Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.7 no.36 México Jul./Ago. 2016
Articles
Fusarium oxysporum Schltdl. and Fusarium solani (Mart.) Sacc. cause wilting of Pinus spp. seedlings in the nurserya b
1Posgrado en Horticultura, Departamento de Fitotecnia, Universidad Autónoma Chapingo. México.
2Departamento de Parasitología Agrícola. Universidad Autónoma Chapingo. México.
3Programa de Fitopatología, Colegio de Postgraduados, Campus Montecillo. México.
Conifers can suffer significant damage due to diseases in the nursery, primarily root rot and wilting of the seedlings; in Pinus spp. they are recognized as an ailment in various regions of the world. These symptoms -along with initial stages of yellowing of Pinus patula and P. pseudostrobus plants- were observed during 2013 and 2014 in a forest nursery in the state of Guerrero, Mexico. Thus, the present study was proposed, with the objective of identifying the phytopathogenic fungi associated with the problem through the morphological characterization of their asexual reproductive structures, analysis of the sequences of ITS regions of the rDNA, and pathogenicity tests, as well as of determining the incidence and severity of each of the pathogenic species of interest. Based on the seedlings of the two species of interest showing signs of damage, isolated specimens of the Fusarium genus were obtained. The morphological characterization, pathogenicity test, and analysis of sequences of ITS regions of these organisms proved that F. solani and F. oxysporum are the fungal species that cause symptoms of wilting of Pinus plants. No significant difference was observed between the incidence and the severity of the disease caused by these agents.
Key words: Fusarium oxysporum Schltdl.; Fusarium solani (Mart.) Sacc.; incidence; pathogenicity; Pinus patula Schiede ex Schltdl. & Cham.; Pinus pseudostrobus Lindl
Las coníferas pueden tener daños importantes por enfermedades en vivero, entre las que destacan la pudrición de raíz y marchitez de plántulas. En Pinus spp. se les reconoce como un padecimiento en diversas regiones del mundo. Durante 2013 y 2014 se observaron tales síntomas, además de amarillamiento de plantas en estados iniciales de Pinus patula y P. pseudostrobus en un vivero forestal en el estado de Guerrero, México. Así, se propuso el presente estudio, cuyos objetivos consistieron en identificar a los hongos fitopatógenos asociados con ese problema, mediante la caracterización morfológica de las estructuras de reproducción asexual; análisis de secuencias ITS del ADNr y pruebas de patogenicidad; además de, determinar la incidencia y severidad de cada una de las especies de patógenos de interés. A partir de las plántulas con manifestaciones de daño de las dos especies de pino, se obtuvieron ejemplares aislados pertenecientes al género Fusarium. La caracterización morfológica, prueba de patogenicidad, y análisis de secuencias ITS de tales organismos evidenciaron que F. solani y F. oxysporum son las especies fúngicas responsables de ocasionar los síntomas de marchitez de plantas de Pinus. No se observó diferencia significativa entre la incidencia y la severidad de la enfermedad causada por dichos agentes.
Palabras clave: Fusarium oxysporum Schltdl.; Fusarium solani (Mart.) Sacc.; incidencia; patogenicidad; Pinus patula Schiede ex Schltdl. & Cham.; Pinus pseudostrobus Lindl
Introduction
The forest area of Mexico exceeds 144 million hectares, amounting to 74 % of the national territory. Almost 120 million hectares are severely exploited without correct management, resulting in significant deterioration (Cibrián et al., 2007).
The Mexican pine (Pinus patula Schiede ex Schltdl. & Cham.) is one of the main species used for intensive commercial plantations in Mexico and abroad, due to its exceptionally rapid growth rate, to the good shape of its stem and to the favorable characteristics of its timber for cellulose products (Dvorak et al., 2000). White pine (Pinus pseudostrobus Lindl.) has a wide natural geographical distribution and provides good-quality wood and high productivity (Perry, 2009).
In recent years, it has been deemed convenient to acknowledge the importance of forest diseases in four settings: natural forests, plantations, forest nurseries, and trees in urban areas. The damping-off or witlting of the seedlings is one of the first diseases that the nursery keepers face, causing between 15 and 40 % of loss (Cibrián et al., 2007). Besides being a limiting factor for the production of certain species, particularly conifers, it is one of the most serious obstacles to the large-scale production of plants (Torres, 2003).
