Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.7 no.38 México Nov./Dez. 2016
Article
Histopathology of Pinus patula Schiede ex Schltdl. et Cham. And Pinus pseudostrobus Lindl. roots infected by Phytophthora cinnamomi Rands, 1922
1Departamento de Parasitología Agrícola, Universidad Autónoma Chapingo. México.
2Centro de Desarrollo de Productos Bióticos, Instituto Politécnico Nacional. México.
3Fitopatología, Campus Montecillo, Colegio de Postgraduados. México.
4Escuela de Ciencias Agrarias, Universidad Nacional. Costa Rica.
Phytophthora cinnamomi is one of the most devastating plant pathogens worldwide, since it causes root rot and death of many plant species. The aim of this study was to determine the histological damage induced by this organism in roots of Pinus patula and P. pseudostrobus. Both Pinus species were inoculated by immersion of roots in a suspension of mycelial fragments. Roots of control trees were immersed in sterile distilled water. Root samples were taken every four days after inoculation for 20 days. Microscopic symptoms occurred more rapidly in P. pseudostrobus, although the two species exhibited fragile and necrotic roots. Also, the histological changes at the root of both species were presented with an increase in polyphenol content, degradation of cell walls and necrosis of the periderm. In the case of P. patula, plants showed wilting of the canopy, followed by a yellowing of needles and finally with the death of the tree. For P. pseudostrobus symptoms were similar, but in this case the decay of needles was gradual and subsequently wilt thereof was observed until the total death of the tree.
Key words: Anatomic changes; oomycete; pathogenicity; Phytophthora cinnamomi Rands; 1922; Pinus patula Schiede ex Schltdl. Et Cham.; Pinus pseudostrobus Lindl
Phytophthora cinnamomi es uno de los fitopatógenos más devastadores a nivel mundial, ya que ocasiona pudriciones de raíz y la muerte de numerosas especies vegetales. El objetivo de este estudio fue determinar los daños histológicos inducidos por este organismo a nivel radicular en Pinus patula y Pinus pseudostrobus. Se inocularon 48 árboles de 11 meses de edad de ambas especies forestales mediante la inmersión de sus raíces en una suspensión de fragmentos miceliales y como testigo, otras que fueron sumergidas en agua destilada estéril. Se tomaron muestras de tales estructuras cada cuatro días después de la inoculación por 20 días. Los síntomas microscópicos se manifestaron con mayor rapidez en P. pseudostrobus, aunque en las dos especies las raíces se tornaron frágiles y se necrosaron. Asimismo, los cambios histológicos se presentaron como un aumento en el contenido de polifenoles, degradación de las paredes celulares y necrosamiento de la peridermis. En P. patula, las plantas mostraron un marchitamiento de la copa, seguida por un amarillamiento de las acículas y, finalmente, en la muerte del árbol. Para P. pseudostrobus los síntomas fueron similares, pero el decaimiento de las acículas fue gradual y, posteriormente, se observó marchitez de las mismas hasta la muerte total del ejemplar.
Palabras clave: Cambios anatómicos; oomicete; patogenicidad; Phytophthora cinnamomi Rands; 1922; Pinus patula Schiede ex Schltdl. et Cham.; Pinus pseudostrobus Lindl
Introduction
Phytophthora cinnamomi Rands, 1922 is a soil pathogen that causes root rot, which in the last century devastated 200 000 hectares of natural vegetation in Australia, and destroyed more than 400 host species (Malajczuk, 1979). It is also responsible for serious annual losses of chestnut (Castanea sativa Miller), cranberry plants (Vaccinium myrtillus L.) and ornamental plants within the forest nurseries of the United States of America. This pathogen has also been reported in Argentina, Spain, South Africa, South America and Taiwan where it attacks a large number of hosts (Zentmyer, 1980; Robin et al., 1992; Brasier et al., 1993; Erwin and Ribeiro, 1996). In Mexico, Ph. cinnamomi has caused extensive damage to areas producing avocado (Persea americana L.), as in Atlixco, Puebla, where the crop has disappeared from large regions (Téliz and Mora, 2007). In addition, it affects species in El Arrayanal forests, in the state of Colima, where it caused the death of oak trees (Tainter et al., 2000); the same is true in Tecoanapa, Guerrero (Alvarado et al., 2008).
