room temperature.
Determination of the chemical composition
The extractive determination was performed according to Sluiter et al. (2008), using ultrapure water and absolute ethanol as solvents in two sequential stages. The cellulose, hemicellulose and lignin were extracted using the procedures of the National Renewable Energy Laboratory (Sluiter et al., 2012), with a modification (Mussatto et al., 2011): 500 mg of the material was hydrolized using H2SO4 at 72 % (p/p), during 7 minutes at 50 °C. Subsequently, the hydrolizate was diluted to 4 % (p/p) with distilled water. A second hydrolysis was carried out by autoclaving the reaction mixture at 121 °C for 1 h; the resulting solution was sieved through 0.2-µm filters, and the liquid fraction was analyzed with HPLC in order to determine the levels of glucose (cellulose), arabinose, galactose, mannose and xylose (hemicellulose), according to Mussatto and Roberto (2006).
The content of acid-soluble (ash-free) lignin was calculated as described by Mussatto and Roberto (2006). The solid fraction recovered after filtration is considered to be acid-insoluble lignin. The total lignin value was calculated based on the total amount of acid-insoluble and soluble lignin.
The ash content was estimated through incineration of the samples at 550 °C for 4 h (Horwitz and Latimer, 2005).
Antioxidant potential
The extracts (liquors) of A. lechuguilla were obtained using 2 grams (on a dry basis) of the sample with 40 mL of each solvent: 1) methanol, 2) ethanol and 3) acetone at 60 % (v/v) in all three cases. Each treatment was carried out at a temperature range of 60 to 65 °C, during 90 min with magnetic agitation. The control consisted in the same amount of sample subjected to treatment in an autoclave at 120 °C for 90 min, with 40 mL of NaOH at 2 %. The four extracts were then separated by centrifugation at 2 500 rpm for 20 min and sieved through 0.22 μm filters.
The antioxidant capacity of the extracts was determined by two spectrophotometric methods: the stable free radical assay (DPPH) and the ferric reducing antioxidant power assay (FRAP) were adapted for a 96-well microplate (Martins et al., 2012). The total antioxidant capacity (TAC) was evaluated using the method described by Prieto et al. (1999).
Structural characterization
The FTIR spectra were recorded with the FTIR spectrometer (Agilent, Cary 630, USA); the FTIR in the band mode of absorption were in the range of 650-4 000 cm-1, with a resolution of 4 cm-1 and 32 explorations. Consecutively, the resulting FTIR spectra were analyzed and standardized and are represented in the software Origin (OriginLab®, 2016)
Surface morphology
A. lechuguilla particles were observed using scanning electron microscopy (SEM), in a microscope with a field-emission electron gun at an ultra-high resolution (Nova 200 Nano SEM, FEI Company). Prior to the analysis, the samples were covered with a thin film (35 nm) of Au-Pd (80-20 % by weight). The images were obtained by applying a voltage of 10 kV acceleration, with increases of 200 and 2 000.
Results and Discussion
Chemical Groups and Bonding Layout of the Components
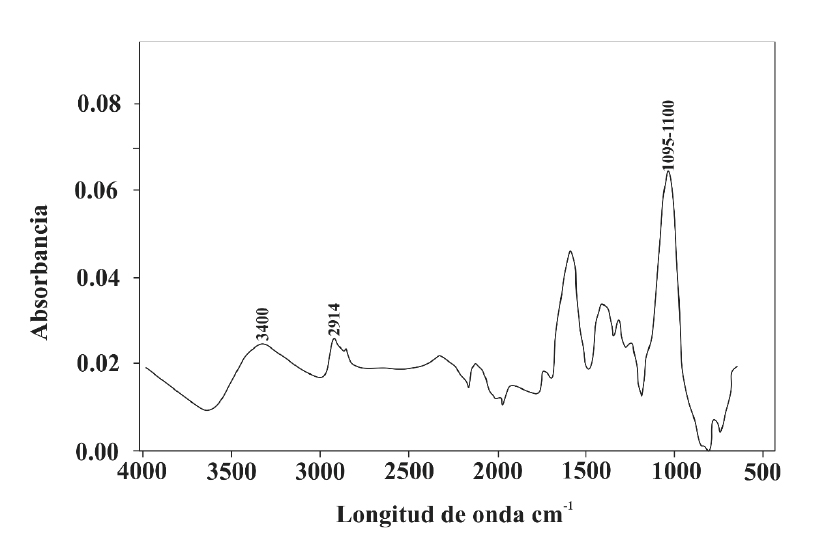
The FTIR analysis confirmed that the buds of A. lechuguilla have absorption bands that are typical of lignocellulosic materials, although their magnitudes differ (Adel et al., 2010).
