Introduction
Japanese quail (Coturnix coturnix Japonica) has recently received more attention in the poultry industry due to increased meat and egg yield, especially in European and Latin American countries. Moreover, quail has become an important model animal for research due to its short life cycle and great resistance to many poultry diseases1,2. In quail feeding, alternative compounds are used today. Citrus have an additional advantage compared other sources due to the presence of bioactive compounds (flavonoids), used as functional components for providing health benefits. Bioflavonoids such as hesperidin and naringenin are abundant as an inexpensive by-product of citrus cultivation3. Flavonoids are secondary metabolites of plant origin called antioxidant heterocyclic organic compounds divided into 14 different subgroups according to the chemical structure and positions of the components on rings A, B and C4. Hesperidin (5, 7, 3’-trihydroxy-4’-methoxy-flavanone 7-rhamno glucoside), one of the primary flavonoids found in citrus fruit5, was formed from hesperidin and rutinose6,7, and have many biological properties, including antimicrobial, antioxidant and vascular activities8. These compounds prevent or delay the oxidation of structures such as proteins, lipids, carbohydrates. Flavonoids can be conjugated with glucuronic acid, and O-methylation or sulfate ester formation may occur to facilitate. This type of biotransformation occurs in the lower parts of the gastrointestinal tract. Some of the flavonoids that are not absorbed in the small intestine and other compounds are secreted by bile. They also contribute to the degradation process in the colon, in which microorganisms disrupt the ring structures9. In general, data on the intestinal absorption of hesperidin are insufficient, and its fate in the intestine is still unclear10.
In recent studies, it has been reported that when hesperidin is added to quail rations, it increases meat quality at the end of fattening, positively affects fatty acids in meat, and egg quality parameters in layer quails11,12. Other studies reported that it promotes the activity of cellular antioxidant enzymes such as Superoxide Dismutase (SOD), Hemeoxygenase-1 (HO-1) and catalase (CAT)13-17.
In this study, it was hypothesized that the addition of hesperidin to the quail diet would have positive effects on blood parameters, antioxidant concentration in tissues, intestinal histomorphology and fecal microflora.
Material and methods
Chemicals
Hesperidin (molecular formula: C28H34O15, cas no: 520-26-13, purity 91%, Chem-Impex International Company, USA) was purchased commercially in powder form.
Animals and dietary treatments
This study was carried out with the Ethics Committee Approval number 327/2022 of Sivas Cumhuriyet University Animal Experiments Local Ethics Committee. Japanese quails (300 quails, weighing 40-45 g and 1-2 wk old) were obtained from breeders in Sivas (39°42'34.8"N 37°01'13.0"E). The animals were kept in metal cages (Çimuka, Türkiye) (height: width: depth) (20*45*90 cm) with light for 21 h and darkness for 3 h at room temperature (25 ± 2 °C) for 35 d. The study was carried out with three main groups, 20 quails with 5 sub-repeats in each group and a total of 300 quails. The first treatment (control) (0 g hesperidin/kg feed), second treatment (HES1) (1 g hesperidin/kg feed), third treatment (HES2) (2 g hesperidin/kg feed) was added to with the basal diet through 35 d.
The diets used in the study were formulated according to the recommendations of NRC18. (Table 1). The granular feed and water were given ad libitum. Starting the study, an adaptation period for the environment and the feed was applied for one week. The dose of hesperidin was determined as is reported in previous study3. Quails were divided into three groups as control and study groups to study parameters such as blood parameters, antioxidant activity, intestinal histomorphology, and fecal microbial counts.