This phytopathogenic oomycete is very aggressive to plants, and has repercussions on diverse economic aspects (Hardham, 2005). The typical symptoms of this desease from root damage are foliar chlorosis, their wilting and rapid detachment, leading to a reduction in transpiration (Erwin and Ribeiro, 1996; Moralejo et al., 2009). It enters the plant through the root and also invades the base of the trunk of its host, but the plants have defense mechanisms that prevent the organism from advancing, limiting its infection; some of these defenses are preformed biochemical barriers and phenolic compounds. Plant resistance to root-affecting pathogens is frequently assessed histologically (Glazebrook, 2005; Obwald et al., 2014). Therefore, the objectives of this study were to determine the susceptibility of Pinus patula Schiede ex Schltdl. et Cham. and P. pseudostrobus Lindl. to infection by Phytophthora cinnamomi, as well as performing a histological analysis of the root to describe the damage induced by this fungus.
Materials and Methods
A total of 48 trees of 11 months of age were donated by the Molino de las Flores forest nursery with 24 individuals per species (P. patula and P. pseudostrobus), which were kept in greenhouse conditions, to be inoculated later. The experiment was carried out in two stages: the first phase consisted of the incorporation of Phytophthora cinnamomi in the plants, while the second one was based on the histological process of the inoculated samples; the aforementioned forest species were selected because of their wide range of adaptation and their commercial importance.
Pathogenicity test
The inoculation method of substrate with mycelium was applied, which consists of taking colonies of Phytophthora cinnamomi to obtain inoculum at a concentration of 3.3 x 108 mycelial fragments. The trees were inoculated bare root by immersion in the suspension of mycelial fragments for 3 h; 12 plants of each species were used as a control, to which their root was put just into sterile distilled water. After 3 h, each tree was planted on a sterile substrate composed of Peat Moss and sterile forest soil in a ratio of 3: 1. The plants were subjected to 100 % relative humidity and at 22 ± 3 °C for 72 h and were kept in the greenhouse under normal handling conditions.
Before the onset of symptoms, root samples of 10 mm in length were obtained from both inoculated and control trees. Once the first signs of damage were observed, the following samples were taken, for which a root cut of approximately 1 cm was made, which was immersed in a fixative solution based on a mixture of formaldehyde, glacial acetic acid, distilled water and 96 % ethyl alcohol (FAA). In total, six samples were collected from each species of Pinus at 4-day intervals after inoculation.
Histological analysis
For the processing of the samples for histological studies, the modified procedure described by Leyva et al. (2012) and Tovar et al. (2012) was used. Thus, root samples fixed in the FAA solution were washed with running water for 10 min and infiltrated in an automated tissue processor model 4640-B (Tissue-Tek IITM). Dehydration was carried out in a gradual series of ethyl alcohol (30, 50, 70, 85, 96 and 100 %), after which they were passed through a mixture of absolute ethanol-xylene (1: 1) and three xylene changes at 100 % at 2 h intervals in each of the changes. Inclusion in Paraplast (SigmaTM) was carried out for 96 h.
With the aid of a Spencer 820 (American Optical®) rotating microtome, 10 μm cross sections of each sample were obtained, which were placed in a flotation bath at 45-50 °C. Under these conditions the cuts were spread and adhered to the slides. The cuts were dewaxed in three changes of absolute xylene (3 min each). Subsequently, they were hydrated in ethyl alcohol at 100, 96 and 70 % (3 min at each change), and stained with safranine (SigmaTM) 1 % for 24 h and rinsed with running water until the water became crystalline. The sections were then washed with 50, 70 and 96 % ethyl alcohol (3 min each) plus picric acid and stained with rapid green (Technical ChemistryTM) for 10 s. Afterwards, they were washed with clove oil to remove excess dye and rinsed with a mixture of clove oil, xylene and absolute ethanol. To conclude, the samples were placed in 100 % xylene, mounted on synthetic resin and examined in a composite microscope (Olympus BX41).