The conifer trees can suffer significant damage due to diseases caused by Fusarium spp. in the nursery. These species of phytopathogenic fungi can cause damping-off in both pre- and post-emergence, as well as latent infections that can result in the failure of transplanted seedlings and in their establishment (Cibrián et al. 2007; Gordon et al., 2015). Fusarium oxysporum Schltdl. has been recognized as the main cause of root and hypocotyl rot in the nurseries. Furthermore, the management of root diseases caused by Fusarium in this environment has consisted primarily in soil fumigation before sowing (Gordon et al., 2015).
Based on this, the objectives proposed in this study were to identify the phytopathogenic species that cause the symptoms of root rot and seedling wilt in Pinus patula and P. pseudostrobus through the combination of morphological characterization, analysis of the sequences of the ITS regions of rDNA, and pathogenicity tests, besides determining the incidence and severity caused by the pathogens inducing that disease.
Materials and Methods
Sample collection
During 2013 and 2014, a directed sampling procedure was carried out in a forest nursery located in Chilpancingo, Guerrero, Mexico, where 20 Pinus patula and P. pseudostrobus seedlings with symptoms of root rot and wilting.
Fungi isolation, purification and preservation
In order to isolate the causative agent, fragments of P. patula and P. pseudostrobus roots with symptoms of dry rot were decontaminated by immersion in a sodium hypochlorite solution at 2 % during 3 minutes; the fragments were washed twice in sterilized distilled water and placed in Petri dishes with a 90 mm diameter in 20 mL of potato-dextrose-agar (PDA) culture medium (Difco) with streptomycin (SigmaTM). The dishes were placed in incubation at a temperature of 25 °C, under a regime of 12 h light/12 h darkness.
After three days of incubation, the fungal colonies which occurred most frequently were transferred to Petri dishes with a fresh PDA culture medium and placed in incubation under the conditions described above. Once the colonies produced spores, the isolates were purified using the monospore culture technique, transferring a simple spore to a fresh PDA culture medium. Fungal isolates were preserved through the transference of mycelial disks (of 5 mm diameter) to 2 mL cryogenic tubes with 1.5 mL glycerin at 20 % (v/v) and stored at a temperature of -80 °C.
Morphological characterization
The fungal isolates most frequently separated were identified through the morfological characterization of the asexual reproduction structures. For this purpose, they were transferred to a PDA culture medium and incubated at 25 °C under continuous light. After 12 days of inclubation, semipermanent preparations were made in glycerin at 100 % in order to examine their components under the Olympus BX41 compound microscope. 100 macroconidia and 100 microconidia were observed; their shape, size, color and number of septa were recorded. For the formation of phyalides, the fungal isolates had to be cultivated in a potato-agar culture medium for four days. Subsequently, a humid chamber was prepared in a 90 mm Petri dish with blotting paper moistened to saturation point with sterilized distilled water; 5 mm PDA disks with mycelium growth were transferred and incubated during three days, after which the phyalides produced in the humid chamber were observed under the described compound microscope. The fungi were morphologically identified at genus level using specialized codes (Barnett and Hunter,1998); their identification at species level was based on the descriptions by Leslie and Summerell (2006).
Pathogenicity test
The pathogenicity of six isolates morphologically identified as Fusarium was determined through the inoculation of 70 one-year-old Pinus patula and P. pseudostrobus seedlings. The inoculation was carried out by immersing the roots in a suspension of conidia (1 x 106 spores mL-1) during 10 hours. Ten seedlings of each species, whose roots were placed in sterilized distilled water during 10 h, served as controls. After this step was concluded, the seedlings were individually planted in flowerpots with peat moss and agrolite (2:1) and placed in a nursery at a temperature of 20 to 25 °C and a relative humidity of 50-80 %. The pathogenicity test was carried out twice.
