Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.8 no.40 México mar./abr. 2017
Articles
Phenology and tree architecture of Calyptranthes schiedeana O. Berg, Lysiloma acapulcense (Kunth) Benth and Tabebuia chrysantha (Jacq.) G. Nicholson in agroecosystems of Veracruz
1Programa Agroecosistemas Tropicales, Colegio de Postgraduados. México. Correo-e: eleonora.moreno@colpos.mx
2Universidad Autónoma del Estado de Hidalgo. México.
3Colegio de Postgraduados, Campus Montecillo. México.
4Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana. México.
This paper describes a study of the phenology and tree architecture of Calyptranthes schiedeana, Lysiloma acapulcense and Tabebuia chrysantha, species of the low deciduous forest with a cultural importance and a potential for agroforestry. Ten trees of each of these taxa dispersed within the agroecosystems of veracruz, Mexico, were selected, and their phenology was observed every 14 days (from February 2014 to March 2015). Changes occurred in: 1) the development of floral buds, 2) flowering, 3) fruiting, 4) seed dispersal, 5) presence, 6) fall, and 7) shooting of leaves, all of them related to temperature and precipitation. In March 2014 the tree architecture was assessed, and the type of growth was determined. The phenology of the taxa was characteristic of the low deciduous forest, although L. acapulcense tended to keep its leaves for a longer time and to fruit with lower levels of soil moisture. C. schiedeana required precipitation in order to form leave buds (r = 0.60, P = 0.0004), while the development of these dependend on the decrease of temperature (r = 0.58, P = 0.0008). Fruiting in L. acapulcense diminished gradually with lower temperatures (r = -0.90, P < 0.0001); this species was also prominent for its higher, larger crown with longer branches; besides, it adopted the Troll growth model, while T. chrysantha exhibited the Leeuwenberg model, and C. schiedeana, the Attims model. It is concluded that the three species have different phenologic patterns and architectural models, but they can all adapt to agroforestry systems by being associated to crops.
Key words: Multipurpose trees; flowering; fruiting; architectural model; low deciduous forest; agroforestry systems
Se estudió la fenología y arquitectura de Calyptranthes schiedeana, Lysiloma acapulcense y Tabebuia chrysantha, especies de selva baja caducifolia, con importancia cultural y potencial para la agroforestería. Se seleccionaron 10 árboles de cada taxón dispersos dentro de agroecosistemas de veracruz, México; y se observó su fenología con una periodicidad de 14 días (febrero 2014-marzo 2015). Se registraron cambios en: 1) formación de yemas florales, 2) floración, 3) fructificación, 4) dispersión de semillas, 5) presencia, 6) caída, y 7) brote de hojas; los cuales se relacionaron con la temperatura y precipitación. En marzo del 2014 se evaluó la arquitectura arbórea y se definió el tipo de crecimiento. La fenología de los taxa correspondió con la característica de selva baja caducifolia; aunque L. acapulcense tendió a mantener hojas durante mayor tiempo y fructificar cuando la humedad en el suelo fue menor. C. schiedeana requirió de precipitación para desarrollar yemas foliares (r = 0.60, P = 0.0004); mientras que su formación en T. chrysantha dependió del descenso en la temperatura (r = 0.58, P = 0.0008). La fructificación en L. acapulcense fue disminuyendo con temperaturas bajas (r = -0.90, P < 0.0001), esta última también destacó por su copa situada a mayor altura (P < 0.0001 ), más grande (P < 0.0001) y con ramas más largas (P < 0.0001); además, adoptó el modelo de crecimiento Troll; T. chrysantha presentó el modelo Leeuwenberg y C. schiedeana el de Attims. Se concluye que las tres especies tienen distintos patrones fenológicos y modelos arquitectónicos, pero pueden adaptarse a sistemas agroforestales asociándolos a cultivos.
Palabras clave: Árboles multipropósito; floración; fructificación; modelo arquitectónico; selva baja caducifolia; sistemas agroforestales
Introduction
Multipurpose trees are cultivated in order to make more than one significant contribution to the production and service functions within agroecosystems. A wide diversity of these native species, which have a high cultural value for society, are distributed in various ecological regions (Aguilar and Condit, 2001). However, the implementation of local taxa in agroforestry systems requires knowledge of their characteristics: phenology, habits and form of growth (Wood and Burley, 1995), productive capacity, ability to recycle nutrients, nitrogen fixation, resistance to pests and diseases, and the preference of the producers that will use it (Huxley, 1983; Owino, 1992).
The phenology of species is helpful to make decisions regarding the appropriate canopy management to ensure production; furthermore, the canopy is intended to provide varying levels of shade throughout the year in synchronization with the phenological cycle of the crops (Somarriba, 2005). Tree architecture is another important aspect when agroforestry systems are implemented, because the shape and characteristics of the crown determine the way in which the tree will grow, as well as its efficiency in the production of timber (Sestras, 2004) and that of the associated crops (Beer et al., 2003).
Disturb occurs in the low and medium rainforests of central veracruz as a result of agriculture and stockbreeding (Pennigton and Sarukhán, 2005; Gómez et al. 2010); the implementation of agroforestry systems with multipurpose native species has been proposed in order to restore them. Some of the main species in these ecosystems --Calyptranthes schiedeana O. Berg (Myrtaceae), Lysiloma acapulcense (Kunth) Benth (Fabaceae) and Tabebuia chrysantha (Jacq.) G. Nicholson (Bignoniaceae)-- are characteristic of secondary vegetation and are prevalent in mature forests. They have a high cultural importance and are also essential for wild life, although they are scarce in the region at present (Suárez et al., 2012).
Although the relevance of planting native trees is recognized, there is little information regarding their ecology, biology and performance; therefore, the options of trees for restoration purposes and for the forestry industry are limited (Condit et al., 1993; Wishnie et al., 2007). Given the need to become familiar with the native vegetation of the region that has a potential to become integrated into agroforestry systems, the purpose of the present study is to describe the phenological patterns and the tree architecture of C. schiedeana, L. acapulcense and T. chrysantha within agroecosystems with dispersed and clustered trees in pasturelands, agricultural plots and living fences where their characteristics may be assessed in similar conditions to those that prevail in the sites for their potential establishment in agroforestry systems.