Results and Discussion
Pathogenicity test
Phytophthora cinnamomi induced different symptoms according to the infection time in the two artificially inoculated Pinus species, whereas the control plants showed no appreciable change and remained asymptomatic (Figure 1A and 2A). Four days after inoculation (dai), P. patula plants maintained their vigor and appearance (Figure 1B), in addition to showing no signs of the pathogen. At 8 dai the trees showed severe decay of needles, from the upper section of the tree (Figure 1C). At 12 dai, together with the decay of the tree, yellowing of the needles was observed, and at the base of the tree some of them dried and got a yellow and light brown coloration (Figure 1D). At 16 dai the dehydration of needles became more evident and the top of the stem took a greyish colouration (Figure 1E). At 20 dai, the trees were dry in more than three-quarters and had an intense brown color in addition to loss of vigor and at 24 dai were completely dry (Figure 1F).

Figure 1 Symptoms caused by Phytophthora cinnamomi Rands, 1922 in Pinus patula Schiede ex Schltdl. et Cham. seedlings observed at different days after inoculation (dai). (A) Control plant, B) Plant at 4 dai, C) Plant at 8 dai, D) Plant at 12 dai, E) Plant at 16 dai, F) Plant at 20 dai.
On the other hand, the inoculated P. pseudostrobus did not show signs indicating the presence of the pathogen, in addition to maintaining their tone and vigor until 4 dai (Figure 2B). At 8 dai it was possible to distinguish a very slight decay of needles, which began to lose turgor (Figure 2C). At 12 dai the trees experienced a visible decay of needles with incipient yellowing (Figure 2D). At 16 dai the weakening of the same prevailed and a light brown color began to appear in them (Figure 2E). At 20 dai these structures no longer had turgor and most of the tree changed to a brown tone (Figure 2F).

Figure 2 Symptoms caused by Phytophthora cinnamomi Rands, 1922 in Pinus pseudostrobus Lindl. seedlings observed at different days after inoculation (dai). (A) Control plant, B) Plant at 4 dai, C) Plant at 8 dai, D) Plant at 12 dai, E) Plant at 16 dai, F) Plant at 20 dai.