The incidence of the disease was determined by the recorded symptoms and the number of dead trees. With these data, the area under the progress curve of the disease (AUDPC) using the trapezoidal integration method (Campbell and Madden, 1990). The severity of the disease was estimated for each fungal isolate and in each of the two Pinus species using the formula of Townsend and Hueberger:
Where:
n = Degree of infection according to the scale
v = Number of plants per category
N = Maximum degree of infection
V = Total number of plants
According to the various degrees of infection, an apparent severity rating scale was devised as follows: Class 0 = 0 % damage (asymptomatic plant); Class 1 = 20 % damage (fallen needles); Class 2 = 40 % damage (chlorosis or yellowing); Class 3 = 60 % damage (dry tips); Class 4 = 20 % damage (wilting); Class 5 = 20 % damage (dead plant). With the results obtained based on the interactions of each of the isolates and the two assessed pine species, disease progress curves were built and adjusted to epidemiological models (Logistic, Exponential, Monomolecular and Gompertz) in order to determine the parameters of intensity at the onset of the disease (0), and the increase rate (r) and thus, to obtain a signal of the form and development structure of the disease (Campbell and Madden, 1990). The epidemiological models and the AUDPC were adjusted using the SAS 9.3 software (SAS, 2011).
DNA extraction, amplification by PCR and sequentiation
Genomic DNA of six fungal isolates assessed in the pathogenicity test and morphologically identified as belonging to the Fusarium genus was extracted by macerating 50 to 100 mg of mycelium from 10 day old colonies. Subsequently, the protocol indicated in the Plant DNeasy Mini Kit (Qiagen®) extraction kit was followed. The quality of the DNA was verified by electrophoresis in agarose gel at 0.8 % with 0.5 X TBE buffer using 5μL of the DNA and run with 90 volts. The gel was analyzed in a Gel-Docmod 2000 (BIORAD®) transilluminator.
The universal initiators ITS5/ITS4 were used for the PCR (White et al., 1990). The reaction mixture was prepared at a final volume of 25 μL, with a 1X PCR buffer, 2.5 mM MgCl2, 0.2 mMdNTP, 0.4 μM of each primer, 1U of DNA polymerase (BioTecMol®) and 100 ng of DNA. The PCR was carried out in a C1000 (BIORAD®) thermocycler with an initial denaturalization of 2 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 55 °C, 1 min at 72 °C, and a final extension of 10 min at 72°C. The amplified products were verified by electrophoresis in agarose gel at 0.8 %. The gel was analyzed in a Gel-Docmod 2000 (BIORAD®) transilluminator.
The fragments amplified with the ITS5/ITS4 initiators were purified using the DNA clean and concentrator protocol (Zymo Research®). For this purpose, 5 volumes of the DNA Binding buffer were added in a 1.5 mL microcentrifuge tube and mixed by inversion. The mixture was transferred to a Zymo-Spin column in a 2 mL colection tube. It was centrifuged for 30 s at 8 000 rpm, and the supernatant was discarded. 200 μL of the Wash DNA buffer were added to the column and centrifuged for 30 s at 8 000 rpm. The supernatant was discarded, and the mixture was transferred to a new 2 mL tube. 60 μL of Elution DNA buffer were added directly to the column, and the product was incubated during 1 min. The column was transferred to a new 1.5 mL tube and the DNA was diluted. The purified DNA fragments were sent to the Corean enterprise Macrogen® for sequencing. The sequences thus obtained were compared in the database at the NCBI using the BLASTn tool.
Results and Discussion
Morphological identification
Six isolates were obtained from the symptomatic vegetal material; of these, two types were found to be the most frequently occurring: the first showed white-yellow mycelium growth, and the second, initially white mycelium growth that, however, turned purple-red with time; both growths were velvety. These types of colonies have been recorded for Fusarium species (Nelson et al., 1983; Leslie and Summerell, 2006).
The isolate with purple-violet mycelium growth in a PDA culture medium (Figure 1A-B) had 19-43 x 3.1-5.4 μm sickle-shaped, hyaline, thin-walled macroconidia with 3-5 septa and with a foot- shaped basal cell (Figure 1C). Elliptic to oval unicellular microconidia (Figure 1D). Short, sharp unbranched monophyalides (Figure 1E). Intercalary and terminal chlamydiospores in pairs (Figure 1F). All the characteristics agreed with those recorded by Leslie and Summerell (2006) for Fusarium oxysporum.
