Introduction
Different environmental factors can cause stress in poultry farming. Environmental temperature is an important factor in poultry production since it affects the performance of the animal and causes economic problems1-4. In general, the thermoneutral temperature has been reported as 16-25 ˚C in poultry5. It has been reported that physiologically stress occurs if the ambient temperature remains above the thermoneutral temperature6. When exposed to stress, adrenocorticotropic hormone (ACTH) is secreted depending on CRH that secretes from the hypothalamus. ACTH provides the secretion of corticosteroids and adrenaline. Thus; glucose, lipid and protein metabolisms are regulated by secreting high amounts of corticosteroids into the environment as metabolic adaptation during heat stress7-9. Metabolism, nutrition and environmental conditions are effective on the acid-base balance of the body. The most important parameters that indicate the acid-base state of the blood are blood pH, bicarbonate (HCO3 -), and the concentrations of sodium (Na+), potassium (K+) and chlorine (Cl−) ions. Monovalent minerals play an important role for the acid-base balance10-12. Animals maintain homeostasis under heat stress conditions through vasodilatation, convection, and evaporation13. Initially, environmental stress factors alter metabolic functioning in poultry and causes the production of glucose to maintain homeostasis during the presence of stressors. At heat stress, air sacs play an important role in gas exchange, as they increase air circulation to the surface which results in evaporation that causes heat to spread14.
Due to stress, oxidation occurs in the structure of proteins and DNA in the blood and the tissues. As a result of heat stress, an increase in heat shock proteins is observed15. Heat shock proteins (HSP) are a family of proteins produced by cells in response to stressors that are or are not related to temperature16. HSPs are an important family of proteins that have been preserved throughout evolution and are expressed in all living things from prokaryotes to eukaryotes. HSPs have performed tasks such as folding newly synthesized proteins in the cell, preventing protein aggregation, stabilizing proteins, and eliminating misfolded proteins. HSPs are divided into five main classes according to their molecular mass: small HSPs (<40 kDa), HSP60 (60 kDa), HSP70 (70 kDa), HSP90 (90 kDa) and HSP100 (100 kDa). Each HSP has different isoforms, and they are localized in different parts of the cell. The Hsp70 molecular chaperone plays a central role in protein quality control. By binding to Hsp70 protein substrates, they help them fold, break down, transfer, regulate, and prevent clustering. Hsp70 substrate binds to hydrophobic regions in proteins and helps the newly synthesized proteins and partially folded proteins to fold correctly17-21.
Previous studies reported that heat stress causes poor performance in the animal and suppresses the immune system22. Following the heat stress; decrease in live weight, paleness in the color of meat23, low immunity, fluid-electrolyte balance and irregularity in blood pH24, even cases such as sudden death can be observed in broilers. When heat stress occurs in broilers, acid-base balance disturbance and respiratory alkalosis may occur25.
Hesperidin is an effective antioxidant that reduces oxidative stress. It also inhibits lipid peroxidation26,27. It has been reported that the concentration of lactate dehydrogenase and heat shock protein (Hsp70), which are markers of heat stress, decreases with the addition of hesperidin to poultry rations28. It has been reported that in order to overcome the negative effects of heat stress on Japanese quails, a good nutrition strategy should be administered29. Diets supplemented with hesperidin provide an alternative to the use of synthetic additives, can improve the lipid profile of chicken meat, and ensure higher quality poultry meat production30,31. Additionally, recent studies have reported that the contribution of hesperidin to the ration has positive effects on meat quality, egg quality and intestinal micro flora in quails32-34.
Heat stress has been shown to have adverse effects on broilers, including increased feed consumption as well as reduced growth rate and vitality of broilers35. In addition, it may decrease the quality of the products obtained from broilers by increasing their abdominal fat36. In current study, the effects of hesperidin, a citrus by-product, on blood parameters and HSP 70 levels will be determined.