Materials and Methods
The study was carried out in various localities of the Paso de Ovejas municipality, veracruz (19°1’58.27’’-19°12’54.18’’ N, 96°25’25.28’’-96°30’46.29’’ W), between the altitudes of 84 and 203 masl, with a mean annual temperature of 24 °C and an annual precipitation of 973 mm (Inegi, 2009). The vegetation in this area is a low deciduous forest, and its importance is derived from its structure, floral diversity and number of endangered species (Castillo-Campos, 2005).
T. chrysantha, L. acapulcense and C. schiedeana --belonging to the group of heliophytes-- were considered (Pennigton and Sarukhán, 2005); they were selected for their cultural importance and their scarcity in the study area (Bautista, 2009; Suárez et al., 2012). Ten mature individuals of each species were chosen in 14 sites used for agriculture and stockbreeding (surface areas of 0.5 to 30 ha), selected within a radius of approximately 18 km. The assessed trees were located in living fences, dispersed in pasturelands and agricultural plots or in copses, in association with other taxa.
Register of phenophases and tree architecture
The phenology was assessed every 14 days, from February 2014 to March 2015. The following changes in the phenological phases were registered: 1) development of floral buds, 2) flowering, 3) fruiting, 4) seed dispersal, 5) presence of leaves, 6) falling of leaves, and 7) shooting of leaves (Mila et al. 2009; Pineda-Herrera et al. 2012). The progress of phenological stages was recorded based on a 0 to 100 scale in five intervals: 0, 1-25, 26-50, 51-75 and 76-100 % (Pineda-Herrera et al., 2012).
The tree architecture was determined in 20 stem specimens for each species (DBH > 2.5 cm), located in the sites described above (some of which were also included in the phenology part). In order to establish the dendometric context of the architecture, the stem diameter (at 1.3 m from the base), crown diameter (30 m Bahco measuring tape), crown height and total height were measured using (360 Brunton clinometer). The architectural model of the three species was determined as suggested by Hallé et al. (1978) and Interián-Ku et al. (2009). The growth of the main axis was qualitatively assessed based on its (monopodial or sympodial) pattern and its (determined or undetermined) shape; the branching was assessed based on its (orthotropic or plagiotropic) orientation and symmetry; the branch insertion angle (upward, of 16 to 45°, or diffused, of 46 to 75°); the length of the branches (long or short); the reproductive structures by (axillary or apical) position; the (total or partial, adaptive or traumatic) reiterations of the branches, and the insertion angle and length of the branches, Image Tool version 3.0 (IT, 2002).
As part of the architecture, the insertion angles of the leaves were also measured, using a protractor, and so was the leaf area (Portable Area Meter LI-3000C). Attributes of the outline, cover and architecture of the crowns (i.e. form, segmentation, division and depth) were taken into account (Trichon, 2001). In addition, the density of the foliage (LAI-2000, LI-COR Biosciences), the low-ground cover and the cover outside the crown were estimated in 10 individuals of each taxon during the rainy season, in order to quantify the degree in which the shade shed by each species modifies the soil cover (Bonham, 1989).
Data analysis
The average occurrence of each phenophase per species was estimated and charted by (fortnightly) sampling date. Pearson’s parametric correlation coefficients were calculated using the values of the phenophases, the accumulated precipitation and the mean temperature of the air (for the corresponding 14-day period). After verifying the normal distribution of the variables, variance analyses, including the species effect of the tree, were carried out using the GLM procedure, and LS Means tests adjusted to Tukey, utilizing the SAS software (SAS, 2010).
Results
Phenology of the species
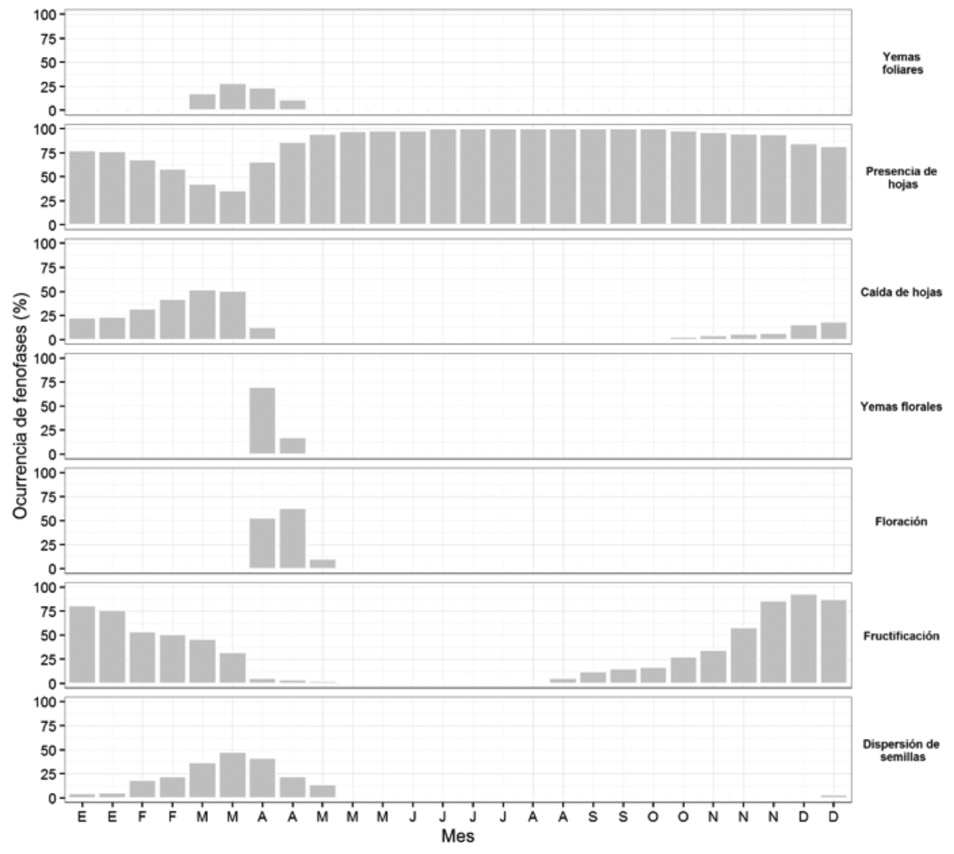
Calyptranthes schiedeana kept its leaves throughout the year, showing a rapid development of leaf buds in May, until its crown was completely covered in June. The falling of the leaves began in January and became more intense in April and May. Once the tree crown was covered with foliage, it soon began to develop floral buds, in June, and to fruit slowly, in July; the fruiting became more intense in October, with increased fruit dispersal (Figure 1). The precipitation was necessary for this species to develop leaf buds (r = 0.60, P = 0.0004), and the temperature influenced the fruiting ( r = 0.46, P = 0.0108).