The damages that the aerial part of the plants of P. patula evidenced were ostensible from the decline of the tree, which consisted of a wilting of the crown; to this continued a yellowing of the needles to later become brown and then redish-brown, which continued until the tree was completely dry. The needles became fragile and easily detached, and the roots became brittle and necrotic.
In P. pseudostrobus the symptoms were similar, with the difference that the decay of the needles was gradual, i. e. slower, however, afterwards their yellowing was verified until the tree was dead. Similarly, the roots became brittle and necrotic. The previous aspect coincided with that described by Zentmyer (1980) and Chavarriaga et al. (2007) for specimens of Pinus spp. infected by Phytophthora cinnamomi.
Histological analysis of roots of P. patula
Control trees did not register significant changes throughout the process. The histological cut corresponding to the roots of the controls revealed the presence of the periderm, made up of a layer of lignified cells (sclereids) and the hypodermis located below the previous layer (Figure 3A). Phloem and xylem exhibited no alteration or fungal structures (Figure 3B).
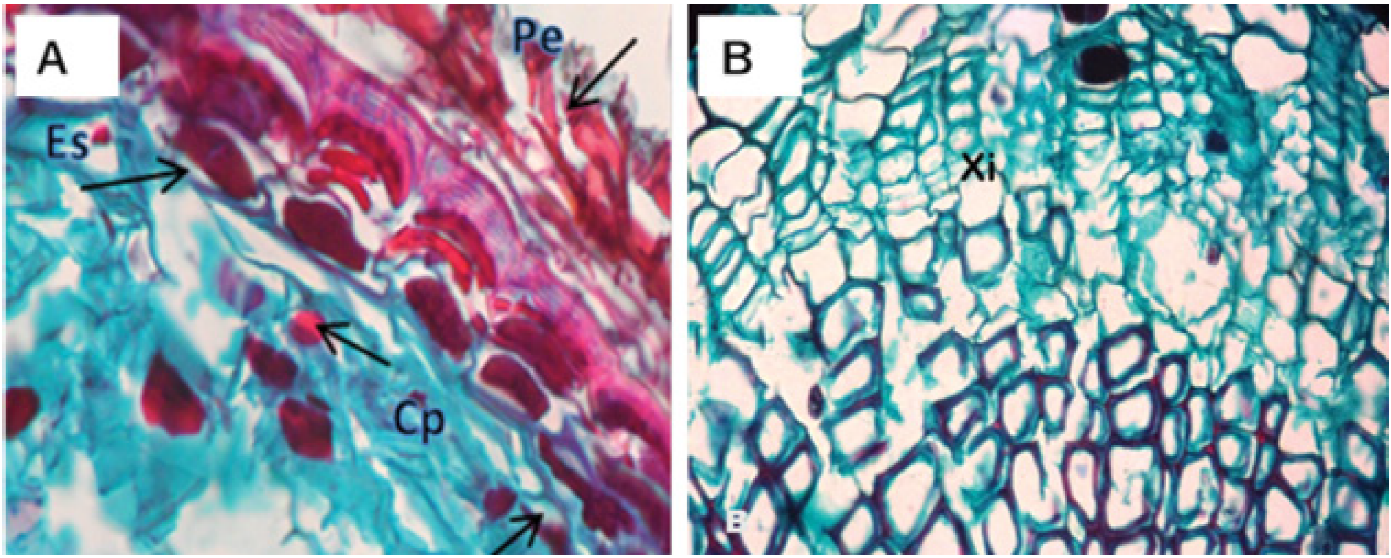
Figure 3 Cross-sectional micrograph of a healthy root of Pinus patula Schiede ex Schltdl. et Cham. A) View of periderm (Pe), content of polyphenols (Cp) and sclereids (Es). B) Phloem (Flo) and xylem (Xi).
The histological analysis of roots collected at 4 dai did not show any significant modifications, since the cellular structure (periderm, phloem and xylem) remained in order. However, evidence of polyphenols was observed in xylem cells (Figure 4A). At 8 dai, in the latter, there was wall detachment, but no vascular bundle obstruction, nor existence of fungal bodies, and, as in the previous sample, the polyphenol content was very scarce (Figure 4B). At 12 dai, a reduction in the polyphenols in the xylem was observed, which were dense and larger than in the materials collected at 8 dai, in addition to necrosis in the periderm, although the latter still retained its structure (Figure 4C and D). In the samples