Material and methods
In the study, 160 quails (Coturnix coturnix japonica, male) at the age of 6 wk with a live weight of 150-200 g were housed in cages for 1 week of exercise and for 5 wk of experimental period with 10 quails per cage, a total of 42 d. Quails (45cm width X 20cm height X 90cm length) were housed in cages. The study design consists of 4 groups with 40 animals and 4 subgroups within each group. Thermoneutral (24 ± 0.1 ˚C) groups are NC (0 g hesperidin/kg basal feed) and NHES3 (3 g hesperidin /kg basal feed) and heat stress (34 ± 0.1 ˚C) groups are HC (0 g hesperidin/kg basal feed) and HHES3 (3 g hesperidin /kg basal feed) were randomly generated. The hesperidin (C28H34015, cas no: 520-26-13, 91 % purity, Chem-Impex International Company, USA) used in the study was commercially available. The rations used in the experiment were formulated according to the recommendations of the NRC37 (Table 1).
Table 1 Diet compositions used in the experiment
| Diets***** | ||||
|---|---|---|---|---|
| Thermoneutral | Heat stress | |||
| Ingredients, % | NC | NHES3 | HC | HHES3 |
| Wheat | 52.03 | 52.03 | 52.03 | 52.03 |
| Maize | 10.42 | 10.42 | 10.42 | 10.42 |
| Vegetable oil | 2.76 | 2.76 | 2.76 | 2.76 |
| Soybean meal, %48 | 27.52 | 27.52 | 27.52 | 27.52 |
| Limestone* | 5.55 | 5.25 | 5.55 | 5.25 |
| Dicalcium phosphate | 1.17 | 1.17 | 1.17 | 1.17 |
| Salt | 0.26 | 0.26 | 0.26 | 0.26 |
| Vitamin-mineral premix** | 0.25 | 0.25 | 0.25 | 0.25 |
| L threonine | 0.03 | 0.03 | 0.03 | 0.03 |
| Hesperidin*** | - | 0.30 | - | 0.30 |
| Calculated values | ||||
| Dry matter, % | 90.30 | 90.30 | 90.30 | 90.30 |
| Crude protein, % | 19.96 | 19.96 | 19.96 | 19.96 |
| Crude ash, % | 9.80 | 9.50 | 9.80 | 9.50 |
| Crude cellulose, % | 2.86 | 2.86 | 2.86 | 2.86 |
| Ether extract, % | 4.56 | 4.56 | 4.56 | 4.56 |
| Metabolic energy, kcal/kg | 2900 | 2900 | 2900 | 2900 |
| Calcium, % | 2.50 | 2.38 | 2.50 | 2.38 |
| Available phosphorus, % | 0.35 | 0.35 | 0.35 | 0.35 |
| Methionine +cystine, % | 0.64 | 0.64 | 0.64 | 0.64 |
| Lysine, % | 1.00 | 1.00 | 1.00 | 1.00 |
| Threonine, % | 0.74 | 0.74 | 0.74 | 0.74 |
| Tryptophan, % | 0.27 | 0.27 | 0.27 | 0.27 |
* *Limestone was reduced and added instead of hesperidin in the experimenting groups.
**Vitamin-Mineral premix contained per kg: mg: retinol (vit A) 3, tocopherol (vit E) 30, menadione (vit K3) 5, thiamine (vit B1) 1, riboflavin (vit B2) 5, pyridoxin (vit B6) 3, nicotinic acid 30, pantothenic acid 10, folic acid 0.8, ascorbic acid (vit C) 10, choline chloride 450, Co 0.2, I 0.5, Se 0.3, Fe 25, Mn 120, Cu 10, Zn 100; μg: cholecalciferol (vit D3) 62.5, cobalamin (vit B12) 20, biotin 100 μg.
*** Hesperidin obtained from Chem-Impex Int. company, molecule formula (C28H34O15), cas no (520-26-13), purity grade 91% (Chem-Impex, Wood Dale, IL, USA).
****NC= Control (0g hesperidin/kg feed), (24 ± 0.1 ˚C); NHES3: thermoneutral temperature (24 ± 0.1 ˚C), (3g hesperidin/kg feed); HC= heat stress temperature (34 ± 0.1 ˚C); HHES3= heat stress temperature (34 ± 0.1 ˚C), (3g hesperidin/kg feed).
