Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias pecuarias
versão On-line ISSN 2448-6698versão impressa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.14 no.2 Mérida Abr./Jun. 2023 Epub 26-Jun-2023
https://doi.org/10.22319/rmcp.v14i2.6003
Articles
Stover chemical composition in three corn cultivars after sterilization or colonization with Ganoderma lucidum mycelia
a Colegio de Postgraduados-Campus Puebla. Boulevard Forjadores de Puebla No. 205, Santiago Momoxpan, 72760, San Pedro Cholula, Puebla, México.
b Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Campo Experimental La Posta, Km. 22.5 Carretera Federal Veracruz-Córdoba. 94277, Medellín, Veracruz, México.
Nutritional quality in grain by-products such as corn stover can be improved with processes such as steam sterilization and fungus inoculation. The stover of two native corn cultivars and one commercial hybrid cultivar were steam sterilized or inoculated with mycelium of the white-rot fungus Ganoderma lucidum. The experimental design was completely random with a 3x4 factorial arrangement, one additional treatment and four replicates. The four treatments were untreated stover, sterilization and immediate drying, sterilization and drying after 15 d, and colonization with G. lucidum for 15 d; pure mycelia were also analyzed to establish values for the fungus. Five variables were measured: in vitro dry matter digestibility (IVDMD), neutral detergent fiber (NDF), acid detergent fiber (ADF), lignin and crude protein (CP). The three cultivars differed (P<0.0001) in terms of digestibility, with cultivar A having the highest values. Digestibility was lowest (P<0.05) in the G. lucidem-colonized stovers (P<0.05), intermediate in the untreated stovers and highest in the sterilized stovers. Contents of NDF, ADF, lignin, and CP differed (P<0.0001) between the cultivars and treatments (P<0.0001). Cultivar A had less NDF than the other cultivars. The untreated stovers had less NDF than the sterilized and G. lucidem-colonized stovers. For both ADF and lignin, the untreated stovers had the lowest values, the sterilized stovers had intermediate values and the colonized stovers had the highest. Crude protein (CP) differed between the cultivars (P<0.0001), and the colonized stovers had the highest values (P<0.05). Inoculation of corn stover with Ganoderma lucidum mycelia did not improve digestibility after fifteen days colonization, but slightly increased crude protein content.
Keywords Digestibility; White-rot fungus; Landrace corns; Hybrid corns
Se evaluó la calidad nutritiva del rastrojo de dos cultivares criollos de maíz y un híbrido, colonizados por micelio de Ganoderma lucidum. El diseño experimental fue completamente al azar con arreglo factorial 3x4 con un tratamiento adicional y cuatro repeticiones. Cada cultivar tuvo rastrojo colonizado por el hongo hasta los 15 días, rastrojo en su estado natural (sin tratar), a tiempo cero después de la esterilización, a 15 días después de la esterilización y el micelio puro (adicional). Se determinó digestibilidad in vitro (DIVMS), fibra detergente neutro (FDN) y ácido (FDA), lignina y proteína cruda (PC). Los cultivares difirieron (P<0.0001) en digestibilidad, el criollo A presentó valores mayores. Los rastrojos colonizados tuvieron menor (P<0.05) digestibilidad; los rastrojos sin tratar tuvieron valores medios y los esterilizados fueron los más digestibles. La concentración de FDN, FDA, lignina, y PC difirió (P<0.0001) en los cultivares y las condiciones del rastrojo (P<0.0001). El criollo A tuvo menos FDN que los otros cultivares. Los rastrojos en su forma natural tuvieron menos FDN que los esterilizados y los colonizados. En la FDA los rastrojos en su forma natural tuvieron concentración baja, los esterilizados una concentración media y los colonizados la concentración mayor, situación que fue similar para lignina. En PC los cultivares fueron diferentes (P<0.0001), siendo los rastrojos colonizados los que tuvieron valores mayores (P<0.05). En conclusión, la colonización del rastrojo por el micelio de Ganoderma lucidum no aumentó la digestibilidad a los 15 días de colonización, lo que mejoró ligeramente fue la concentración de proteína cruda.