Because of their complex nature, their FTIR spectra are often separated into two main sections; -OH and -CH, which extend through the region of 3 800-2 700 cm-1, and the digital fingerprint region that is assigned to the stretching vibrations of various groups of components of these lignocellulosic materials to 1 800-800 cm-1 (Poletto et al., 2012).
The broad peak between 3 400 cm-1 is related to the O-H stretching vibrations of the hydroxyl group. The signals between 2 920 cm-1 and 2 850 cm-1 are commonly observed in samples of lignocellulosic biomass and correspond to the methyl and methylene groups (-CH3 and CH4), respectively. However, for A. lechuguilla samples these signs appeared at 2 914 cm-1, which may be due to the greater extractive content of this raw material, as some compounds in organic extractives, such as methyl esters of fatty acids and esters of phenolic acid, contain methyl and methylene groups (Poletto et al., 2012).
The signals recorded at 1 735 cm-1, 1 375 cm-1, 1 240 cm-1, 1 165 cm-1 and 1 060 cm-1 are characteristic of the stretching and twisting of various groups of carbohydrates. For the A. lechuguilla bands attributable to symmetric and asymmetric stretching of the C-O-C in glycosidic bonds, they occurred at 1 100 cm-1 and 1 095 cm-1 and correspond to the β-1,4-glycosidic bonds that unite the glucose units in cellulose and hemicellulose (Figure 1).
Surface morphology
The images obtained using scanning electron microscopy revealed a rigid structure in A. lechuguilla (Figure 2). The small fractures observed can be attributed to the milling process (Figure 2a), and the small crystalline forms (Figure 2b) would be small crystals of calcium oxalate, commonly present in this type of biomass (Pérez-Pimienta et al., 2016).
Chemical composition
After the elimination of the extractive compounds, the material lost 45.34 % of its original weight, which corresponds to the non-structural components such as sucrose, nitrate/nitrites, protein, ash, chlorophyll, and fatty compounds of the plant (Sluiter et al., 2012).
The relative moisture of the material, after the drying process, was 4.33 %. The cellulose fraction was calculated based on the glucose detected using HPLC (0.55 g L-1). The hemicellulose was determined on the basis of the concentration of xylose (0.22 g L-1), galactose (0.18 g L-1), arabinose (0.068 g L-1) and mannose (0.08 g L-1). The cellulose, hemicellulose, and lignin contents (dry basis) in the samples were 17.72 %, 17.15 and 7.32 %, respectively (Table 1).
Antioxidant potential
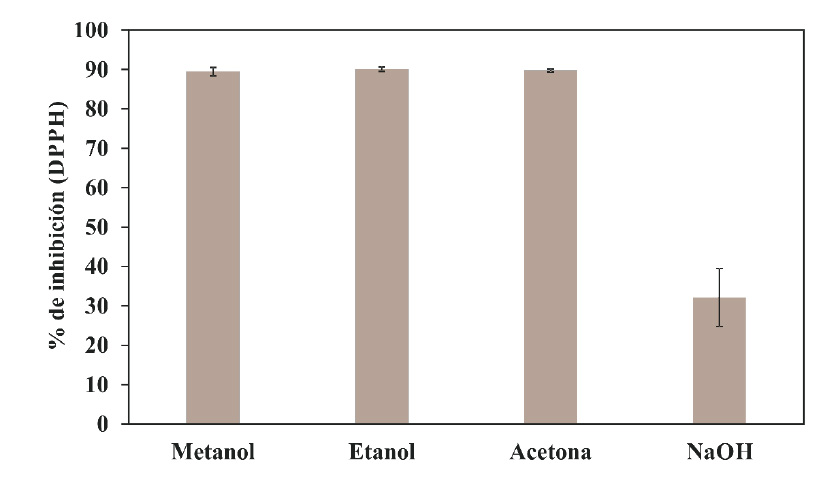
The antioxidant potential of A. lechuguilla was assessed using three of the most common testing methods (DPPH, FRAP and CAT). DPPH has been widely used as a fast, reliable and reproducible parameter to measure the in vitro antioxidant capacity of pure compounds as well as extracts from plants (Burda and Oleszek, 2001; Ara and Nur, 2009). The results of the assay DPPH are shown in Figure 3; the values are provided in terms of % of inhibition by DPPH. It is evident that the treatments with solutions of methanol, ethanol and acetone were of the order of 89.47 %, 90.09 % and 89.75 %, respectively; while the liquor from the treatment with NaOH had a low DPPH value (32.148 %) (Figure 3).