In the study, granular feed and water were given ad libitum to animals. During the study period, there was a relative humidity of 50-60 % in the cages. Fluorescent lamps were used for the lighting of the trial room, and a timer (Cata CT 9181, China) was used during the trial to provide a 16-h light 8-h darkness along with sunlight. In order to create heat stress in the cages, electric heaters were used for heating up the compartments. The trial cage was kept at the room temperature, while the group subjected to stress using the electrical thermostat control heaters during the trial period was kept at 34 ± 0.1 ˚C, and the quails in the thermoneutral group were kept at 24 ± 0.1 ˚C. During the experimentation period, the relative humidity of the trial room was constantly measured with a hygrometer and kept under control. Electric fans were used to regulate air circulation and get rid of the accumulated dust and harmful gases in the cages.
Ethical approval
This study has been conducted with the permission of Tokat Gaziosmanpaşa University, Animal Experiments Local Ethics Committee dated 20.05.2021 and numbered 51879863-36.
Biochemical analysis of blood gas and serum
At the end of the trial, 3 animals were randomly selected from each subgroup, which equals to 12 from each group and a total of 48 overall. Blood samples from the Vena saphena brachialis were taken before slaughter, and the blood gas values were determined by photometric method using a commercial kit (epoc BGEM blood test, Germany). Immediately after the blood samples were taken, they were centrifuged for 10 min at 3,000 rpm, and then the serum collected at the top was transferred to 2 ml Eppendorf tubes. The serums were frozen and stored for analysis in a freezer at -80 °C. Biochemical values were detected in blood serum samples using an autoanalyzer device (Mindray BS200, China).
Hsp70 gene expression analysis
At the end of the study, tissue samples of 2-3 g were taken from liver, kidney, and breast muscles from each animal under hygienic conditions. The tissue and blood samples were then stored at -80 °C for Hsp70 gene analysis. After 0.9 ml of physiological saline was added to the 0.1 g tissue sample weighed, the tissue samples (0.1 g) were homogenized in a homogenization buffer (0.15 M NaCl, 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, 1 mM PMSF, 0.1 M). E-46, 0.08 µM of aprotinin, 0.1 µM of leupeptin and 0.1% NP-4038) and homogenates were centrifuged at 4 °C for 20 min at 12,000 ×g using an Ultra-turrax homogenizer on ice. The supernatant was collected and stored at -20 °C until protein determination. The amount of protein was determined using ELISA (BT-LAB, E0124Ch). The standard curve and immunological detection of proteins have been carried out mainly according to the manufacturer's instructions.
Results
Within the scope of the experiment, there is a statistically significant difference between thermoneutral and heat stress groups in terms of pCO2, pO2, pH, HCO3, Na, K, Cl concentrations among the blood parameters (P<0.05); however, all groups yielded the same results in terms of hematocrit and hemoglobin (P>0.05). In blood gas parameters, pCO2 was the lowest in heat stress group HC; it was highest in the HHES3 group (P<0.05). pO2 was highest in the HC group, which is the heat stress group, and lowest in the HHES3 group (P<0.05). Blood pH was the lowest in the HHES3 group; it was highest in the HC group (P<0.05). The blood Na and Cl concentrations were the lowest in the heat stress groups; was highest in thermoneutral groups (P<0.05). The K concentration was the lowest in the HC group and the highest in the HHES3 group (P<0.05) (Table 2).
Table 2 The effect of adding hesperidin to quail diets at thermoneutral and heat stress on blood gas parameters
| Thermoneutral (24 ˚C) | Heat stress (34˚C) | ||||
|---|---|---|---|---|---|
| NC | NHES3 | HC | HHES3 | p | |
| Hgb, g/dL | 12.34±0.53 | 12.40±0.50 | 10.80±0.07 | 11.20±0.58 | 0.06 |
| PCO2, mmHg | 37.99±0.18b | 38.13±0.14b | 31.24±0.06c | 48.85±2.20a | 0.001* |
| PO2, mmHg | 46.81±0.69c | 50.16±0.50ab | 52.36±0.02a | 40.09±1.16d | 0.001* |
| Hct, % | 36.53±1.62 | 36.26±1.45 | 31.81±0.30 | 32.70±1.66 | 0.05 |
| pH | 7.42±0.01b | 7.41±0.001b | 7.53±0.01a | 7.31±0.02c | 0.001* |
| HCO3, mmol/L | 23.84±0.01b | 24.13±0.06b | 25.92±0.44a | 24.60±0.03b | 0.001* |
| Na, mmol/L | 162.97±0.74a | 159.23±0.56b | 144.41±0.15d | 147.85±0.83c | 0.001* |
| K, mmol/L | 4.55±0.09b | 4.73±0.05b | 3.94±0.01c | 5.93±0.17a | 0.001* |
| CI, mmol/L | 129.35±1.33a | 118.81±1.79b | 109.00±0.001c | 108.48±0.14c | 0.001* |
Hgb= hemoglobin, PCO2:= partial carbon dioxide pressure, PO2= partial oxygen pressure, Hct= hematocrit, pH= potential of hydrogen, HCO3= hydrogencarbonate, Na= natrium, K= potassium, Cl= chloride.