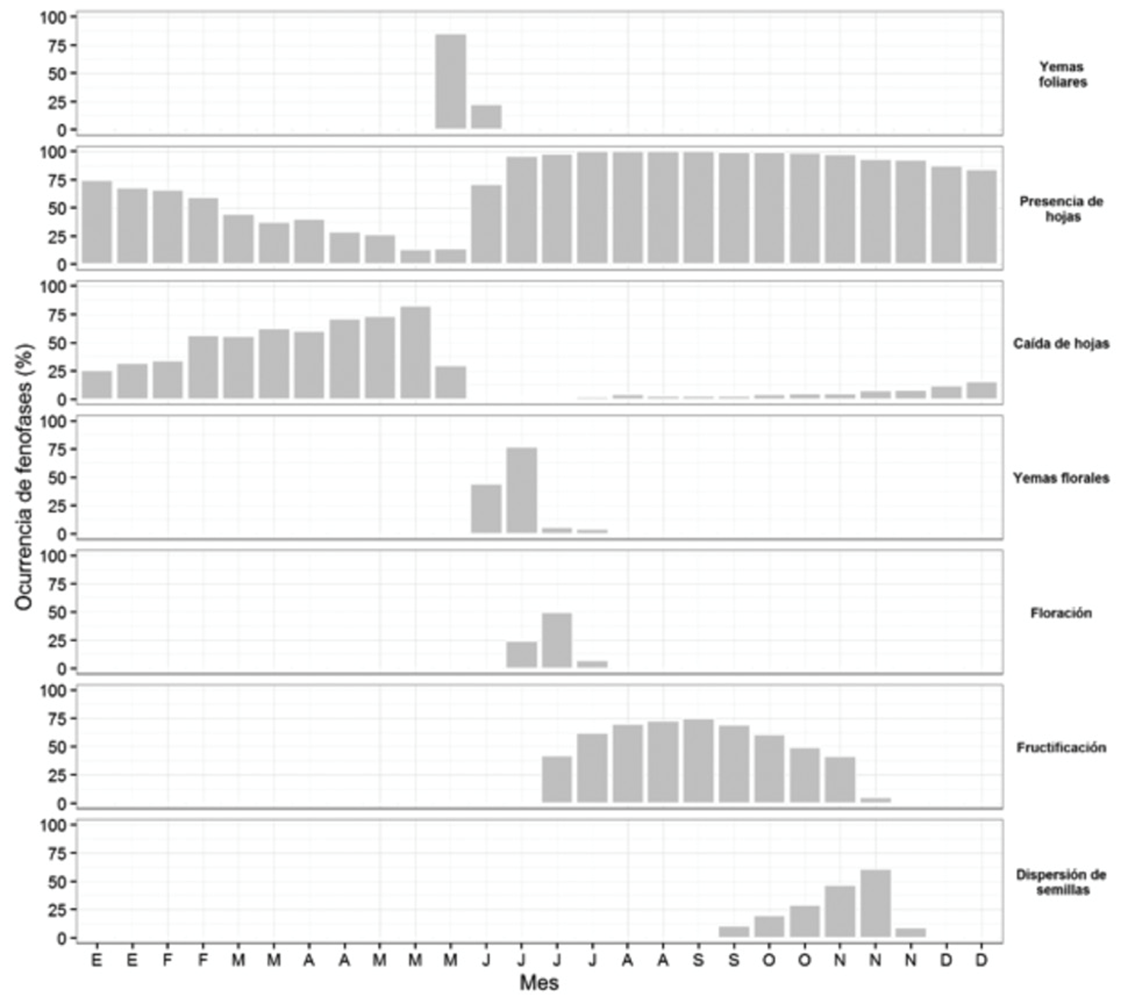
Figure 1 Occurrence of seven phenophases of Calyptranthes schiedeana O. Berg, evaluated every 14 days during the 2014-2015 period.
Tabebuia chrysantha reached its maximum development of leaf buds in May, and its largest leaf cover from June to December, with the highest defoliation in February, being completely leafless in April and May. The development of floral buds was observed from June to March, when flowering occurred; the fruiting and fruit dispersal occurred in April (Figure 2). The decrease of the temperature had an impact on the development of floral buds (r = - 0.58, P = 0.0008), and the falling of the leaves (r = - 0.67, P< 0.0001), while the increase in precipitation was related to the formation of leaf buds (r = 0.45, P = 0.0107).
Lysiloma acapulcense, like C. schiedeana, kept its leaves the year around, developed leaf buds in March --the most intense period of leaf fall (February-March)--, while in April (the month with the lowest precipitation) a quick transition from the development of floral buds to flowering was observed. Fruiting occurred mainly in November, and seed dispersal, in April of the following year (Figure 3). Fruiting in L. acapulcense diminished with low temperatures (r = -0.90, P < 0.0001).