collected at 16 dai it was noticed thinning of the periderm, null presence of sclereids and stability in the content of polyphenols (Figure 4E). At 20 dai, a rough appearance of the xylem walls was found, which is atypical in healthy tissue (Figure 4F). Also, at 32 dai, trees showed generalized necrosis in the root periderm and tissues such as xylem and phloem were no longer recognizable (Figure 4G).
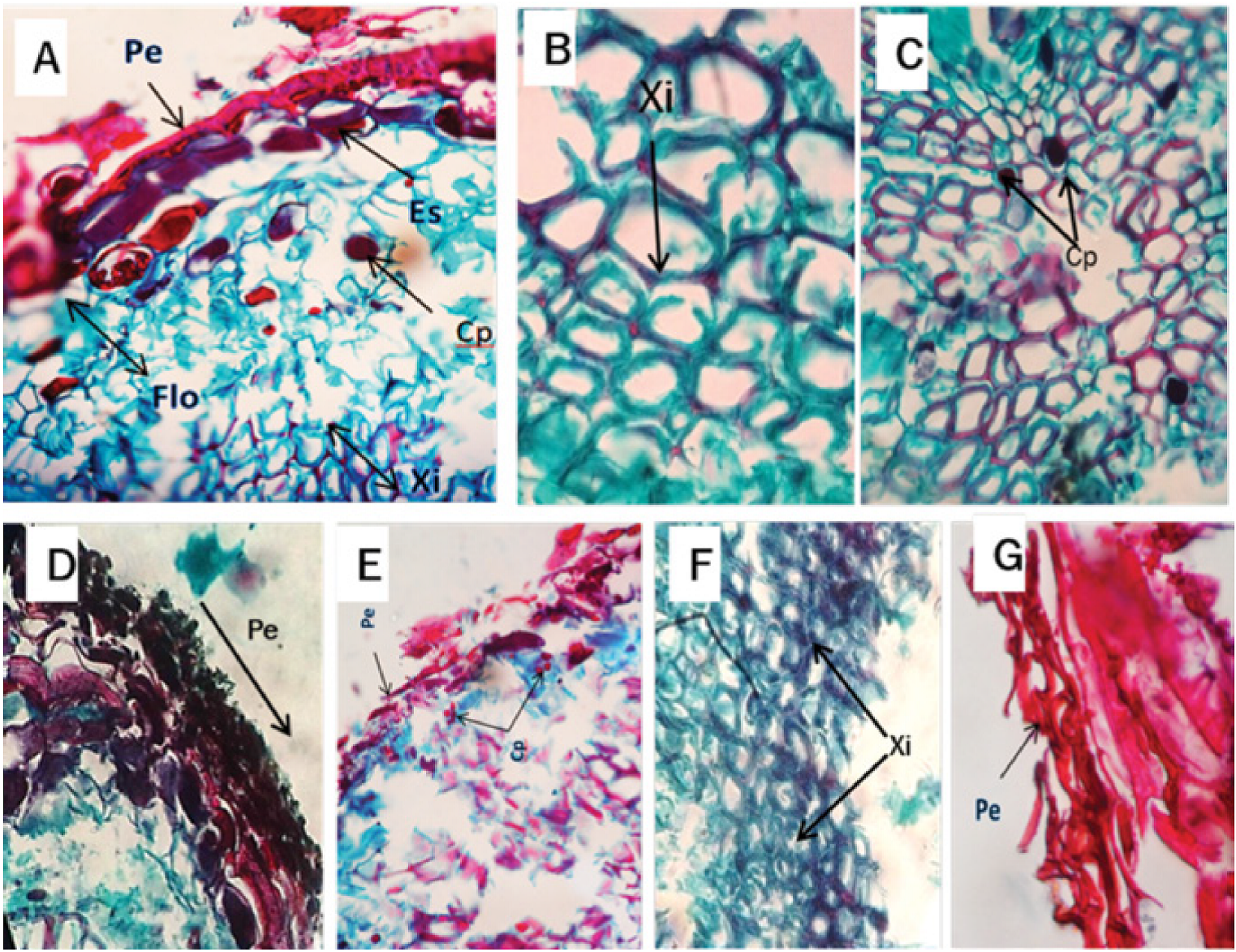
Figure 4 Cross-sectional micrograph of the Pinus patula Schiede ex Schltdl. et Cham. root infected by Phytophthora cinnamomi Rands, 1922. A) Periderm (Pe), sclereids (Es) phloem (Flo), content of polyphenols (Cp) and xylem (Xi) at 4 days after inoculation (dai). B) Root cross section with alterations of the tissues, observing detachment of the xylem walls (Xi) at 8 dai. C and D) Root cross section with presence of polyphenols (Cp) in the xylem at 12 dai. E) Periderm (Pe) and content of polyphenols (Cp) at 16 dai. F) Root cross section showing xylem (Xi) with rough walls at 20 dai. G) Periderm (Pe) at 32 dai.
Histological analysis of roots of P. pseudostrobus
As in P. patula, control trees showed no significant changes at any time. The structure of the histological cut corresponding to its roots was constituted by the periderm, which in turn was conformed by a layer of lignified cells (sclereids), below which the hypodermis was identified. The phloem did not suffer alterations and the xylem did not exhibit cells with polyphenols or fungal structures.
At 4 dai, the histological sample of the inoculated trees of P. pseudostrobus showed moderate presence of polyphenols in the xylem (Figure 5A and B). At 8 dai it revealed a large number of them in both the xylem and the phloem, in addition to the formation of tyloses in the xylem vessels (Figure 5C). At 12 dai, there was a thinning of the periderm, a greater number of polyphenols and even an increase in size in phloem cells (Figure 5D). At 16 dai the histological section of the xylem showed a thickening of its walls (Figure 5E). At 20 dai, a complete cellular disorganization of the root elements was verified (Figure 5F) and at 32 dai the trees died.