*There is a statistically significant difference between the experimental groups (P<0.05).
In addition, serum alkaline phosphatase (ALP) enzyme concentration in the blood serum parameters was the lowest in the HHES3 group; was highest in the HC group (P<0.05); however, all groups are similar in terms of other parameters (Table 3).
Table 3 Effects of adding hesperidin to quail diets at thermoneutral and heat stresss on blood serum parameters
| Thermoneutral (24 ˚C) | Heat stress (34 ˚C) | ||||
|---|---|---|---|---|---|
| NC | NHES3 | HC | HHES3 | P | |
| Glucose, mg/dl | 169.62±30.94 | 144.50±32.89 | 204.44±35.20 | 165.39±23.14 | 0.59 |
| Triglyseride, mg/dl | 1156.98±69.06 | 983.02±176.00 | 1225.63±3.60 | 1022.87±141.10 | 0.48 |
| HDL, mg/dl | 59.40±20.54 | 26.83±13.27 | 81.90±17.21 | 43.98±11.74 | 0.20 |
| Total cholestrol, mg/dl | 313.03±43.29 | 262.43±15.81 | 268.06±18.46 | 256.43±10.26 | 0.33 |
| Total protein, mg/dl | 4.52±0.24 | 4.69±0.27 | 5.32±0.51 | 4.72±0.16 | 0.31 |
| Albumin, g/dl | 1.90±0.11 | 1.87±0.06 | 1.79±0.06 | 1.79±0.08 | 0.69 |
| Globulin, g/dl | 3.40±0.41 | 2.82±0.21 | 2.94±0.13 | 2.73±0.16 | 0.23 |
| ALT, u/l | 6.50±0.92 | 5.67±0.21 | 8.44±1.09 | 6.14±1.03 | 0.17 |
| AST, u/l | 194.00±22.52 | 181.83±10.64 | 216.33±17.64 | 199.86±26.28 | 0.67 |
| ALP, u/l | 845.93±161.17ab | 622.83±154.0b | 1272.91±146.42a | 507.16±67.60b | 0.001* |
| Ca, mg/dl | 26.21±3.11 | 22.17±3.18 | 21.36±1.14 | 22.40±2.67 | 0.60 |
| Mg, mg/dl | 7.11±0.44 | 6.91±0.51 | 6.77±0.27 | 6.88±0.30 | 0.94 |
| P, mg/dl | 11.60±1.11 | 12.03±1.46 | 12.27±0.81 | 12.68±0.89 | 0.90 |
HDL= high density lipoprotein, ALT= alanine transaminase: AST= aspartate transaminase: ALP= alkaline phosphatase, LDH= lactate dehydrogenase, Ca= calcium, Mg= magnesium, P= phosphor.
*There is a statistically significant difference between the experimental groups (P<0.05).
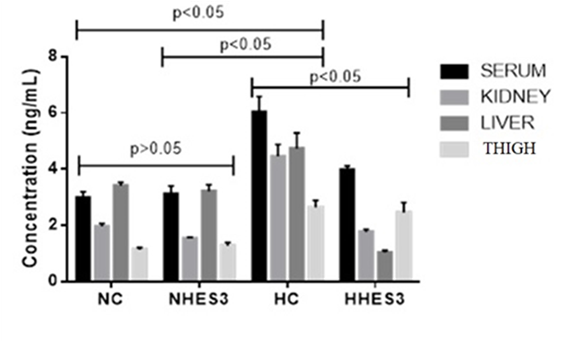
In terms of HSP 70 parameter, it was the lowest in thigh tissue in thermoneutral groups (P>0.05). Concentration was similar in all tissues in thermoneutral groups (P>0.05). In the heat stress groups, the serum concentration was highest in the HC group, but lower in the HHES3 group (P<0.05). Concentration in liver and kidney tissues was high in the HC group under heat stress, while it was significantly lower in the HHES3 group. In addition, in the heat stress groups, the Hsp70 concentration in the liver tissue was the lowest in the HHES3 group (P<0.05) (Figure 1).