Palabras clave Digestibilidad; Hongo pudrición blanca; Maíces criollos; Maíces híbridos
Introduction
Corn stover is a common ingredient in ruminant diets in arid and tropical regions, mainly when dry or cold conditions restrict plant growth and reduce green forage availability. However, ruminants can make only limited use of corn stover because it has low nutrient concentrations and low digestibility1. Among the many methods of improving stover digestibility is the use of edible or functional fungi, which can transform plant tissue cell structure2.
White-rot fungi have been applied as an alternative for improving stover nutritional quality2. For example, species in the Pleurotus genus can degrade lignin through their multienzymatic system which acts on complex molecules that are difficult for ruminants to degrade3,4,5. Species such as Ceriporiopsis subvermispora, Lentinula edodes, Pleurotus eryngii and Pleurotus ostreatus, increase substrate crude protein content but reduce nutrient concentrations6. Strains of Pleurotus florida, P. ostreatus, P. pulmonarius and P. sajor-caju, have been used to treat corn stover7; indeed, P. sajor-caju is known to increase crude protein content and metabolizable energy while reducing lignin content.
The white-rot fungus Ganoderma lucidum also degrades lignin, normally within three to five months post inoculation8. However, this fungus produces polysaccharides such as mannose, xylose, arabinose, galactose, glucose and rhamnose9-12, as well as chitin13, all of which can increase fiber content. In addition, when used in corn stover it can cause the autoclave sterilization process to produce net negative results since nitrogenous compounds in the stover are solubilized6. The fungus strain used to improve stover quality is therefore vital because each requires a carbohydrate source and a certain time to colonize the substrate, and its long-term consumption must be safe for animals14. To date, no data is available on the effects of G. lucidum on corn stover during short colonization times. The present study objective was to analyze the chemical composition of corn stover from two landrace cultivars and one hybrid, when unprocessed, sterilized and dried at two different times, or inoculated with G. lucidum.
Material and methods
Corn cultivars
Two landrace corn cultivars (named A and C) and the hybrid cultivar Aspros 1503® (assigned the letter B), all with white grains, were planted under natural rainfall conditions, in the municipality of Cuautinchan, Puebla state, Mexico. The three cultivars were planted in a 200 m x 50 m area, following a randomized block design, with four replicates. The landraces were selected from the previous harvest of two producers located in the same municipality, both known for conserving and improving corn cultivars following traditional practices. The hybrid seeds, also from the previous year’s harvest, were purchased from an Aspros seed distributor in the same municipality.
Stover preparation
The corn grain was harvested 170 d after planting. At this time, five plants were taken from each replicate, and ground in a two-centimeter mill with sieve. Average stover dry matter (DM) yield was 300 g per replicate. The material for each replicate was divided into four parts corresponding to the four treatments: untreated stover (untreated); sterilized stover dried immediately (sterilized/dried); sterilized stover stored in moisture for 15 d (sterilized/dried at 15 d); and sterilized stover inoculated with G. lucidum (G. lucidum-colonized).
Sterilization was done with an autoclave (All American®) at 121.5 °C for 25 min. To evaluate nutrient leaching, immediately after sterilization one portion of sterilized stover was dried at 60 °C to constant weight in a forced-air oven (Thermo Scientific® Model 3478); this is the sterilized/dried treatment. To evaluate the effect of storage in moisture, which can generate hydrolysis reactions that modify stover nutritional quality, a second portion of sterilized stover was placed in Petri dishes (20 g DM per dish) for 15 d and then dried; this is the sterilized/dried at 15 d treatment. A third portion of sterilized stover (20 g DM) was placed in a Petri dish and inoculated with G. lucidum (G. lucidum-colonized).
Fungus strain preparation and stover inoculation
The fungus was G. lucidum CP-145, obtained from the Center for Biotechnology of Edible, Functional, and Medicinal Fungi (Centro de Biotecnología de Hongos Comestibles, Funcionales y Medicinales) of the Colegio de Postgraduados, Puebla Campus. The strain was cultured in Petri dishes in potato dextrose agar (PDA) culture medium for 8 d, sufficient time for growth of mycelium. Five discs (5 mm diam.) with mycelia were used to inoculate the stover. The inoculated stover was monitored every three days until 15 d post inoculation, the time required for maximum mycelial colonization and the average reported time required to attain the highest fungal enzymatic activity15.