Table 1 Ingredients and nutrient composition of quail diet
| Ingredients (%) | Diets1 | ||
|---|---|---|---|
| C | HES1 | HES2 | |
| Corn | 44.29 | 44.29 | 44.29 |
| Wheat grain | 14.80 | 14.80 | 14.80 |
| Soybean meal, % 48 | 37.65 | 37.65 | 37.65 |
| Limestone2 | 0.81 | 0.71 | 0.61 |
| Dicalcium phosphate | 0.74 | 0.74 | 0.74 |
| DL-methionine | 0.50 | 0.50 | 0.50 |
| L-threonine | 0.50 | 0.50 | 0.50 |
| Salt | 0.42 | 0.42 | 0.42 |
| L-lysine, hydrochloride | 0.04 | 0.04 | 0.04 |
| Vitamin-mineral mix3 | 0.25 | 0.25 | 0.25 |
| Hesperidin4 | 0.00 | 0.10 | 0.20 |
| Calculated values | |||
| Dry matter, % | 89.73 | 89.63 | 89.53 |
| Crude protein, % | 24.05 | 24.05 | 24.05 |
| Ether extract, % | 1.85 | 1.85 | 1.85 |
| Crude ash, % | 5.30 | 5.30 | 5.30 |
| Crude cellulose, % | 2.89 | 2.89 | 2.89 |
| Metabolic energy, kcal/kg | 2.900 | 2.900 | 2.900 |
| Methionine+Cystine, % | 1.27 | 1.27 | 1.27 |
| Lysine, % | 1.31 | 1.31 | 1.31 |
| Threonine, % | 1.37 | 1.37 | 1.37 |
| Tryptophan, % | 0.32 | 0.32 | 0.32 |
| Calcium, % | 0.61 | 0.57 | 0.53 |
| Available phosphorus, % | 0.31 | 0.31 | 0.31 |
1Diets: C - basal diet, HES1 - basal diet with 1 g/kg hesperidin, HES2 - basal diet with 2 g/kg hesperidin.
2hesperidin replaced limestone in the same amount in the groups with hesperidin addition.
3contained per kg: mg: retinol (vitamin A) 3, tocopherol (vitamin E) 30, menadione (vitamin K3) 5, thiamine (vitamin B1) 1, riboflavin (vitamin B2) 5, pyridoxin (vitamin B6) 3, nicotinic acid 30, pantothenic acid 10, folic acid 0.8, ascorbic acid (vitamin C) 10, choline chloride 450, Co 0.2, I 0.5, Se 0.3, Fe 25, Mn 120, Cu 10, Zn 100; μg: cholecalciferol (vitamin D3) 62.5, cobalamin (vitamin B12) 20, biotin 100; 4 obtained from Chem-Impex Int. company, molecule formula (C28H34O15), cas no (520-26-13), purity grade 91% (Chem-Impex, Wood Dale, IL, USA).
Slaughter procedures
At the end of the study (35 d) (n=8*3 groups total 24 animals), quails were not fed for 6 h and were slaughtered in a commercial slaughterhouse under hygienic conditions. The blood of slaughtered quails was taken into blood collection tubes and centrifuged at 3,000 rpm for 10 min. The serum was transferred to 2 ml of microcentrifuge tubes (Eppendorf, Germany) and kept in a freezer at -80 °C until the analysis were performed. The carcasses were placed in plastic bags one by one after the internal organs were evacuated and kept at +4˚C for 24 h. The blood serum, the thigh (Musculus gastrocnemius) and the liver tissues were kept in a freezer at -80 oC until the analysis were performed.
Blood serum parameters
Tchol (Total cholesterol), BUN (Blood Urea Nitrogen), ALT (Alanine amino Transferase), AST (Aspartate amino Transferase), GGT (Gamma Glutamyl Transferase), ALP (Alkaline Phosphatase), TP (Total Protein), TG (Triglyceride), CK (Creatin Kinase), LDH (Lactate Dehydrogenase), Ca (Calcium), Mg (Magnesium) P (Phosphorus), Urea, Kreatin, Albumin biochemical values were determined in serum samples using an auto-analyzer device (Mindray BS200, China).
Tissue parameters
Liver, and thigh tissues were homogenized. Subsequently, GSH levels were determined using the ELABSCIENCE (E-BC-K030-M); SOD levels were detected using the ELABSCIENCE (E-BC-K022-M) CAT levels were determined using the ELABSCIENCE (E-BC-K031-M) MDA (Malondialdehyde) was measured using the ELABSCIENCE (E-BC-K298-M) LPO levels were detected using the ELABSCIENCE (E-BC-K176-M) corresponding to the absorbances of the serum and tissue samples calculated.