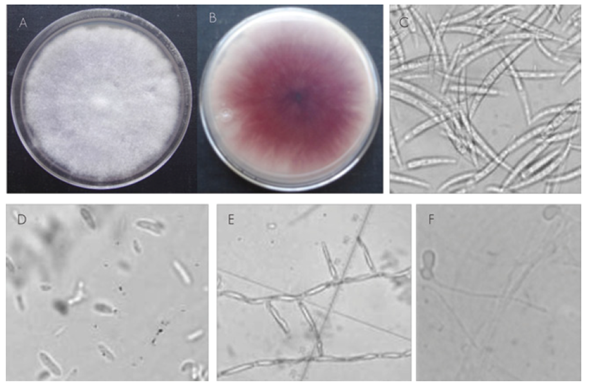
A) Eight-day colony; B) Reverse of the colony with a purple-red color; C) Macroconidia; D) Microconidia; E) Phialides; F) Chlamydospores
Figure 1 Morphology of colonies and asexual reproductive structures of Fusarium oxysporum Schltdl. taken from Pinus spp. with root rotting symptoms.
On the other hand, the fungal isolate with white-yellow cottony mycelium growth (Figure 2A-B) exhibited robust hyaline, fusiform macroconidia with 5-6 septa and with a slightly curved and pointed apical cell (Figure 2C). The microconidia were hyaline, oval and kidney-shaped, with 1-2 septa (Figure 2D), and produced in long monophyalides (Figure 2E). The chlamydospores were quickly formed; they were oval, intercalary between hyphae or terminals in the side branches of the hyphae, and occurred individually and in pairs.
All the characteristics agreed with the findings of Leslie and Summerell (2006) for the Fusarium solani (Mart.) Sacc. species (Teleomorfo: Haematonectria haematococca).
Analysis of the sequences of ITS regions
The sequences obtained from the DNA of the six fungal isolates morphologically identified as Fusarium were compared with the sequences submitted to the database of the GenBank, and five of them (KU056813, KU056814, KU056816, KU056817, KU056818) showed 99 % identity with F. oxysporum, while only one (KU056815) exhibited 99 % with those of Fusarium solani. This confirmed the results of the morphological characterization, which indicates that both species of the genus of interest are causative agents of root diseases in Pinus spp. in Mexico.
Fusarium oxysporum has been recognized as an infectious agent of Pinus strobus L. in the United States of America (Ocamb and Juzwik, 1995; Ocamb et al., 2002); P. elliottii Engelm. and P. taeda L. in Argentina (Lori and Salerno, 2003); P. wallichiana A. B. Jacks in India (Hassan et al., 2011); P. halepensis Miller in Algeria (Lazreg et al., 2014), and P. tecunumanii F. Schwerdtf. ex Eguiluz & J. P. Perry in Colombia (Herron et al., 2015). Fusarium solani has also been associated with Pinus strobus tissues in the United States of America (Ocamb and Juzwik, 1995); P. radiata D. Don in New Zealand (Dick and Dobbie, 2002); P. elliottii and P. taeda in Argentina (Lori and Salerno, 2003); P. tropicalis Morelet in Cuba (Guerra et al., 2004), and P. halepensis in Algeria (Lazreg et al., 2014).
Pathogenicity test
Fifteen days after inoculation (DAI), all the plants inoculated with the spores’ suspension exhibited symptoms of wilting, while the control seedlings remained asymptomatic. Fusarium colonies were re-isolated from the inoculated specimens and exhibited the same morphological characteristics as the originally inoculated colonies, whereby Koch’s postulates were completed. This confirmed that Fusarium oxysporum and F. solani are the species of phytopathogenic fungi that cause the symptoms of root rot and wilting of P. patula and P. pseudostrobus seedlings in Guerrero, Mexico. Similarly, Herron et al. (2015) carried out successful pathogenicity tests with F. circinatum Nirenberg & O’Donnell, F. marasasianum Herron, Marinc. & M. J. Wingf., F. parvisorum Herron, Marinc. & M. J. Wingf. and F. sororula Herron, Marinc. & M. J. Wingf. isolates in Pinus patula seedlings in Colombia, while Latiffah et al. (2009) identified F. solani and F. oxysporum as the prevailing species in the soils of a forest area in Malasya, but did not determine the pathogenicity of the isolates.