The results expressed in mM Fe(II) g-1 biomass (on a dry basis) were 0.51, 0.51, 1.6 and 0.46, respectively, for the extracts obtained through treatment with methanol, ethanol, acetone and NaOH respectively.
Although the solutions used in the treatments are binary water-solvent mixtures, not all have the same physical-chemical characteristics, since the polarity of each solvent (or mixture of these) has a direct relationship to the amount and quality of antioxidant compounds that can be extracted by it (Aliyu et al., 2012). The order of hierarchy in terms of polarity for this work is Acetone> Methanol>Ethanol. This is related to the concentration of antioxidants detected using the FRAP method in liquors resulting from the extractions with solvents, as the highest concentration was obtained with the acetone method (1.60 mM Fe(II) g-1 of biomass), and the values of the liquors obtained with methanol and ethanol, both of which exhibit a very similar polarity, were 0.51 mM Fe(II) g-1 of biomass. Finally, although no solvent was used to extract it, the biomass treated with NaOH had a value of 0.46 mM Fe(II) g-1 because a fraction of antioxidant compounds were extracted by virtue of the interaction of the temperature used and the water present in the solution.
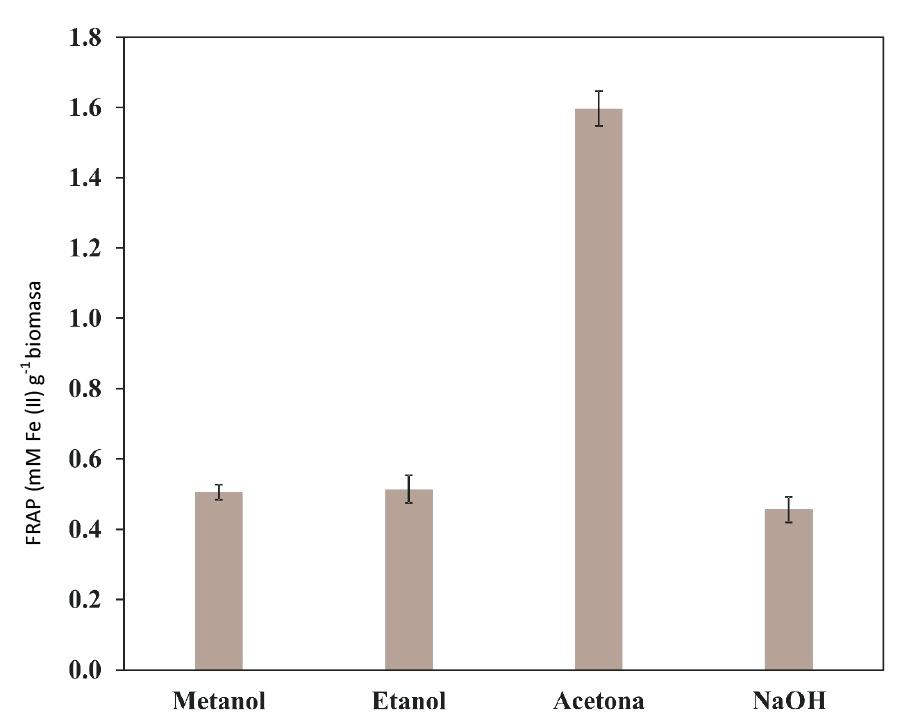
Figure 4 Antioxidant capacity of various extracts of Agave lechuguilla Torr. detected using the FRAP method.