Intestinal histomorphology
Ileum, cecum and colon samples were taken into tissue containers for histological preparation in a 10% buffered formula and washed in tap water for 48 h; 5 µm thick sections were taken from the tissues, followed by the histological tissue follow-up method that was applied routinely, were blocked in the paraffin wax. Hematoxylin - eosin staining was performed on sections to determine the overall histological structures and perform histometric measurements19. The stained preparations were examined under a research microscope (Zeiss Primo Star, Germany) and their photos were taken. Villus height, villus width and crypt depth of the 10 pieces obtained from different parts of three sections belonging to each animal were measured using the ImageJ software. The villus height was measured at the vertical distance from the villus peaks to the starting point of the crypts, while the villus widths were obtained from measurements taken at the medium height of the villus. The depth of the crypt was calculated as the vertical distance from the villus-crypt junction to the lower boundary of the crypt20.
Fecal microbial counts
One gram of fecal content from each quail was aseptically collected and homogenized with 9 mL of 0.1% peptone water. Serial 10-fold dilutions were made in sterile peptone water from 10-1 to 10-6 and 0.1 mL from last three dilutions were plated in duplicate onto respective selective medias.
Escherichia coli counts were performed on Tryptone Bile X-Glucuronide (TBX) agar and plates were incubated for 24 h at 37 °C. Enterococci were cultured on Slanetz Bartley agar (SB, Oxoid CM377, England) and enumerated after 24-48 h of incubation at 37 ºC. Enterobacteriaceae and coliforms were grown on violet red bile glucose agar (VRBG, Oxoid CM485, England) and violet red bile agar (VRB, Oxoid CM107, England) respectively, using the pour plate technique and enumerated after 24-48 h of incubation at 37 ºC. Tryptose Sulfite Cycloserine Agar (TSC Agar) Base (Merck 1.11972, Germany) was utilized for the Clostridium count. The plates were incubated for 24 h at 45 °C under anaerobic conditions, anaerobic indicator (Mitsubishi Gas Chemical, America) was included to monitor the atmospheric condition. Petri dishes (90 mm*15 mm) (Fırat Plastic, Türkiye) observed 30 to 300 colonies were counted using a colony counter21. The microbial counts were expressed as log10 CFU per gram of fecal contents.
Statistical analysis
The obtained data were evaluated using the SPSS 20.0 statistical package program. The data showed normal distribution in all parameters. One-way analysis of variance (ANOVA) was used and Bonferroni and Tamhane’s T2 multiple comparison test was used for comparisons between groups (P<0.05).
Results
Blood serum parameters
Serum alanine transaminase (ALT) and lactate dehydrogenase (LDH) in the HES1 and the HES2 groups, were no different compared to the control group (P<0.05). Aspartate transaminase (AST) decreased in the HES2 group compared control group and HES1(P< 0.05). Amylase, on the other hand, showed an increase in HES2 compared to the control group (P<0.05) (Table 2). No significant differences were found between the experiment groups in the parameters of Tchol, TG, GGT, ALP, CK, BUN, Albumin, TP, Urea, Creatine, Glucose, Ca, Mg and P (P>0.05).