Discussion
There are many studies on the effect of heat stress on the addition of vitamins, amino acids and minerals in poultry feed3,5. Current study was conducted to observe the effects of hesperidin, a flavonoid included in the diet, on blood biochemistry and expression of Hsp70 in quails exposed to heat stress.
Depending on the increase in environment temperature, some changes occur in the blood and metabolism. In case of rapid breathing, high loss of carbon dioxide, a decrease in partial CO2 pressure (pCO2) in the blood and an increase in blood pH occur. Hyperventilation alters the acid-base balance in poultry through the development of respiratory alkalosis39. In the current study, pCO2 pressure was lowest in the HC group while pO2 pressure was highest. While the highest pCO2 pressure was seen in the HHES3 group, the pO2 pressure was the lowest. While pCO2 and pO2 pressures, which were similar in the thermoneutral group, increased in the heat stress groups, pCO2 increased as expected, the pO2 decreased. Attia et al3 reported that the addition of amino acids to the ration in broilers at heat stress was slightly higher blood pH in the heat stress control group. Depending on the ambient temperature, the highest pH in the HC group and the decrease in pH in the HHES3 group may be associated with hesperidin supplementation. In current study, a decrease in blood pH level below the neutral pH level (7.35), an increase in pCO2 to 48 mmHg, and a decrease in PO2 to 40 mmHg in the HHES3 group at heat stress is seen as a table of respiratory acidosis. Additionally, blood HCO3 concentration is highest in the HC group, while it is at the level of thermoneutral groups in the HHES3 group. No compensation effect on blood pH due to the addition of hesperidin has been observed.
While the blood hemoglobin concentration was lowest in the HC group, it approached that in the thermoneutral groups in the HHES3 group. In previous studies, it has also been reported that the hemoglobin concentration in the blood that occurs at normal temperature tends to decrease due to an increase in heat stress40,41.
In general, Na, K and Cl concentrations are important for blood acid base balance in terms of pH. The blood pH rises with the formation of respiratory alkalosis. In the current study, blood pH was highest in the HC group from the heat stress groups, as expected, depending on the alkalosis status. However, blood pH shifted to neutral pH in the HHES3 group, which was thought to be related to the contribution of hesperidin. Contrary to the thermoneutral groups, the Na concentration, which is an important cation, is not expected to be the lowest in the HC group. Likewise, the K concentration in the HC group has also showed a decrease. Although Na and K concentrations showed a general similarity in the normal and heat stress groups in current study, higher blood K and lower blood Cl concentration have been observed in the HHES3 group with a low pH. Similarly, in a study conducted on the effects of heat stress on dairy cattle, it has been reported that a decrease in Na and K concentrations in the rumen fluid causes urinary excretion of Na and loss of K in the skin42. The normal interval for the concentration of chlorine in the blood is between 97 and 107 mEq/L. It has been reported that, when stress occurs in the body, electrolyte levels may become irregular; hence, an increase in the chlorine concentration in the blood occurs43. However, in current study, while the blood chlorine concentration is above the normal levels at thermoneutral groups, it is thought that the blood chlorine concentration of an animal under heat stress had hit the upper limit as a result of compensation.
It has been reported that gluconeogenesis is stimulated by increasing the number of free radicals in the environment due to heat stress, secreting the ACTH and cortisol hormones, and preventing insulin release from β-cells in the pancreas; thus, increasing the serum glucose levels44. Rudich et al45 have reported in their study that oxidative stress conditions negatively affect insulin secretion. In current study, it has also been determined that the blood glucose level is lower at thermoneutral groups than it is at HC group. As a result, as in previous studies46,47, blood glucose concentration increased in the HC group under heat stress; and hesperidin in the NHES3 group and the lowest concentration in the HHES3 group in heat stress compared to the HC group. It has been reported that stress caused by the administration of adrenocorticotropic hormone (ACTH), one of the stress hormones, increases the blood glucose, cholesterol, and high-density lipoprotein (HDL) levels, but reduces the triglyceride level48. Moeni et al49 reported that chromium contribution to broiler rations reduces blood triglyceride, cholesterol and LDL levels but increases cholesterol and HDL concentrations.