Figure 5 shows the results for the total antioxidant capacity measured with the phosphomolybdenum method, which is based on the reduction of Mo (IV) to Mo (V) for the extract, with the subsequent formation of the green phosphate Mo (V) complex at an acidic pH. This model evaluates the antioxidant capacity of both water-soluble and fat-soluble compounds, hence the name “total antioxidant capacity” (Aliyu et al., 2012). The records indicate that there is a relationship between the method of extraction and the solvent used in the concentration of antioxidants in the resulting liquor; that is to say, that the extraction using the acetone method concentrated 1.75 mg of antioxidant compounds equivalent to α-tocopherol, which are capable of reducing the oxidant compound in the reaction matrix.
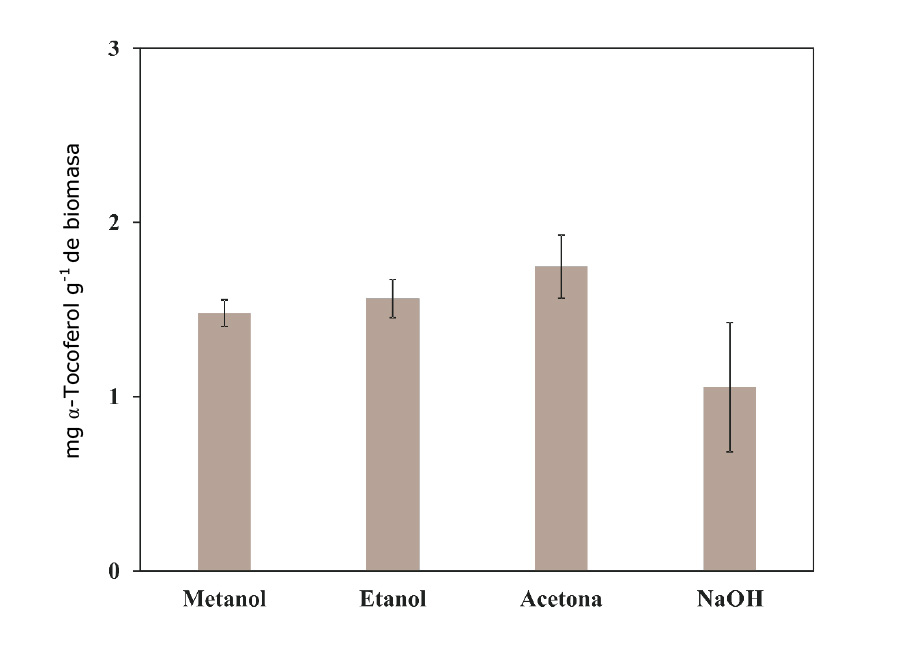
Figure 5 Antioxidant capacity of various extracts of Agave lechuguilla Torr. detected using the Total Antioxidant method.
Each of the applied methods evaluates the antioxidant potential of a biological sample in different ways, and each one of these is based on a variety of reactions. Despite the fact that the DPPH assay showed little difference in the three different extractions, there is a correlation between the results of the total antioxidant capacity and testing with FRAP, in which the acetone extract exhibited higher values than the methanol and ethanol extracts. This would mean that, under the same conditions, the antioxidant compounds detected in A. lechuguilla have more affinity with acetone. Furthermore, the temperature conditions of the extractions were favorable for the acetone, whose boiling point is lower than that of methanol and ethanol; thus, higher vapor pressures are generated in the containers, which leads to higher gas-solid extraction rates.