Table 2 The effect of hesperidin added to quail diets on blood serum biochemistry
| Control | HES1 | HES2 | ||
|---|---|---|---|---|
| Mean± SEM | Mean± SEM | Mean± SEM | P | |
| Tchol, mg/dL | 239.82±9.09 | 262.38±7.22 | 259.23±5.32 | 0.12 |
| TG, mg/dL | 265.35±24.77 | 248.40±25.59 | 247.43±26.77 | 0.86 |
| ALT, U/L | 5.50±0.65ab | 4.25±0.48b | 6.60±0.25a | 0.01* |
| AST, U/L | 237.50±6.89a | 243.00±5.51a | 194.60±9.66b | 0.001* |
| GGT, U/L | 2.00±0.58 | 2.40±0.40 | 3.50±0.50 | 0.14 |
| ALP, U/L | 265.73±22.42 | 279.37±26.89 | 261.70±26.62 | 0.89 |
| CK, U/L | 2.422±197.57 | 2.614±211.07 | 2.650±242.93 | 0.78 |
| LDH, U/L | 711.93±36.69ab | 678.36±27.69b | 767.81±13.88a | 0.03* |
| Amylase, U/L | 243.50±15.50b | 302.75±12.15ab | 355.50±18.68a | 0.01* |
| BUN, mg/dL | 4.00±0.00 | 4.67±0.33 | 4.71±0.36 | 0.26 |
| Albumin, g/dL | 1.83±0.11 | 1.55±0.17 | 1.83±0.10 | 0.24 |
| TP, g/dL | 6.26±0.71 | 3.97±0.21 | 4.74±0.55 | 0.08 |
| Creatin, mg/dL | 0.18±0.02 | 0.16±0.01 | 0.19±0.04 | 0.82 |
| Glucose, mg/dL | 56.58±9.66 | 61.60±5.40 | 59.20±2.20 | 0.93 |
| Ca, mg/dL | 10.52±0.20 | 8.87±0.76 | 9.99±0.10 | 0.16 |
| Mg, mg/dL | 7.58±0.64 | 6.18±0.50 | 7.56±0.24 | 0.09 |
Tchol= total cholestrol, BUN= blood urea nitrogen, ALT= alanine amino transferase, AST= aspartate amino transferase, GGT= gamma glutamyl transferase, ALP= alkaline phosphatase, TP= total protein, TG= triglyceride, CK= creatin kinase, LDH= lactate dehydrogenase, P= phosphorus.
*There is a statistically significant difference between the experimental groups (P<0.05)
Tissue parameters
GSH level increased in thigh, liver and serum tissue in HES1 group compared to control group (P<0.05). In thigh tissue, it decreased in the HES2 group compared to the control group. LPO level increased in thigh and serum tissue in HES1 group compared to control group. In thigh tissue, it decreased in the HES2 group compared to the control group. and increased in HES2 groups in serum. In liver tissue, it decreased in HES1 and HES2 groups compared to the control group (P<0.05). The CAT level in the thigh tissue was decreased in the hesperidin groups compared to the control group (P<0.05). It increased in liver tissue (P>0.05). In serum tissue, it decreased in HES1 and increased in HES2 (P<0.05). MDA level decreased in thigh, liver and serum in HES1 and HES2 groups compared to control group (P<0.05). On the other hand, while SOD level decreased in hesperidin groups in thigh tissue compared to the control group (P<0.05); increased in HES1 and HES2 groups in liver tissue (P<0.05). In the serum tissue, it decreased in the HES1 group compared to the control group, but increased in the HES2 group (P>0.05) (Table 3).
Table 3 The effect of hesperidin added to the diet on oxidant parameters in thigh, liver and blood
| Control | HES1 | HES2 | P | ||
|---|---|---|---|---|---|
| Mean± SEM | Mean± SEM | Mean± SEM | |||
| GSH, µmol/L | Thigh | 10.79±0.49 | 12.61±0.16 | 10.04±0.75 | 0.06 |
| Liver | 6.33±1.01c | 12.77±0.65b | 19.62±0.65a | 0.001* | |
| Serum | 51.68±1.34b | 75.16±1.24a | 70.88±0.42a | 0.01* | |
| SOD, U/ml | Thigh | 1.03±0.04a | 0.81±0.04b | 0.85±0.07b | 0.02* |
| Liver | 1.09±0.02 | 1.11±0.03 | 1.16±0.01 | 0.14 | |
| Serum | 0.37±0.12 | 0.32±0.13 | 0.42±0.11 | 0.84 | |
| CAT, U/ml | Thigh | 211.62±0.72a | 101.55±1.16b | 61.56±0.91c | 0.001* |
| Liver | 76.13±1.31b | 88.40±4.18ab | 96.69±3.43a | 0.01* | |
| Serum | 54.30±1.06ab | 51.33±1.25b | 61.65±1.03a | 0.02* | |
| MDA, µmol/L | Thigh | 758.53±42.62a | 642.30±43.17ab | 379.71±40.09b | 0.02* |
| Liver | 1.091.16±40.52a | 318.98±39.98b | 308.31±31.66c | 0.001* | |
| Serum | 777.69±36.18a | 438.24±35.80b | 298.32±38.67b | 0.01* | |
| LPO, µmol/L | Thigh | 278.79±1.46a | 285.13±1.15a | 267.28±2.34b | 0.03* |
| Liver | 257.09±1.39a | 241.16±0.78b | 249.30±1.29a | 0.01* | |
| Serum | 241.56±1.56b | 279.88±1.42a | 284.71±1.52a | 0.001* |
GSH= reduced glutathione, SOD= superoxide dismutase, CAT= catalase, MDA= malondialdehyde, LPO= lipid peroxide.