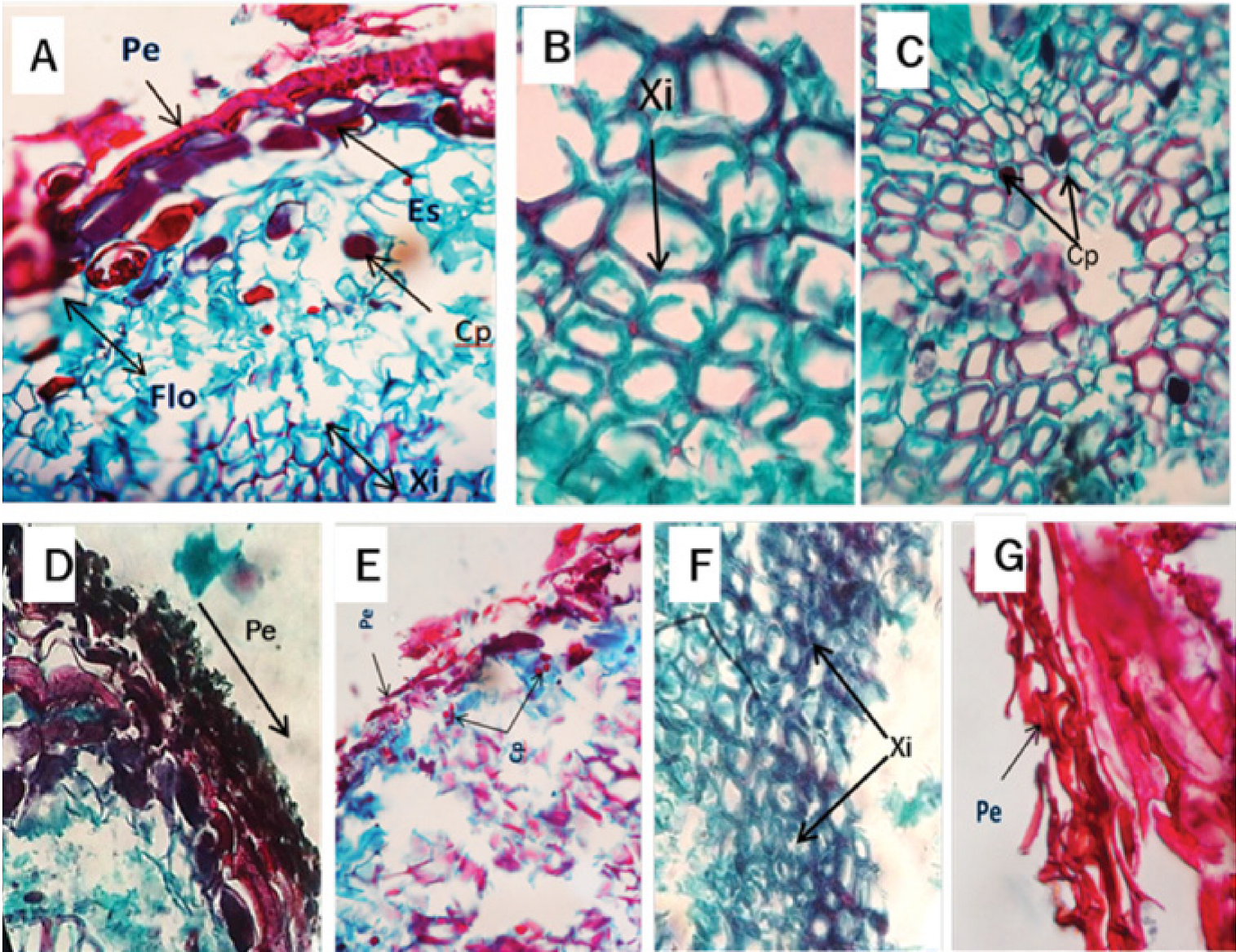
Figure 5 Cross-sectional micrograph of the root of Pinus pseudostrobus Lindl. infected by Phytophthora cinnamomi Rands, 1922. A and B) Presence of polyphenols (Cp) in the xylem at 4 days after inoculation (dai). C) Tilides (Ti) in the xylem at 8 dai. D) Periderm (Pe), content of polyphenols (Cp) and phloem (Flo) at 12 dai. E) Normal xylem (XiN) and thickened xylem (XiE) at 16 dai. F) Periderm (Pe) at 20 dai.