Lower antioxidant capacity values were obtained for the sample treated with NaOH than for all the other samples. The difference between the samples treated with solvents and the sample treated with NaOH may be attributed to the longer operation time allotted to the former, as, according to the literature, the extraction of this type of compounds depend not only on the temperature but also on the time during which the herbaceous samples are in contact with the solvent (Ben Hamissa et al., 2012). While for the treatment with NaOH, the temperature was higher and the total operation time of the process was longer than for the treatments with solvents, the reactions involving antioxidants extracted from plants are pH dependent, as electron transport is limited in a highly oxidized environment -i.e. in an environment saturated with OH-, which tends to degrade phenolic compounds and to generate highly oxidizing substances due to delignification; therefore, the resulting liquor will have a lower antioxidant capacity (Yokoyama et al., 2007; Aliakbarian et al., 2009).
Conclusions
The composition of A. lechuguilla biomass is characteristic of herbaceous materials, which contain a smaller amount of structural carbohydrates than timber samples. The biomass of A. lechuguilla contains a significant number of extractive compounds (>45 %) because this species grows in arid conditions with scarce precipitation; therefore, it requires preservation mechanisms involving in some way such materials as antioxidant compounds or fats. The liquors resulting from the extractions with solvents have a considerable amount of antioxidant compounds, contained precisely in the fraction of extractives --a finding that opens up new possibilities of study for this non-timber forest resource.
Although the alkaline treatment decreases the antioxidant capacity of the liquors, this process for the extraction of compounds with a high added value can be adapted to the system for the production of biofuels like second-generation ethanol (2G) and conform to a biorefinery model. However, its application will require further, more detailed studies and a technical-economic feasibility assessment.
Acknowledgments
The authors would like to thank the Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, Sagarpa (Department of Agriculture, Livestock, Rural Development, Fisheries and Food) and the Consejo Nacional de Ciencia y Tecnología, Conacyt (National Council for Science and Technology) for the financial support granted.
REFERENCES
Abdel-Gawad, M. M., M. M. El-Sayed and E. S. Abdel-Hameed. 1999. Molluscicidal steroidal saponins and lipid content of Agave decipiens. Fitoterapia 70: 371-381. [ Links ]
Adel, A. M., Z. H. A. El-Wahab, A. A. Ibrahim and M. T. Al-Shemy. 2010. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresource Technology 101:4446-4455. [ Links ]
Aliakbarian, B., F. Dehghani and P. Perego. 2009. The effect of citric acid on the phenolic contents of olive oil. Food Chemistry 116: 617-623. [ Links ]
Aliyu, A. et al. 2012. Free radical scavenging and total antioxidant capacity of methanol extract of Ethulia conyzoides growing in Nigeria. Romanian Biotechnological Letters 17: 7458-7465. [ Links ]
Almaraz-Abarca, N. et al. 2013. Variability of the foliar phenol profiles of the Agave victoriae-reginae complex (Agavaceae). Botanical Sciences 91: 295-306. [ Links ]
Andrade-Cetto, A. and M. Heinrich. 2005. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. Journal of Ethnopharmacology 99: 325-348. [ Links ]
Ara, N. and H. Nur. 2009. In vitro antioxidant activity of methanolic leaves and flowers extracts of Lippia alba. Research Journal of Medicine and Medical Sciences 4:107-110. [ Links ]
Ávila-Fernández, Á. et al. 2011. Production of functional oligosaccharides through limited acid hydrolysis of agave fructans. Food Chemistry 129: 380-386. [ Links ]