*There is a statistically significant difference between the experimental groups (P<0.05).
Intestinal histomorphology
In terms of histomorphology in the intestine, in ileum tissue, villus height increased in HES1 and HES2 groups compared to the control group (P<0.05) (Table 4). villus height increased in HES1 and HES2 groups compared to the control group in the caecum and colon tissue (P<0.05). However, crypt depth in colon tissue decreased in HES1 and HES2 groups compared to control group (P<0.05) (Figure 1).
Table 4 The effect of hesperidin added to the diet on villus and crypt depth in the ileum, caecum and colon
| Control | HES1 | HES2 | P | ||
|---|---|---|---|---|---|
| Mean±SEM | Mean±SEM | Mean± SEM | |||
| Ileum | VH | 222.42±13.53 | 234.53±21.69 | 264.24±22.43 | 0.33 |
| VW | 67.21±1.97 | 64.70±5.47 | 67.10±2.54 | 0.86 | |
| CD | 30.50±1.24 | 31.71±1.82 | 35.02±3.55 | 0.41 | |
| Caecum | VH | 71.40±3.79b | 85.56±4.24a | 79.86±1.28ab | 0.03* |
| VW | 41.33±2.47 | 42.76±1.28 | 47.81±1.55 | 0.06 | |
| CD | 44.45±3.45 | 38.15±1.78 | 41.80±1.58 | 0.22 | |
| Colon | VH | 226.38±18.2b | 298.58±22.10ab | 312.50±28.75a | 0.04* |
| VW | 60.88±2.01b | 73.70±3.84a | 62.54±3.13ab | 0.02* | |
| CD | 53.47±4.65a | 40.24±2.89b | 42.62±2.39ab | 0.04* |
VH= villus height, VW= villus width, CD= crypt depth.
*There is a statistically significant difference between the experimental groups (P<0.05).
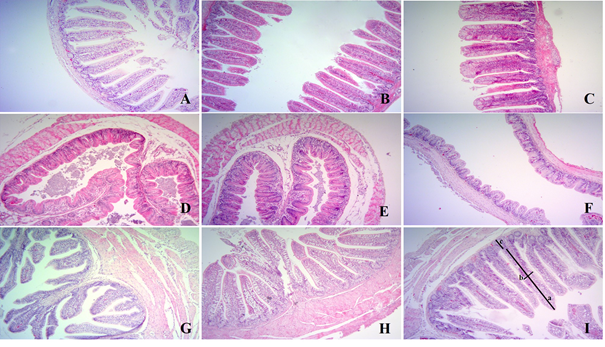
Control group: ileum (A), caecum (D), colon (G), HES1 group: ileum (B), caecum (E), colon (H), HES2 group: ileum (C), caecum (F), colon (I) tissue. a: villus hight, b: villus width, c: crypt depth. Hematoxylin-eosin staining X10.
Figure 1 The effect of hesperidin addition to diet on ileum caecum and colon tissues
Fecal microbial counts
In terms of fecal microflora, Clostridium spp. number decreased in HES1 and HES2 groups compared to the control group (P<0.05) (Table 5). However, there is no significant difference between the experiment groups in terms of Escherichia coli, Enterococcus spp., Coliform spp. and Enterobactericeae counts (P>0.05).