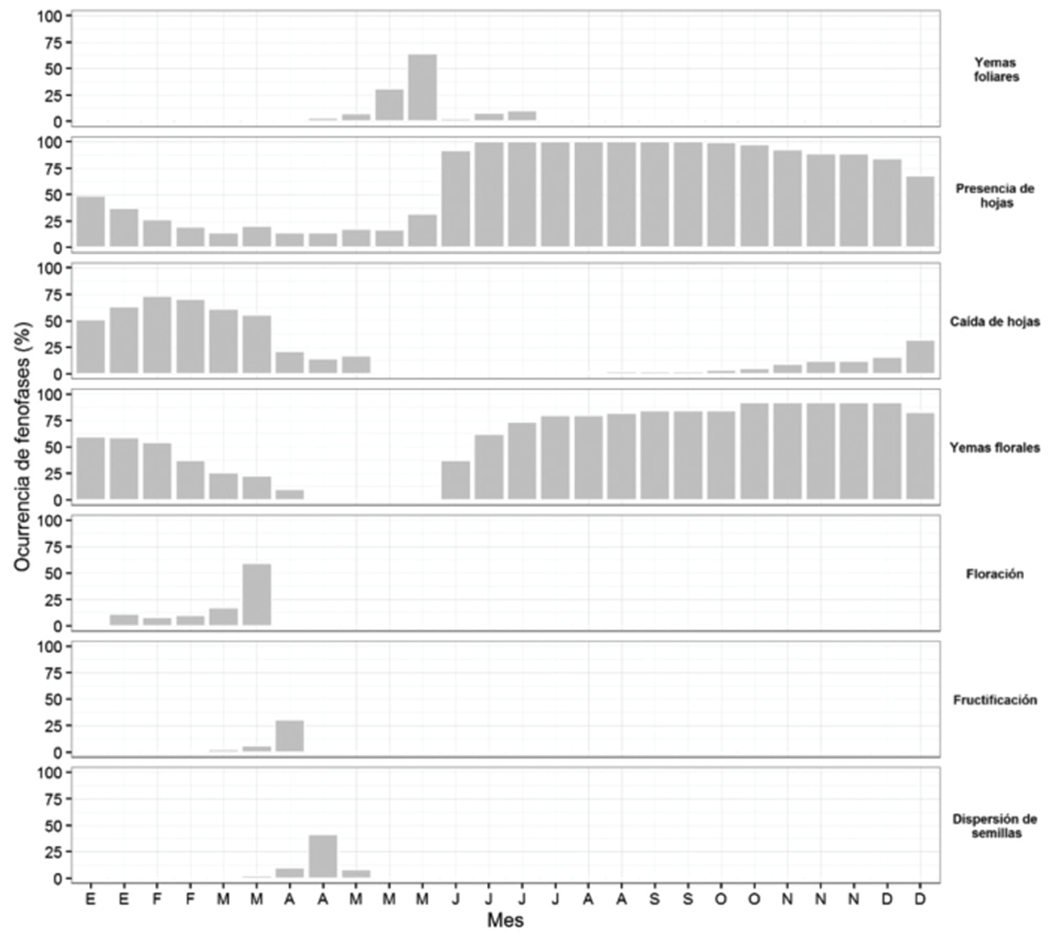
Figure 2 Occurrence of seven phenophases of Tabebuia chrysantha (Jacq.) G. Nicholson, evaluated every 14 days during the 2014-2015 period.
Architecture of the species
Calyptrantes schiedeana exhibited a determined, sympodial growth pattern of the main axis and a proleptic merisystematic activity; the branching patter was sympodial, with a plagiotropic orientation and symmetry; the orientation of the first order branches in relation to the main axis is upward (29.5°), with axillary reproductive structures, and the morphology of the inflorescences is monopodial, with total and adaptive reiterations.
Lysiloma acapulcense exhibited a sympodic growth of the main axis, a determined shape and a proleptic merisystematic activity; the branches are sympodial and plagiotropic, and first order branches have a diffused orientation (51.2°). The reproductive structures were in an axillary position, and the morphology of the inflorescences was monopodial, with total and adaptive reiterations. Tabebuia chrysantha exhibited a monopodial growth factor, a determined shape and a proleptic meristematic activity, with a sympodial branching pattern and an orthotropic orientation and symmetry; the first order branches were upward (44.1°). The position of the reproductive structures was apical (terminal), and the inflorescences had a sympodial morphology, with total and adaptive reiterations.
The first order branches of Lysiloma acapulcense and T. chrysantha had the same angle (P = 0.067), which surpassed that of C. schiedeana branches (P < 0.0001); likewise, the angles of the second order branches differed between C. schiedeana and T. chrysantha (P = 0.044), and those of the third order branches were similar in all these species (P = 0.943). Clearly, L. acapulcense is a larger tree, with longer branches (P < 0.0001) than T. chrysantha (P < 0.0001) and C. schiedeana (P< 0.0001), the latter of which had the shortest branches (Table 1).
Table 1 Branch architecture of Calyptranthes schiedeana O. Berg, Lysiloma acapulcense (Kunth) Benth and Tabebuia chrysantha (Jacq.) G. Nicholson pole trees.
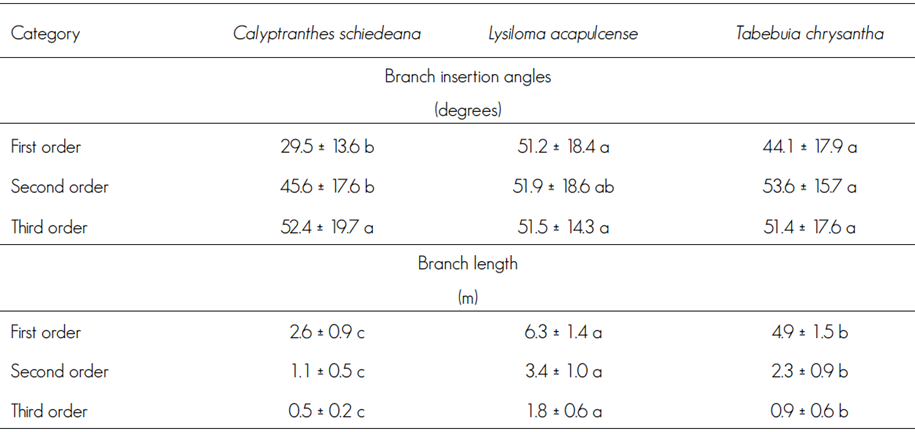
Different letters on the same line indicate statistical differences, Tukey (P<0.05).
All three taxa exhibited the same leaf insertion angle (P = 0.642); the leaves of L. acapulcense were compound (consisting of leaflets and small subleaflets), with a larger leaf area (P = 0.05) and leaf blade length (P = 0.05); however, this species exhibited a lower leaf density index. Like T. chrysantha, C. schiedeana had smaller, simple leaves and a higher leaf density index (Table 2).
Table 2 Leaf architecture and dasometric measures of Calyptranthes schiedeana O. Berg, Lysiloma acapulcense (Kunth) Benth and Tabebuia chrysantha (Jacq.) G. Nicholson pole trees.