Ben Hamissa, A. M., M. Seffen, B. Aliakbarian, A. A. Casazza, P. Perego and A. Converti. 2012. Phenolics extraction from Agave americana (L.) leaves using high-temperature, high-pressure reactor. Food and Bioproducts Processing 90: 17-21. [ Links ]
Brackenbury, T. D. and C. C. Appleton. 1997. A comprehensive evaluation of Agave attenuata, a candidate plant molluscicide in South Africa. Acta Tropica 68: 201-213. [ Links ]
Burda, S. and W. Oleszek. 2001. Antioxidant and antiradical activities of flavonoids. Journal of Agricultural and Food Chemistry 49: 2774-2779. [ Links ]
Castillo, Q. D., R. C. A. Berlanga y P. A. Cano. 2005. Recolección, extracción y uso de la fibra de lechuguilla (Agave lechuguilla Torr.) en el estado de Coahuila. CIR Noreste Centro. INIFAP. Publicación Especial Núm. 6. Saltillo, Coah., México. 13 p. [ Links ]
Castillo, Q. D., A. O. Mares y G. E. E. Villavicencio. 2011. Lechuguilla (Agave lechuguilla Torr.) planta suculenta de importancia económica y social de las zonas áridas y semiáridas de México. Boletín de la Sociedad Latinoamericana y del Caribe de Cactáceas y otras Suculentas 8: 6-9. [ Links ]
Castillo, Q. D., R. J. T. Sáenz, V. M. Narcia y R. J. A. Vázquez. 2013. Propiedades físico-mecánicas de la fibra de Agave lechuguilla Torr. de cinco procedencias bajo plantaciones. Revista Mexicana de Ciencias Forestales 4: 78-91. [ Links ]
Chen, P. Y. et al. 2011. Cytotoxic steroidal saponins from Agave sisalana. Planta Médica 77: 929-933. [ Links ]
De Paula, M., T. Lacerda, M. Zambon and E. Frollini. 2012. Adding value to the Brazilian sisal: acid hydrolysis of its pulp seeking production of sugars and materials. Cellulose 19: 975-992. [ Links ]
De Rodríguez, D. J. et al. 2011. In vitro antifungal activity of extracts of Mexican Chihuahuan Desert plants against postharvest fruit fungi. Industrial Crops and Products 34: 960-966. [ Links ]
Escamilla-Treviño, L. 2012. Potential of plants from the genus Agave as bioenergy crops. BioEnergy Research 5: 1-9. [ Links ]
García, E. 1973. Modificaciones al sistema de clasificación climática de Köppen, Segunda Edición. Instituto de Geografía. UNAM. 146 p. [ Links ]
García, M. D., A. M. Quílez, M. T. Sáenz, M. E. Martínez-Domínguez and R. de la Puerta. 2000. Anti-inflammatory activity of Agave intermixta Trel. and Cissus sicyoides L., species used in the Caribbean traditional medicine. Journal of Ethnopharmacology 71: 395-400. [ Links ]
García, M. D., M. T. Saenz, R. Puerta, A. Quilez and M. A. Fernandez. 1999. Antibacterial activity of Agave intermixta and Cissus sicyoides. Fitoterapia 70: 71-73. [ Links ]
Hernández S., R., E. C. Lugo C., L. Díaz J. and S. Villanueva. 2005. Extraction and indirect quantification of saponins from the Agave lechuguilla Torrey. e-Gnosis 3: 1-9. [ Links ]
Horwitz, W. and G. W. J. Latimer. 2005. Official methods of analysis of 729AOAC International. 18th ed. 730AOAC International. Gaithersburg, MD, USA. http://www.eoma.aoac.org/ (20 de agosto de 2016). [ Links ]
Idarraga, G., J. Ramos, V. Zuñiga, S. Turgut and Y. Raymond. 1999. Pulp and paper from blue agave wastes from Tequila production. Journal of Agricultural and Food Chemistry 47: 4450-4455. [ Links ]
Iñiguez-Covarrubias, G. et al. 2001. Utilization of by-products from the tequila industry. Part 2: Potential value of Agave tequilana Weber azul leaves. Bioresource Technology 77: 101-108. [ Links ]
Man, S., W. Gao, Y. Zhang, L. Huang and C. Liu. 2010. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 81: 703-714. [ Links ]
Martins, S., C. N. Aguilar, J. A. Teixeira and S. I. Mussatto. 2012. Bioactive compounds (phytoestrogens) recovery from Larrea tridentata leaves by solvents extraction. Separation and Purification Technology 88: 163-167. [ Links ]
Marroquín F. et al. 1981. Estudio ecológico dasonómico de las zonas áridas del norte de México. 2ª Edición. Instituto Nacional de Investigaciones Forestales. Publicación Especial Núm. 2. México, D. F., México. 166 p. [ Links ]