In the histological analysis carried out on P. patula plants infected with Phytophthora cinnamomi, phloem and, to a lesser extent, xylem damage, were observed. This behavior is typical of the infection of species of the genus Phytophthora generally developed as a result of the invasion of hyphae on the bark (Brummer et al., 2002; Oh y Hansen, 2007; Horta et al., 2010; Portz et al., 2011). In addition, xylem exhibited cellular detachment, indicating severe loss of turgidity and degradation of cell walls was probably recognized by extracellular enzymes such as pectins, which are produced by Phytophthora species (Brummer et al., 2002). Also, polyphenols and periderm necrosis were detected, which is common in infections induced by Phytophthora spp. (Erwin and Ribeiro, 1996).
On the other hand, the inoculated plants of P. pseudostrobus showed the less affected xylem in comparison to the phloem, in addition to that there was a great concentration of polyphenols, mainly in the xylem. This could be explained by the synthesis and accumulation of phenols increases after infection has occurred; several of these compounds are potent inhibitors of various hydrolytic enzymes including the pectolytics. Tyloses were observed in the vessels of the xylem, which because of their size and abundance, can obstruct the vessels completely and thus block the progression of the pathogen. In general, plant varieties that form few tyloses or none against the pathogen are always susceptible to diseases (Agrios, 2005).
The tissues showed thickening of the cell walls due to a plasmolysis from a serious imbalance in the osmoregulation of the roots, as well as necrosis of the periderm.
Despite confirming various changes in the internal tissues of the root, no hyphae of Ph. cinnamomi were detected, perhaps due to several of Phytophthora spp. Are capable of directly infecting the trunk of certain host species by means of lenticels, adventitious roots, or wounds, and then rapidly invade and destroy the bark and phloem tissue. Such is the case of the action of the pathogen on Eucalyptus marginata Donn ex Sm. (Hardy et al., 1996) and E. calophylla Lindl. (O’Gara et al., 1997), which invaded the specimens by the trunk. However, studies by Ruiz et al. (2015) in six-month-old Quercus ilex L. plants inoculated with Ph. cinnamomi recorded colonization of apoplast and penetration into cortical cells. Upon reaching the parenchymal tissues of the central cylinder, the fungus developed different reproductive structures within the cell and caused some host responses such as thickening of the cell wall and accumulation of phenolic compounds.
Conclusions
The roots of Pinus pseudostrobus and Pinus patula infected with Phytophthora cinnamomi showed diverse histological alterations such as increase of polyphenol content, degradation of cell walls, presence of tyloses and necrosis of the periderm. The alterations were more evident in the trees of P. pseudostrobus.
Acknowledgements
The authors would like to thank Dr. Elizabeth Cárdenas Soriano for the support given during the interpretation of results. Likewise, to Dr. Guadalupe Valdovinos Ponce, from Colegio de Postgraduados for facilitating the laboratory equipment to perform the histological analysis.
REFERENCES
Agrios, G. N. 2005. Plant pathology. Elsevier Academic Press. San Diego, CA, USA. 922 p. [ Links ]
Alvarado R., D., R. L. Saavedra y S. A. Almaraz. 2008. Primer reporte de Phytophthora cinnamomi Rands asociado al encino (Quercus spp.) en Tecoanapa, Guerrero, México. Agrociencia 42: 565-572. [ Links ]
Brasier, C. M., F. Robredo and J. F. P. Ferraz. 1993. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathology 42: 140-145. [ Links ]
Brummer, M., M. Arend, J. Fromm, A. Schlenzig and W. F. Obwald. 2002. Ultrastructural changes and immunocytochemical localization of the elicitin quercinin in Quercus robur L. roots infected with Phytophthora quercina. Physiological and Molecular Plant Pathology 61: 109-120. [ Links ]