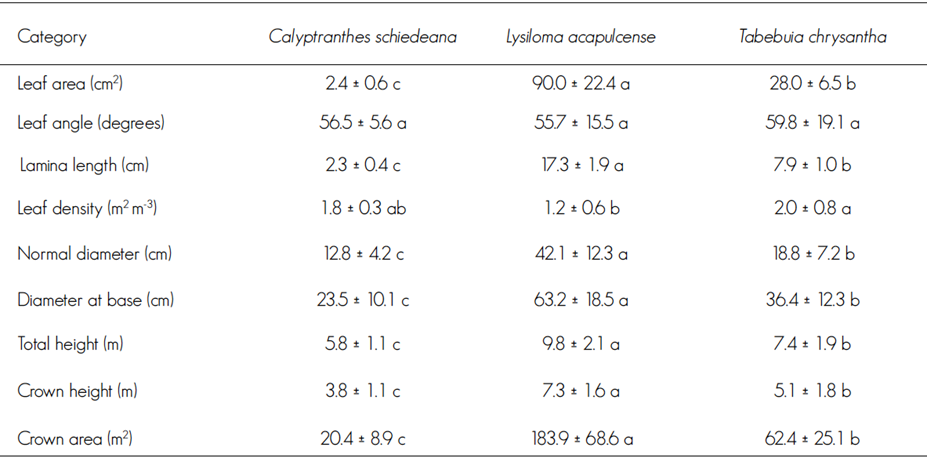
Different letters on the same line indicate statistical differences, Tukey (P < 0.05).
Lysiloma acapulcense stood out for its larger normal diameter (P < 0.0001), diameter at base ( P< 0.0001) and height (P < 0.0001) than those of other taxa; also, its crown is higher (P < 0.0001) and larger (P < 0.0001), while C. schiedeana had the smallest dimensions (Table 2).
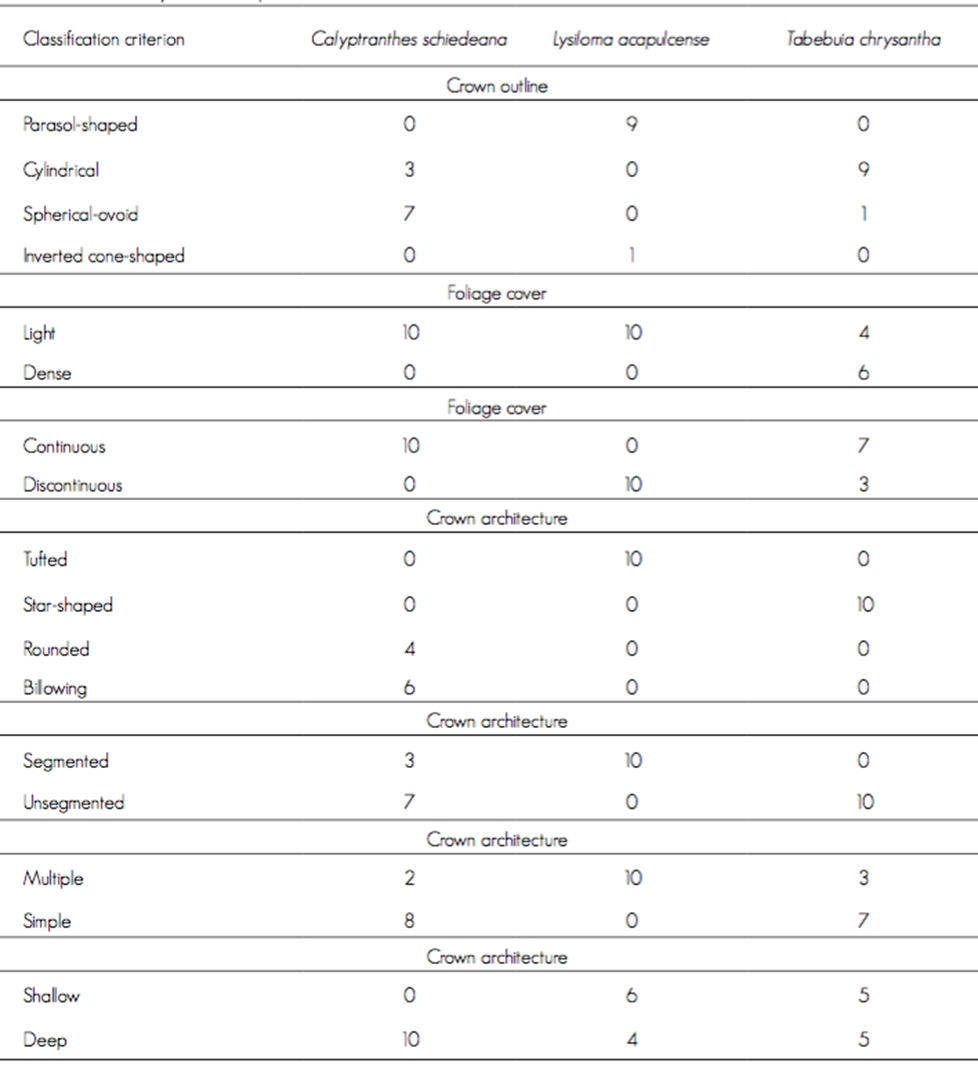
The crown architecture varied: the crown of L. acapulcense is parasol-shaped, that of T. chrysantha is cylindrical, and that of C. schiedeana is mostly spherical-ovoid (Table 3). The arrangement of the foilage is evenly light and discontinuous in L. acapulcense, while in C. shiedeana it ranges from light to continuous, and in T. chrysantha, the leaf cover is less well defined. The crown architecture of Lysiloma acapulcense was observed to be tufted, segmented, multiple, and ranging from shallow to deep; the crown of T. chrysantha is star-shaped, unsegmented, ranging from compound to simple and from shallow to deep, and that of C. schiedeana is maily segmented and billowing, simple and deep (Table 3). Due to the management and characteristics of the sites where the trees were located, certain aspects of the architecture turned out to be unclearly defined, and therefore, individuals of the same species exhibited differente patterns.