Meneses, N. G., S. Martins, J. A. Teixeira and S. I. Mussatto. 2013. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Separation and Purification Technology 108: 152-158. [ Links ]
Mussatto, S. I. and I. C. Roberto. 2006. Chemical characterization and liberation of pentose sugars from brewer's spent grain. Journal of Chemical Technology and Biotechnology 81: 268-274. [ Links ]
Mussatto, S. I., L. M. Carneiro, J. P. Silva, I. C. Roberto and J. A. Teixeira. 2011. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydrate Polymers 83: 368-374. [ Links ]
Narcia V., M., D. Castillo Q., J. A. Vázquez R. y C. A. Berlanga R. 2012. Turno técnico de la lechuguilla (Agave lechuguilla Torr.) en el noreste de México. Revista Mexicana de Ciencias Forestales 3(9): 81-88. [ Links ]
Narváez-Zapata, J. A. and L. F. Sánchez-Teyer. 2009. Agaves as raw material: recent technologies and applications. Recent Patents Biotechnology 3: 185-191. [ Links ]
OriginLab®. 2016. Origin. Ver. 9.0. Origin Lab Corporation. MA, USA. n/p. [ Links ]
Peana, A. T., M. D. Moretti, V. Manconi, G. Desole and P. Pippia. 1997. Anti-inflammatory activity of aqueous extracts and steroidal sapogenins of Agave americana. Planta Medica 63: 199-202. [ Links ]
Pérez-Pimienta, J. A. et al. 2016. Fractional pretreatment of raw and calcium oxalate-extracted agave bagasse using ionic liquid and alkaline hydrogen peroxide. Biomass and Bioenergy 91: 48-55. [ Links ]
Podolak, I., A. Galanty and D. Sobolewska. 2010. Saponins as cytotoxic agents: a review. Phytochemistry Reviews 9: 425-474. [ Links ]
Poletto, M., A. J. Zattera and R. Santana. 2012. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. Journal of Applied Polymer Science 126: 337-344. [ Links ]
Prieto, P., M. Pineda and M. Aguilar. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry 269: 337-341. [ Links ]
Sánchez, E., N. Heredia and S. García. 2005. Inhibition if growth and mycotoxin production of Aspergillus flavus and Aspergillus parasiticus by extracts of Agave species. International Journal of Food Microbiology 98: 271-279. [ Links ]
Secretaría del Medio Ambiente y Recursos Naturales (Semarnat). 1996. Norma Oficial Mexicana NOM-008-SEMARNAT-1996. Procedimientos, criterios y especificaciones para realizar el aprovechamiento, transporte y almacenamiento de cogollos. https://www.gob.mx/cms/uploads/attachment/file/86382/Temario_Convocatoria_SEMARNAT_2016_10.pdf (20 de agosto 2016). [ Links ]
Singh, B., T. K. Bhat and B. Singh. 2003. Potential therapeutic applications of some antinutritional plant secondary metabolites. Journal of Agricultural and Food Chemistry 51: 5579-5597. [ Links ]
Sluiter, A., B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker. 2012. Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory. Tech. Report. NREL/TP-510-42618. Golden, CO, USA. 15 p. [ Links ]
Sluiter, A., R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton. 2008. Determination of extractives in biomass. Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory. Tech. Report. TP-510-42619. Golden, CO, USA. 12 P. [ Links ]
Sparg, S. G., M. E. Light and J. van Staden. 2004. Biological activities and distribution of plant saponins. Journal of Ethnopharmacology 94: 219-243. [ Links ]
Tropicos, 2017. Missouri Botanical Garden. http://www.tropicos.org (21 de agosto 2016). [ Links ]
Verástegui, Á., J. Verde, S. García, N. Heredia, A. Oranday and C. Rivas. 2008. Species of Agave with antimicrobial activity against selected pathogenic bacteria and fungi. World Journal of Microbiology and Biotechnology 24: 1249-1252. [ Links ]
Yokosuka, A. and Y. Mimaki. 2009. Steroidal saponins from the whole plants of Agave utahensis and their cytotoxic activity. Phytochemistry 70: 807-815. [ Links ]
Yokoyama, T., Y. Matsumoto and G. Meshitsuka. 2007. Detailed examination of the degradation of phenol derivatives under oxygen delignification conditions. Journal of Agricultural and Food Chemistry 55: 1301-1307. [ Links ]
Received: April 10, 2017; Accepted: May 06, 2017











 texto en
texto en