Table 5 The effect of dietary hesperidin on bacterial concentration (gram/Log10) in fecal samples
| Control | HES1 | HES2 | ||
|---|---|---|---|---|
| Mean± SEM | Mean± SEM | Mean± SEM | p | |
| Escherichia coli | 6.51±0.30 | 6.55±0.27 | 6.11±0.43 | 0.61 |
| Enterococcous spp. | 6.37±0.34 | 6.05±0.28 | 6.77±0.25 | 0.27 |
| Coliform spp. | 6.27±0.36 | 6.09±0.25 | 5.44±0.18 | 0.20 |
| Enterobactericeae | 6.43±0.24 | 5.65±0.26 | 6.65±0.24 | 0.88 |
| Clostridium spp. | 6.55±0.07a | 5.93±0.27a | 5.26±0.11b | 0.01* |
*There is a statistically significant difference between the experimental groups (P<0.05).
Discussion
Serum biochemistry
Dietary antioxidant supplements can improve the physiology and yield characteristics of animals by changing metabolic processes17. The current study aimed to investigate the effects of hesperidin on biochemical parameters and antioxidant indices in quails. Citrus flavonoids, such as hesperidin and naringin, obtained from citrus fruits and fruit juices have been associated with lower cholesterol and triglyceride levels in animals in the previous studies22,23. The hypocholesterolemic effect of hesperidin has been reported to be mediated by a decrease in the activity of HMG-Co A reductase24. In the current study, both triglyceride and cholesterol levels no decreased in the groups with hesperidin (P>0.05).
ALT and AST and LDH are enzymes related to liver health, function and it is desirable that they be low. In rats given 5g/kg of flavonoid daily, it was reported that ALT and AST concentrations decreased compared to the control group25. Abdel-Kareem and El-Sheikh26 also reported 250, 500 and 1000 mg/kg, Galal et al27 found that 100 and 150 mg/kg propolis significantly reduced AST and ALT activities in laying hens. In the current study, AST enzyme levels decreased in the reason for the decrease in ALT, AST and LDH with different doses of hesperidin is unknown.
Antioxidant compounds taken from the diet, such as bioflavonoids, can provide some protection against the early stage of diabetes and the development of complications. It has been reported that hesperidin significantly reduces glucose in the blood28. However, unlike previous studies, the serum glucose concentration increased slightly in the current study (P>0.05). To interpret the increase in glucose concentration, it is necessary to look at the plasma insulin concentration.
Tissue oxidant vs antioxidant enzymes
The concentration of SOD enzymes in thigh samples was found to be low, but as high as expected in liver tissue. In general, this effect of hesperidin on the oxidation parameter indicates that it can terminate oxidation by adding hydrogen atoms to free radicals. Under physiological conditions, reactive oxygen species (ROS) production is regulated both endogenously and by antioxidant supplementation29,30. The main endogenous antioxidant enzymes are superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX)31. SOD converts hydrogen peroxide, which is one of the superoxide radicals, into oxygen and water under the action of CAT and/or GPX. While SOD and GPX are formed in many tissues32, CAT is highly present in liver, kidney tissues and red blood cells33. The membranes of immune cells are made of highly polyunsaturated fatty acids, making them highly sensitive to oxidative stress34. Therefore, they present high concentrations of antioxidant enzymes since membrane-related signaling and gene expression are critical for maintaining immune cell functionality32. In the current study, it is believed that the concentrations of thigh and serum tissues and liver and serum tissues in terms of GSH parameter and CAT parameter, respectively, increase in relation to the hesperidin supplementation. In another study, it has been reported that the concentration of hesperidin in animals was lower in terms of SOD activity in rats undergoing a fatigue test35. Lien et al36 and Ting et al37 reported that the concentration of superoxide dismutase (SOD) in the blood serum was high with the addition of hesperidin and naringin (0.5-4.0 g/kg) to broiler rations.