Discussion
All three taxa showed characteristic phenological patterns of low deciduous and sub-deciduous forest species, althought they tended to differ in certain aspects. L. acapulcense did not lose absolutely all its leaves at any point, and when it reached 75 %, new shoots began to cover its crown again (in the spring), while most specimens of C. schiedeana and T. chrysantha lost all of their foliage (during the dry seasons), as do many deciduous species whose leaves fall at the onset of hydric stress (Wright, 1996), and which produce new leaves one month before the beginning of the rainy season (Opler et al., 1980). Notably, defoliation lasted longer in L. acapulcense than in other taxa; this indicates differences in the hydric stress tolerance mechanism between the taxa (Sánchez et al., 2004).
The flowering times did not coincide: C. schiedeana flowered during the summer dry season (August), while the other species flowered during the dry season. However, they all had short, clearly defined flowering gaps. Furthermore, the seed dispersal occurred at a different rate: during the dry season (spring) for L. acapulcense and T. chrysantha, and in winter for C. schiedeana. In deciduous species, leaf growth does not coincide with the ripening of the fruits, which occurrs in spring and fall. This is because the distribution of resources (nutrients) for organ production takes place during the most favorable periods of the year (Opler et al., 1980).
In general, the studied species had different phenological patterns although they shared the same physiographic region, and can be identified with one of the three strategies (competitors, stress tolerator and ruderal) proposed by Grime (2001). The pioneers bloom throughout the year, while those of late successional phases do so in annually marked periods, or even every two or three years (Plana, 2000). The phase difference of blooming and fruiting peaks between the species reduces the competition for common dispersal agents and pollinators within a vegetal community (Snow, 1965), and furthermore, it defines the diet of the consumers according to the availability of food (Van Schaik et al.,1993). Therefore, the coincidence of biotic factors with the phenological cycles determines the intensity or occurrence of the phenophase peaks; climate cycles define the time in which the biotic processes related to phenology occur. In this study, soil management may have exerted some effect on the phenological patterns described above, although the scope of the research did not allow to discern their magnitude.
In studies carried out in Costa Rica and Mexico, T. chrysantha has been observed to bloom during the dry season (January-May) (Borchert et al., 2004), and, like T. neochrysantha A. H.Gentry and T. rosea (Bertol.) Bertero ex A. D.C., it produces flowers immediately after the falling of its leaves (Borchert, 1983); furthermore, these species coincide with most low forest trees, which bloom during short periods (7-9 weeks) and extreme draughts (Opler et al., 1980; Kochmer and Handel, 1986).
The phenological pattern of L. acapulcense differed from that cited by Pennington and Sarukhán (2005), as its blooming and fruiting were more premature than in the tropical rain forest, where taxa of the Fabaceae family bloom at various times of the year (Ochoa-Gaona et al., 2008). L. watsonii Rose, an evergreen taxon, shares flowering with L. acapulcense (April-May). However, the leaf fall period coincided partially with that observed by Pennington and Sarukhán (2005), lasting two months (February-March) in the study area.
Only certain phenologic events were related to climatic variables. The development of leaf buds in C. schiedeana and T. chrysantha exhibited an evident positive relationship with precipitation: leaf buds were formed as the rainy season advanced.
Conversely, L. acapulcense did not seem to depend on precipitation, as its buds emerged during the season with least rain and the highest temperatures. This response may be due to the fact that L. acapulcense has a deeper root system that allows it greater access to the moisture of the soil (Meinzer et al., 1999), and therefore it tolerates draughts well.
Leaf fall, flowering, the development of floral buds and fruiting were all related to the temperature. The fruiting and defoliation of L. acapulcense and T. chrysantha progressed as the temperatures fell; i.e. they occurred in the fall and the winter. The leaf fall rate during the dry season coincided with the reduction of the soil moisture and the increase in the hydric stress of the trees (Reich and Borchert, 1984).
The taxa exhibited very different architectures. The Troll model adopted by L. acapulcense is prevalent among the Fabaceae family and is characterized by the presence of horizontal axes with continuous overlap (Hallé et al., 1978); its morphometric characteristics allow inferring structural attributes related to the environment: pioneer tree, pinnate laminae, secondary and tertiary branches flexible enough to reduce the aerodynamic resistance to strong gusts of wind; these taxa develop a limited number of small leaves, held by thin and more flexible petioles (Vogel, 1989; Ennos, 1997).
Tabebuia chrysantha acquired the Leeuwenberg model, having erect axes (orthotrops), a determined growth and terminal inflorescences, characteristic of secondary vegetation species and disturbed sites (Hallé et al., 1978). The Attims model was associated with C. schiedeana, with a main axis and orthotropic branches, with continuous growth and branching, and lateral inflorescences.
The model of L. acapulcense resembled the one proposed by Vester (2002) for L. latisiliquum (the Troll model). The Leeuwenberg model representing T. chrysantha has been attributed to T. rosea (Borchert and Tomlinson, 1984; Vester, 2002). However, Vester (2002) attributes the Roux model to C. pallens, which differs from the Attims model of C. schiedeana by the horizontal orientation of its branches.
Tree architecture is the result of the influence of ontogenetic and morphogenetic factors affecting all levels of the organization of the organism, in each stage of its development and during its entire lifetime (Barthélémy and Caraglio, 2007). Thus, the space-time combination of the nature and condition, life cycle and habitat determine the various types of branching (Weberling et al., 2002). However, the architecture of the trees examined in this study differed from that of the trees growing in undisturbed sites because the competition conditions in agricultural soils are different from those existing in vegetal communities.
In agroforestry there is a wish to manage and shape the shared woody components, rather than to compete for the environmental resources (Huxley, 1999). For this reason, it is necessary to group the utilized woody plants based on their functionality, for which purpose those shapes that best adapt to a particular cirumstance in order to optimize production must be take into account (Donald, 1968). The three species considered in the present research may be regarded as competent and complementary ideotypes, whose structural attributes are compatible with other cultivated plants and with the livestock under the particular conditions of the studied sites.
All three taxa exhibited adequate phenological qualities and tree architecture for inclusion in agroforestry systems. The presence of leaves in L. acapulcense and C. schiedeana during the rainy season (summer) does not interfere with the development of rainfed crops that are highly important in the region (corn and beans), because they have small leaves that ensure less interference to the passage of light. The attributes of the tree architecture of C. schiedeana --its narrow crown, its leaf density index (1.75 m2 m-3) and the soil covered by its canopy (only 7.5 % less than in full sunshine)-- are useful for establishing this species in living fences around pastures.