As reported in previous studies, it was observed that the blood triglyceride concentration was highest in the HC group under heat stress and close in the HHES3 group, while it was close in the NHES3 group. That means that the concentration of triglycerides in the blood has increased due to the heat stress. According to Rashidi et al47, this increase in the level of lipids in the blood is due to heat stress, a decrease in feed consumption, and the provision of energy needs by mobilization of lipid resources. On the other hand, in current study, they reported that the addition of organic chromium and selenium to the ration decreased serum lipid content, similar to the decrease in serum total cholesterol and triglyceride levels in the NHES3 and HHES3 groups compared to the HC group. It was observed that the blood HDL concentration in thermoneutral groups was lower in the NHES3 and HHES3 groups compared to the NC group under heat stress, compared to the HC group. As a result, it is believed that the HDL ratio decreased with the addition of hesperidin.
Additionally, in support of the current work, Moeini et al49 reported that, when stress was created at heat stress (33 ± 3 ˚C), the total cholesterol level decreased in the trial groups where organic chromium was added compared to the control group, depending on the increase in the dose. Similarly, in the current study, the total cholesterol concentration in NHES3 group decreased without dependence on heat stress. The group with the additional hesperidin, which is at a heat stress, has the lowest cholesterol level. It is believed that the decrease in total cholesterol concentration in the HHES3 group compared to both the normal and the heat stress control group occurred due to hesperidin addition of 3 g/kg and the dosage.
Oxidative stress caused by heat stress increases the production of free radicals, which leads to oxidation of the cell membrane, lipid peroxidation that leads to hepatocellular damage, increase in the intracellular enzyme levels, which include aspartate aminotransferase (AST) and Lactate dehydrogenase (LDH). There is a statistically significant difference between normal and heat stress groups in terms of blood serum ALP enzyme levels (P<0.05). In the current study, the blood serum concentration of alkaline phosphatase (ALP) enzyme in the HC group under heat stress increased due to heat stress. However, a significant decrease observed in both the NHES3 group in the thermoneutral groups and the HHES3 group, which is the heat stress group, may be due to hesperidin supplementation. In general, the concentrations of ALT, AST, ALP and LDH enzyme have been observed to change at both thermoneutral and heat stress groups due to the contribution of hesperidin. Mehaisen et al50 observed a similar increase in ALT, AST enzyme concentration in the heat stress control group due to the addition of propolis to the ration on heat stress. In the same study, it was reported that the ALT and AST enzyme levels were decreased with the addition of propolis in the trial groups in a similar way they were in the current study. The results obtained in current study are consistent with previous studies51,52. In current study, the AST level has been observed to be slightly higher at thermoneutral groups than the heat stress groups. However, AST data obtained in the heat stress study conducted by Abdelhady et al53 have reported a lower concentration than current study.
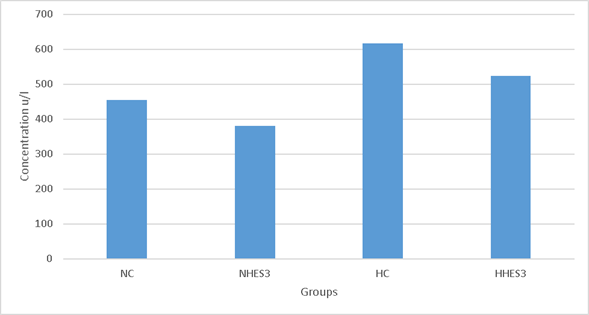
In Figure 2, it was observed that the enzyme level of LDH was lower in heat stress groups compared to thermoneutral groups, but both NHES3 and HHES3 groups were lower than NC and HC groups. Similarly to current study, Al-Mashhadini et al54 have reported that the use of sesame oil on animals exposed to heat stress has reduced the blood LDH enzyme concentration in the group fed with additional sesame oil compared to the control group at normal temperature, that the LDH enzyme concentration increased due to the stress effect in the control group at heat stress, and that the LDH enzyme concentration was found to be lower in the group fed with additional sesame oil than in the control group. Additionally, there are multiple studies reporting that the blood LDH concentration increases due to heat stress in poultry exposed to 41-42 ˚C temperature55,56.