Previous studies reported that a significant decrease in MDA concentration were observed in broilers consuming rations with grape pulp (1.5, 3 and 6 %) in terms of MDA, which is one of the oxidant parameters, compared to those consuming a basal diet38,39. In another study, when broilers were fed a commercial essential oil mixture (0.05 % of the diet), a decrease in MDA was observed in the breast (22.4 %) and thigh 62.3 %) muscles when fed with a commercial essential oil mixture40. In addition, the inclusion of hesperidin (0.15 % or 0.3 %) to the rations of broilers has been reported to reduce the concentration of MDA in the breast muscle41. In the current study, as in the studies noted above, it is denser in the liver due to hesperidin, and the MDA value decreased in the same way in thigh and serum. In addition, a decrease in LPO, which is an essential parameter in terms of tissue damage, is expected from the point of view of the current study due to the dose of hesperidin in thigh and liver tissue. The reason for this is that the body fat composition of adult quails is rich in unsaturated fatty acids, which is seen in studies conducted due to the increase in the dose of hesperidin in recent years11,12.
Intestinal histomorphology
Hesperidin sulfates will be conjugated and absorbed in the duodenum and ileum after oral administration. Previous studies have shown a small amount of absorption of flavonoid glycosides in the small intestine42. The absence of glucuronide detection in intestinal tissue may be due to the restriction of tissue penetration of glucuronide43.
Recent studies have reported that flavonoids in a natural form (flavonoid-rich plants) or the extracts there of was positive effects on immunomodulatory and intestine, including the increasing villus length in broilers and surface area of the small intestine44-46. Kamboh and Zhu20 found that, among broilers consuming added rations of hesperidin, the length of the intestinal villus and the width of the villus (21st and 42nd d) increased, while the depth of the crypt (21 d in the duodenum and ileum, and 42 d in the duodenum) decreased. In the current study, the height and width of the villus in the ileum, cecum and colon also increased relatively. Flavonoid compounds that improve intestinal morphology differences measured in villus height and width have shown that these compounds can stimulate epithelial cell mitosis. That is because longer or thicker (or both) villus are associated with active mitosis, and it is believed that the villus continues long-term absorption without the need for regeneration and a reduced crypt depth. This is because the crypt is considered a villus factory, and that the large crypt presents rapid tissue transformation due to inflammation caused by pathogens, toxins, or both47,48. In addition, increased villus length/crypt depth ratios with genistein and hesperidin can be considered an improvement in the digestive system49. It is that similar results to previous studies will be obtained in the current study.
Fecal microbial counts
In the current study, a lower concentration was determined in hesperidin groups in terms of the population of Clostridium spp. in a similar way to the previous studies. Today, plant extracts commercially used in livestock due to their performance-enhancing and antimicrobial effects are available in pure or mixed form. It is known that phenolic compounds and essential oils obtained from plants have antimicrobial effects16,50. The intestinal flora contained in the colon promotes the absorption of certain nutrients, including polyphenols, from the diet and forms bioactive and absorbable molecules from dietary compounds51. Metabolites, consisting of both polyphenols and flora, can positively effect on health52. Additionally, numerous benefits of polyphenol metabolites derived by microflora on the host53 have been reported.
Flavonoids have the property of low bioavailability and poor absorption. Therefore, they significantly affect intestinal health54,55. Phenolic compounds possess bactericidal53) and bacteriostatic54 properties. They minimize the adhesion of pathogenic bacteria (E. coli, Clostridium spp.) to the intestinal wall and prevent infections in the digestive tract. They also improve the use of nutrients and animals' performance55-58.
Zhang et al59 have reported in their study that polyphenols added in piglet rations did not have a significant effect of feces E. coli and Clostridia spp. numbers on the 11th and 21st d. Kırkpınar et al60 have noted a decrease in Clostridium spp. populations in the ileums of broilers fed with thyme oil, garlic oil and both substances compared to animals in other groups. Supplementation of cranberry extract (80 mg/kg feed), which is a source of phenolic acid, anthocyanin, flavonol and flavan-3-ol, to broiler rations has decreased Enterococcus spp. populations significantly in broilers on the 28th d of the study61.











 texto en
texto en 