Chavarriaga, D., W. J. A. Bodles, C. Leifert, L. Belbahri and S. Woodward. 2007. Phytophrhora cinnamomi and other fine root pathogens in north temperate pine forests. FEMS Microbiology Letters 276(1): 67-74. [ Links ]
Erwin, D. C. and O. K. Ribeiro. 1996. Phytophthora Diseases Worldwide. APS Press. St. Paul, MN, USA. 562 p. [ Links ]
Glazebrook, J. 2005. Contrasting mechanism of defense against biotrophic and necrotropic pathogens. Annual Review of Phytopathology 43: 205-227. [ Links ]
Hardam, A. R. 2005. Phytophthora cinnamomi. Molecular Plant Pathology 6(6): 589-604. [ Links ]
Hardy, G. E. St. J., I. J. Colquhoun and P. Nielsen 1996. The early development of disease caused by Phytophthora cinnamomi in Eucalyptus marginata and Eucalyptus calophylla growing in rehabilitated bauxite mined areas. Plant Pathology 45: 944-54. [ Links ]
Horta, M., P. Caetano, C. Medeira, I. Maia and A. Cravador. 2010. Involvement of the beta-cinnamomin elicitin in infection and colonization of cork oak roots by Phytophthora cinnamomi. European Journal of Plant Pathology 127: 427-436. [ Links ]
Leyva M., S. G., E. Cárdenas S., J. M. Tovar P., J. Huerta E. y H. E. Villaseñor M. 2012. Estimación histopatológica del grado de infección inducido por Stagonospora nodorum en plántulas de trigo. Agronomía 20(1): 7-16. [ Links ]
Malajczuk, N. 1979. Biological suppression of Phytophthora cinnamomi in eucalyptus and avocado in Australia. In: Schippers, B. and W. Gams (eds.). Soil plant pathogens. Academic Press. New York, NY, USA. pp. 635-652. [ Links ]
Moralejo, E., M. J. García and E. Descals. 2009. Susceptibility of Iberian trees to Phytophthora ramorum and P. cinnamomi. Plant Pathology 58: 271-283. [ Links ]
Obwald, W., F. Fleischmann, D. Rigling, J. Diez, A. C. Coelho, A. Cravador, R. J. Dalio, M. Horta, H. Pfanz, C. Robin, G. Sipos, A. Solla, T. Cech, A. Chambery, S. Diamandis, E. Hansen, T. Jung, L. B. Orlikowski, J. Parke, S. Prospero and S. Werres. 2014. Strategies of attack and defense in woody plant-Phytophthora interactions. Forest Pathology 44: 169-190. [ Links ]
Oh, E. and E. M. Hansen. 2007. Histopathology of infection and colonization of susceptible and resistant Port-Orford-Cedar by Phytophthora lateralis. Phytopathology 97(6): 684-693. [ Links ]
O’Gara, E., J. A. McComb, I. C. Colquhoun and G. E. St. J. Hardy. 1997. The infection of non- wounded and wounded periderm tissue at the lower stem of Eucalyptus marginata by zoospores of Phytophthora cinnamomi, in a rehabilitated bauxite mine. Australasian Plant Pathology 26: 135-141. [ Links ]
Portz, R. L., F. Fleischmann , J. Koehl, J. Fromm, D. Ernst, S. F. Pascholati and W. F. Osswald. 2011. Histological, physiological and molecular investigations of Fagus sylvatica seedlings infected with Phytophthora citricola. Forest Pathology 41: 202-211. [ Links ]
Robin, C., M. L. Desprez L. and C. Delatour. 1992. Spatial and temporal enlargement of cankers of P. cinnamomi in red oak. Canadian Journal of Forest Research 22: 362-366. [ Links ]
Ruiz G., F. J., R. M. Navarro C., R. Sánchez C. and A. Pérez de L. 2015. Histopathology of infection and colonization of Quercus ilex fine roots by Phytophthora cinnamomi. Plant Pathology 64:605-616. [ Links ]
Tainter, F. H., J. G. O´Brien, A. Hernández and F. Orozco. 2000. Phytophthora cinnamomi as a cause of oak mortality in the state of Colima, Mexico. Plant Disease 84(4): 394-398. [ Links ]
Téliz O., D. y A. Mora A. 2007. El aguacate y su manejo integrado. Ediciones Mundi-Prensa. México, D.F., México. 321 p. [ Links ]
Tovar P., J. M., J. A. Mora A., C. Nava D., G. Valdovinos P., D. Téliz O., A. Villegas M. and J. Hernández M. 2012. Identification, pathogenicity and histopathology of Lasiodiplodia theobromae on sapote mamey grafts in Guerrero, Mexico. Agrociencia 46(2): 147-161. [ Links ]
Zentmyer, A. G. 1980. Phytophthora cinnamomi and the diseases it causes. The American Phytopathological Society Press. St. Paul, MN, USA. 96 p. [ Links ]
Received: July 07, 2016; Accepted: December 22, 2016











 texto em
texto em 