Lysiloma acapulcense --having leaflets and small subleaflets, horizontal branches that improve the capture of light (King and Maindonald, 1999) and a lower leaf density during the rainy season (1.17 m2 m-3)-- provides an equal or larger cover to the soil under its canopy than under the full sunshine (24.6 %), a feature that distinguishes it from the other two species and which is probably due to the fact that it belongs to the Fabaceae family. Thus, it can be exploited as dispersed trees in pastures, in association with grasses or other fodder taxa, as it favors their development due to the symbiosis established by Fabaceae with soil bacteria (Bloom, 2015). Tabebuia chrysantha --with a higher leaf area index (2.01 m2 m-3) than the other species, its influence on the soil cover (55.8 % less under the canopy) and its broad crown-- is ideal for establishment in living fences, as boundary trees for timber and as dispersed trees in pastures to provide shade for the livestock and protection from water currents and sources (Beer et al., 2003).
Conclusions
The three arboreal species studied herein have a phenology and an architecture that confer upon them the potential to be planted in agroforestry systems, with some differences that involve assigning them different spaces and functions within these systems. The attributes of L. acapulcense make it best suited to be associated with pastures or other crops, dispersed or with a spatial arrangement. C. schiedeana and T. chrysantha may be utilized in spaces such as living fences and as boundary trees for timber so as to avoid interfering with the crops, and at the same time, they may provide environmental services, such as the embellishment of the landscape, reforestation and improvement of the soils, while contributing to the rescue of culturally important native species with value in use.
Acknowledgements
The authors wish to express their gratitude to the Consejo Nacional de Ciencia y Tecnología, CONACYT (National Council for Science and Technology) for the funding provided to carry out this research; to Salomé Quiroz Martínez, Josué Acosta Piña and Wendy Fernández for their support in field work, and to Lizbeth Hernández Landa and Dr. José López Collado, PhD, for their help in preparing the charts
REFERENCES
Aguilar, S. and R. Condit. 2001. Use of native tree species by a Hispanic community in Panama. Economic Botany 55: 223-235. [ Links ]
Barthélémy, D. and Y. Caraglio. 2007. Plant architecture: A dynamic, multilevel and comprehensive approach to plant form, structure ontogeny. Annals of Botany 99: 375-407. [ Links ]
Bautista, T. M. 2009. Sistemas agro y silvopastoriles en El Limón, municipio de Paso de Ovejas, veracruz, México. Tesis de Maestría en Ciencias. Postgrado en Agroecosistemas Tropicales. Colegio de Postgraduados. Tepetates, Manlio Fabio Altamirano, Ver., México. 82 p. [ Links ]
Beer, J., M. Ibrahim, E. Somarriba, A. Barrance y R. Leakey. 2003. Establecimiento y manejo de árboles en sistemas agroforestales In: Cordero, J. y D. Boshier (eds.). Árboles de Centroamérica: Manual para extensionistas. Biblioteca Orton IICA/CATIE. Turrialba, Costa Rica. 1079 p. [ Links ]
Bloom, A. J. 2015. Assimilation of inorganic nutrients. In: Taiz, L. and E. Zeiger (eds.). Plant physiology and development. Sinauer Associates, Inc. Sunderland, MA, USA. pp. 360-361. [ Links ]
Bonham, C. D. 1989. Measurements for terrestrial vegetation. John Wiley and Sons, Inc. CO, USA. 239 p. [ Links ]
Borchert, R. 1983. Phenology and control of flowering in tropical trees. Biotropica 15: 81-89. [ Links ]
Borchert, R. and P. B. Tomlinson. 1984. Architecture and crown geometry in Tabebuia rosea (Bignoniaceae). American Journal of Botany 71: 958-969. [ Links ]
Borchert, R ., S. A. Meyer, R. S. Felger and L. Porter-Bolland. 2004. Environmental control of flowering periodicity in Costa Rican and Mexican tropical dry forests. Global Ecology and Biogeography 13(5): 409-425. [ Links ]
Castillo-Campos, G. 2005. Contribución al conocimiento del endemismo de la flora vascular en veracruz, México. Acta Botánica Mexicana 57: 19-57. [ Links ]
Condit, R., S. P. Hubbell and R. B. Foster. 1993. Identifying fast growing native trees. Forest Ecology and Management 62: 123-143. [ Links ]
Donald, C. M. 1968. The breeding of crop ideotypes. Euphytica 17: 385-403. [ Links ]
Ennos, A. R.1997. Wind as an ecological factor. Tree 12: 108-111. [ Links ]
Gómez, P. A., T. Krômer y R. Castro-Cortes. 2010. Atlas de la flora de veracruz. Un patrimonio natural en peligro. Comisión del Estado de Veracruz para la conmemoración de la Independencia Nacional y la Revolución Mexicana. Veracruz, Ver., México. 528 p. [ Links ]
Grime, J. P. 2001. Plant strategies, vegetation processes, and ecosystem properties. 2nd Edition. Wiley and Sons. Chichester, England. 410 p. [ Links ]
Hallé, F., R. A. A. Oldeman and P. B. Tomlinson. 1978. Tropical Trees and Forests. Springer-verlag Berlin Heidelberg. 331 p. [ Links ]
Huxley, P. A. 1983. Phenology of tropical Woody perennials and seasonal crop plants with reference to their management in agroforestry systems. In: Huxley, P. A. (ed.). Plant research and agroforestry. International Council for Research in Agroforestry. Nairobi, Kenya. pp. 503-525. [ Links ]
Huxley, P. A. 1999. Tropical agroforestry. Blackwell Science. London, England. 384 p. [ Links ]
Image Tool (IT). 2002. Image Tool version 3.0. Dental Diagnosis Science. The University of Texas Health Science Center. San Antonio, TX, USA. n/p. [ Links ]