Incidence a severity of the disease
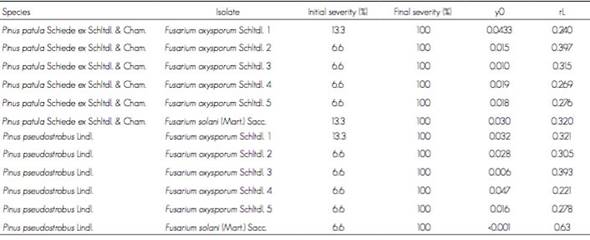
In regard to the incidence of the disease, F. oxysporum isolate 1 had 100 % only in P. pseudostrobus 8 DAI, while F. oxysporum isolate 2 registered the same percentage during the same period for the two Pinus species. Likewise, F. solani had 100 % in both Pinus species 14 DAI (Table 1). This indicates that the F. oxysporum isolate 2 causes disease faster than the other isolates, due to a positive combination of initial intensity of the disease and an increase rate, while this was not the case with the other isolates, in which total infestation occurred less rapidly.
Table 1 Area under the progress curve of the disease (AUDPC) in Pinus patula Schiede ex Schltdl. & Cham. and Pinus pseudostrobus Lindl. seedlings inoculated with five Fusarium oxysporum Schltdl. isolates and a Fusarium solani (Mart.) Sacc. isolate.

When the severity of each Fusarium isolate was determined, F. oxysporum isolate 1 in P. pseudostrobus had the highest value, while the F. oxysporum isolate 2 was the most virulent in P. patula, having killed the trees 21 DAI. Furthermore, it should be emphasized that the F. solani isolate caused the same effect on the inoculated seedlings of both species. This agrees with the findings of Lazreg et al. (2014), who identified seven Fusarium species associated to P. halepensis seedlings, defined significant differences in shoot and root length and in the vigor index in seedlings inoculated with the various isolates; likewise, they pointed out that F. solani stood out from among the other species of the genus for the rapidity with which they colonized the complete roots of the inoculated pine trees.
The logistic model (y = 1 / (1 + ((1-I) / I) * exp (-rL * t)) was the one that best described the progression of symptoms in both pine species in the presence of the Fusarium isolates evaluated over time. In the case of isolate 2 of F. oxysporum in P. pseudostrobus, it was observed that the initial intensity of the disease was higher with respect to P. patula, but the speed of increase of severity was lower. For the F. solani isolate, the two species of pine showed an initial intensity of disease 0.030 % and a rate of increase of severity percentage of 0.32 per day. Therefore, the Fusarium oxysporum isolate 2 was the most virulent, as it grew 0.397 % per day compared to the F. solani isolate, which showed a growth of 0.32 % per day (Table 2). This is indicative that a higher initial intensity disease affects its development; in ABCPE, the initial intensity is related to the amount of inoculum at time 0, but this inoculum will increase as the rate of increase does, which is also subject to the virulence of the pathogen and to the pine species.
Cuadro 2 Ajuste de modelo logístico del progreso de la severidad de cinco aislados de Fusarium oxysporum Schltdl. y un aislado de Fusarium solani (Mart.) Sacc. inoculados en plántulas de Pinus patula Schiede ex Schltdl. & Cham. y Pinus pseudostrobus Lindl.
Similarly, Herron et al. (2015) determined that various Fusarium marasasianum, F. parvisorum and F. sororula isolates caused disease in Pinus patula seedlings; furthermore, they pointed out that there was a variation in the pathogenicity and virulence between fungal isolates of the same species.
Conclusions
Based on the combination of data of the morphological characterization, analysis of sequences of ITS regions and pathogenicity tests, Fusarium solani and F. oxysporum were shown to be the fungal species that cause root rot and wilting of Pinus patula and P. pseudostrobus plants in Guerrero, Mexico. Also, no differences were observed between the incidence and severity rates of the disease caused by five F. oxysporum isolates and a F. solani isolate.