In current study, total protein levels have been observed to have a little bit higher concentration at thermoneutral than at heat stress (Table 3). The addition of hesperidin to the ration in HHES3 group increased the protein level. The total protein level increased under HC groups in heat stress, but a decrease has been observed in the HHES3 group with the additional hesperidin. A high level of total protein at heat stress is associated with an increase in the concentration of heat shock proteins (Hsp70)57,58.
The albumin concentration in the blood serum were a similar concentration at thermoneutral groups as the heat stress groups. As a result, except for a slight decrease in the albumin level from thermoneutral to heat stress, it is believed that the hesperidin addition does not have a positive effect on the albumin concentration in the blood. Effect of vitamin E on heat stress, Şahin59 has reported that heat stress inhibited the total, there is a similar situation in terms of globulin. A lower level of globulin was found at heat stress than normal temperature.
Among the recent studies on heat stress, Al-Mashhadani et al54 have determined the effect of sesame oil on heat stress and found that the concentration of albumin in the blood increased towards heat stress group; however, the group with heat stress and sesame oil has presented a decrease, like current study. Known as a molecular chaperone, Hsp70 is a protein that has been preserved throughout evolution and it is produced by the cells of all living things in response to stress stimuli. Hsp70 levels are quite high at the first times when cellular stress commences. Hsp70 is vital in all stages of cell metabolism, including growth, differentiation, division, and even cell death. In particular, heat stress and the amount of ROS that increases accordingly disrupt the 3-dimensional structures and stability of proteins in cells, leading to their denaturation. Cellular stress factors in the cytosol complicate the protein folding process. Therefore, protein quality control is necessary for the cell to maintain its viability. Hsp70 has functions such as correct folding of newly synthesized protein chains, inter-membrane protein translocation, inhibition of protein aggregation, and targeting decayed proteins for degradation. Thus, Hsp70 has been recognized as an important biomarker for increasing Hsp70 expression levels to maintain cellular integrity in cases of increased stress in the cell and for monitoring heat stress and ROS that increases accordingly17-19. In previous studies, it has been reported that quercetin and several other flavonoids inhibit the induction of Hsp70 caused by cellular level heat shock at the level of mRNA accumulation60. Budagova et al61 reported that quercetin, one of the natural flavonoids of the in vitro cell response to heat stress-induced stress, completely inhibits the synthesis and intracellular accumulation of heat shock protein (Hsp70) in response to hyperthermia. Kim et al62 has reported that fisetin, a dietary flavonoid, can inhibit HSP activity, interact with cancer cell proliferation, and induce apoptosis in their study. Xu et al63 has reported in their study that quercetin may have a cytoprotective role that can act through a mitochondrial pathway during heat stress exposure. In the current study, there was a decrease in Hsp70 levels in quail blood serum, kidney, liver and thigh tissues in the HHES3 group compared to the HC group in the heat stress groups. However, Hsp70 level in liver, kidney and thigh tissues in thermoneutral groups was similar to that of the NC group. According to these results, it is thought that hesperidin supplementation in cases of heat stress has a great potential as an important contribution to reducing the increased stress in tissues due to heat increase, similar to previous studies.
Conclusions and implications
As a result, prevention and treatment of diseases using phytochemicals, in particular flavonoids, are well known. Fruits and vegetables are natural sources of flavonoids. Various flavonoids found in nature have their own physical, chemical and physiological properties. These substances are more widely used in developing countries. As a result of the study, when hesperidin, a flavonoid, was added to the food, compared to the HC group in the HHES3 group, it caused an improvement in hemoglobin, pO2, pH, HCO3, Cl concentrations in case of heat stress. In addition, in the case of heat stress; blood glucose, triglyceride, HDL and total cholesterol concentrations decreased in the HHES3 group compared to the HC group. ALP enzyme concentration showed a significant decrease in the HHES3 group compared to the HC group in the heat stress condition. Hsp70 protein level increased in blood serum, kidney, liver and thigh tissues in HC group with cellular stress during heat stress; however, Hsp70 concentration decreased significantly in the HHES3 group. It is thought that the use of Hesperidin, which is a supplement added to the feed in heat stress, may offer a potential nutritional strategy to overcome the harmful effects of stressors in poultry farming.











 texto en
texto en