Instituto nacional de estadística y geografía (Inegi). 2009. Prontuario de información geográfica municipal de los Estados Unidos Mexicanos. Paso de ovejas, Veracruz de Ignacio de la llave. Clave geoestadística 30126. http://www3.inegi.org.mx/sistemas/mexicocifras/datosgeograficos/30/30126.pdf (5 de abril de 2016). [ Links ]
Interián-Ku, V. M., J. I. Valdez-Hernández, E. García-Moya, A. Romero-Manzanares, M. A. Borja-De-La-Rosa y H. Vaquera-Huerta. 2009. Arquitectura y morfometría de dos especies arbóreas en una selva baja caducifolia del sur de Yucatán, México. Boletín de la Sociedad Botánica de México 85: 17-29. [ Links ]
King, D. and J. H. Maindonald. 1999. Tree architecture in relation to leaf dimensions and tree in temperate stature and tropical rain forests. Journal of Ecology 87: 1012-1024. [ Links ]
Kochmer, J. P. and S. N. Handel. 1986. Constraints and competition in the evolution of flowering phenology. Ecological Monographs 56: 303-325. [ Links ]
Meinzer, F. C., J. L. Andrade, G. Goldstein, N. M. Holbrook, J. Cavelier and S. J. Wright. 1999. Partitioning of soil water among canopy trees in a seasonally dry tropical forest. Oecologia 121: 293-301. [ Links ]
Milla, R., P. Castro-Diez and G. Montserrat-Martí. 2009. Phenology of Mediterranean Woody plants from NE Spain: synchrony, seasonality, and relationships among phenophases. Flora 205: 190-199. [ Links ]
Ochoa-Gaona, S., I. Pérez-Hernández y B. H. J de Jong. 2008. Fenología reproductiva de las especies arbóreas del bosque tropical de Tenosique, Tabasco, México. Revista de Biología Tropical 56: 657-673. [ Links ]
Opler, P. A., G. W. Frankie and H. G. Baker. 1980. Comparative phenological studies of treelet and shrub species in tropical wet and dry forests in the lowlands of Costa Rica. Journal of Ecology 68: 167-188. [ Links ]
Owino, F. 1992. Improving multipurpose tree and shrub species for agroforestry systems. Agroforestry Systems 19: 131-137. [ Links ]
Pennington, T. D. y J. Sarukhán. 2005. Árboles tropicales de México. Manual para la identificación de las principales especies. In: Universidad Nacional Autónoma de México (ed.). Ediciones Científicas Universitarias, México. México, D.F., México. 523 p. [ Links ]
Pineda-Herrera, E., J. I. Valdez-Hernández y M. A. López-López. 2012. Fenología de Schizolobium parahyba y vochysia guatemalensis en una selva alta perennifolia de Oaxaca, México. Botanical Sciences 90 (2): 185-193. [ Links ]
Plana, E. 2000. Introducción a la ecología y dinámica del bosque tropical. Curso sobre gestión y conservación de bosques tropicales. Centro Tecnológico Forestal de Cataluña. http://www.ctfc.es/webcast/ areas/politica_for/docu-ments/ponb.pdf (10 de septiembre de 2015). [ Links ]
Reich, P. B. and R. Borchert. 1984. Water stress and tree phenology in a tropical dry forest in the Lowlands of Costa Rica. Journal of Ecology 72: 61-74. [ Links ]
Sánchez, A. J., F. A. Pariacote, S. Alfonzo y R. Flores. 2004. Arquitectura y fenología de las especies Prosopis juliflora y Acacia tortuosa en el semiárido del estado Falcón, venezuela. Archivos Latinoamericanos de Producción Animal 12: 72-81. [ Links ]
Statistical Analysis System (SAS). 2010. SAS/STAT. User ́s guide version 4.3.0. SAS Institute Inc. Cary, NC, USA. n/p. [ Links ]
Sestras, R. 2004. Horticultural plant breeding (in Romanian). Academic Pres, Cluj-Napoca, Romania. 430 p. [ Links ]
Snow, D. W. 1965. A possible selective factor in the evolution of fruiting seasons in a tropical forest. Oikos 15: 274-281. [ Links ]
Somarriba, E. 2005. ¿Cómo evaluar y mejorar el dosel de sombra en cacaotales? Agroforestería en las Américas. 41: 122-130. [ Links ]
Suárez, A., G. Williams-Linera, C. Trejo, J. I. Valdez-Hernández, V. M. Cetina-Alcalá and H. Vibrans. 2012. Local knowledge helps select species for forest restoration in a tropical dry forest of central veracruz, Mexico. Agroforestry Systems 85: 35-55. [ Links ]
Trichon, V. 2001. Crown typology and the identification of rain forest trees on large-scale aerial photographs. Plant Ecology 153: 301-312. [ Links ]
Van Schaik, C. P., J. W. Terborgh andS. J. Wright. 1993. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics 24: 353-377. [ Links ]
Vester, H. F. M. 2002. Modelos arquitectónicos en la flora arbórea de la Península de Yucatán. Boletín de la Sociedad Botánica de México 71: 45-57. [ Links ]
Vogel, S.1989. Drag and reconfiguration of broadleaves in high winds. Journal of Experimental Botany 40: 941-948. [ Links ]
Weberling, F., T. A. Kraus, C. A. Bianco and R. Malpassi. 2002. Variación y estrategias adaptativas de los sistemas de ramificación de Fabáceas herbáceas. Feddes Repertorium 13: 342-353. [ Links ]
Wishnie, M. H., D. H. Dent, E. Mariscal, J. Deago, N. Cedeño, D. Ibarra, R. Condit and P. M. S. Ashton. 2007. Initial performance and reforestation potential of 24 tropical tree species planted across a precipitation gradient in the Republic of Panama. Forest Ecology and Management 243: 39-49. [ Links ]
Wood, P. J. y J. Burley. 1995. Un árbol para todo propósito. Introducción y evaluación de árboles de uso múltiple para agroforestería. Centro Internacional para Investigación y Agroforestería, Instituto Interamericano de Cooperación para la Agricultura. San José, Costa Rica. 180 p. [ Links ]
Wright, S. J. 1996. Phenological responses to seasonality in tropical forest. In: Stephen, S. M., R. L. Chazdon and A. P. Smith (eds.). Tropical Forest Plant Ecophysiology. Chapman and Hall. New York, NY, USA. pp. 1-17 [ Links ]
Received: July 29, 2016; Accepted: January 09, 2017











 texto en
texto en