REFERENCES
Barnett, H. L. and B. B. Hunter. 1998. Illustrated genera of imperfect fungi. The American Phytopathological Society. St Paul, MN, USA. 218 p. [ Links ]
Campbell, C. L. and L. V. Madden. 1990. Introduction to plant disease epidemiology. Wiley-Interscience. New York, NY, USA. 532 p. [ Links ]
Cibrián T., D., D. Alvarado R. y S. E. García D. 2007. Enfermedades forestales en México/Forest diseases in Mexico. Universidad Autónoma Chapingo-Conafor-Semarnat, USDA Forest Service, Natural Resources Canada, Conafor. Chapingo, Texcoco, Edo. de Méx., México. 587 p. [ Links ]
Dick, M. A. and K. Dobbie. 2002. Species of Fusarium on Pinus radiate in New Zealand. New Zealand Plant Protection 55: 58-62. [ Links ]
Dvorak, W. S., G. R. Hodge, J. E. Kietzka, F. Malan, L. F. Osorio and T. K. Stangen. 2000. Pinus patula. In: Conservation and testing of tropical and subtropical forest tree species by the CAMCORE Cooperative. USA CAMCORE Cooperative, North Carolina State University. Raleigh, NC, USA. 234 p. [ Links ]
Gordon, T. R., C. L. Swett and M. J. Wingfield. 2015. Management of Fusarium diseases affecting conifers. Crop Protection 73: 28-39. [ Links ]
Guerra, C., H. Cruz, I. Vila, A. Duarte y M. O. López. 2004. Principales hongos que afectan a Pinus tropicalis Morelet en Cuba. Fitosanidad 8(2): 9-12. [ Links ]
Hassan D. G., M. A. Beig, F. A. Ahanger, N. A. Ganai and M. A. Ahangar. 2011. Management of root rot caused by Rhizoctonia solani and Fusarium oxysporum in blue pine (Pinus wallichiana) through use of fungal antagonims. Asian Journal of Plant Pathology 5(2): 62-74. [ Links ]
Herron, D. A., M. J. Wingfield , B. D. Wingfield, C. A. Rodas, S. Marincowitz and E. T. Steenkamp. 2015. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Studies in Mycology 80: 131-150. [ Links ]
Latiffah, Z., M. I. Padzilah, S. Baharuddin and Z. Maziah. 2009. Fusarium species in forest soil of Bird Valley. Malaysian Journal of Microbiology 5(2): 132-133. [ Links ]
Lazreg, F., L. Belabid, J. Sánchez, E. Gallego and B. Bayaa. 2014. Pathogenicity of Fusarium spp. associated with diseases of Aleppo-pine seedlings in Algerian forest nurseries. Journal of Forest Science 60(3): 15- 120. [ Links ]
Leslie, J. F. and B. A. Summerell. 2006. The Fusarium laboratory manual. Wiley- Blackwell Publishing. Ames, IA, USA. 388 p. [ Links ]
Lori, G. A. and M. I. Salerno. 2003. Fusarium species on seeds of Pinus taeda and Pinus elliottii in Argentina. Journal of Plant Diseases and Protection 10(5): 437-443. [ Links ]
Nelson, P. E., T. A. Toussoun and W. F. O. Marasas. 1983. Fusarium species: An illustrated manual for identification. Pennsylvania State University Press, University Park, PA, USA. 226 p. [ Links ]
Ocamb, C. M. and J. Juzwik. 1995. Fusarium species associated with rhizosphere soil and diseased roots of Eastern white pine seedlings and associated nursery soil. Canadian Journal of Plant Pathology 17: 325-330. [ Links ]
Ocamb, C. M. , J. Juzwik and F. B. Martin. 2002. Fusarium spp. and Pinus strobus seedlings: root disease pathogens and taxa associated with seed. New Forests 24: 67-79. [ Links ]
Perry, J. P. 2009. The pines of Mexico and Central America. Timber Press. Portland, OR, USA. 234 p. [ Links ]
Statistical Analysis Systems (SAS). 2011. User’s guide. Version 9.3. Cary, NC, USA. 2323 p. [ Links ]
Torres J. J. 2003. Patología forestal. Mundiprensa. Madrid, España. 270 p. [ Links ]
White, T. J., T. Burns, S. Lee and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A., D. Gelfald, J. Sninsky and T. J. White (eds.). PCR protocol: a guide to methods and application. Academic Press, Inc., New York, NY, USA. pp. 315-322. [ Links ]
b Contribution by autor: Leticia Robles Yerena: collection of vegetal materials, isolation of fungi, pathogenicity tests in the nursery, data collection and drafting of the manuscript; Santos Gerardo Leyva Mir: collection of vegetal materials, definition of the experimental design and revision of the manuscript; Armando Cruz Gómez: purification and morphological characterization of the fungi; Moisés Camacho Tapia: DNA extraction and amplification by PCR; Daniel Nieto Ángel: collection of vegetal materials and contribution of laboratory materials; Juan Manuel Tovar Pedraza: definition of the study, analysis of DNA sequences and revision of the manuscript.
Received: June 02, 2016; Accepted: July 29, 2016











 texto em
texto em 