Stover processing
All samples were dried in a forced-air oven at 60 °C to constant weight and ground in a cyclone mill (Foss Tecator®) with 1 mm mesh. The ground samples were stored in resealable plastic bags until nutritional quality analysis.
A separate culture was done of G. lucidum alone to quantify the direct contribution it might make to stover nutritional quality. Before inoculation with G. lucidum, potato dextrose broth (PDB; Difco™) (24 g L-1) was sterilized in an autoclave (All American®) at 121.5 °C and 15 lb ppm-2 for 25 min. The medium was inoculated by depositing five circles (5 mm diam) from an eight-day-old G. lucidum colony grown on PDA medium. It was incubated in an orbital shaker (Thermo Scientific®) at 120 rpm and 27 ºC for 20 d. After harvesting, mycelia were recovered by filtering through Whatman #1 filter paper placed in a Buchner funnel in a Kitazato flask connected to a vacuum pump. The recovered biomass was placed in aluminum trays and dried at 60 °C in a drying oven (Felisa®) for 48 h. It was ground in a ceramic mortar and stored in a resealable plastic bag for subsequent nutritional quality analysis.
Evaluated variables
Five variables were measured in the stover treatments and the G. lucidum culture: neutral detergent fiber (NDF)16; acid detergent fiber (ADF)16; lignin16; in vitro dry matter enzymatic digestibility (IVDMD)17,18; and crude protein (CP)19.
Experimental design and statistical analysis
A completely randomized experimental design was applied including the three cultivars (A, B and C) and the four treatments (untreated, sterilized/dried, sterilized/dried at 15 d and G. lucidum-colonized), as well as the pure fungus culture20. After verifying data normality, an ANOVA was run. Differences between means were identified with a Tukey test, at an α=0.05 significance level. Data analyses were run with the SAS ver. 9.0 statistical program21.
Results
In vitro dry matter digestibility (IVDMD) differed (P<0.0001) between the corn cultivars due mainly to treatment; there was also an interaction (P<0.0001) between two cultivars in terms of treatment (Figure 1).
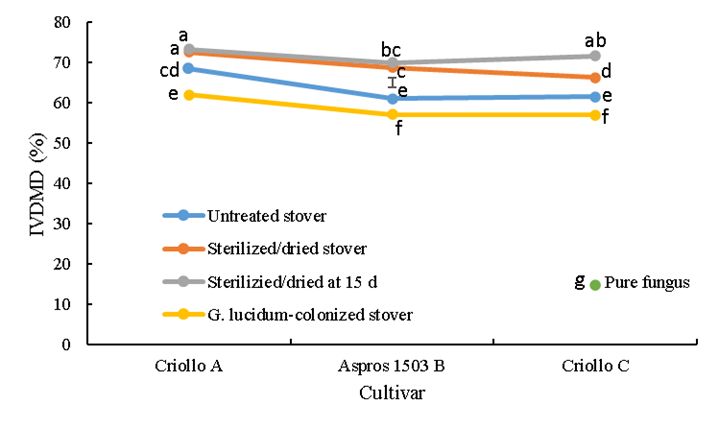
Bars represent honestly significant difference in the comparison of means.
abc Different lowercase letters on bar points indicate significant difference (P≤0.05).
Figure 1 In vitro dry matter digestibility (IVDMD) of the three evaluated corn stovers in four treatments, including colonization with the white rot fungus Ganoderma lucidum
The untreated stover had IVDMD values intermediate (63.7 %) to those of the sterilized (70.4 %) and colonized stovers (58.7 %). Cultivar A was 7.5 % more (P<0.05) digestible than cultivar B and 7.1 % more than cultivar C. Both the sterilized/dried, and sterilized/dried at 15 d treatments had digestibility 6.7 % higher (P<0.05) than the untreated stovers. Among the untreated stovers, cultivar A had the highest (P<0.05) digestibility (73 %) followed by cultivars B (69.3 %) and C (68.9 %).
Compared to the untreated and sterilized stovers, the G. ludicum-colonized stovers had lower (P<0.05) digestibility values: 62.0 % for cultivar A; 57.1 % for B; and 57.0 % for C. Among the colonized treatments, cultivar A had higher (P<0.05) digestibility than cultivars B and C. Pure cultured G. ludicum exhibited the overall lowest digestibility (14.7 %) (Figure 1).
Neutral detergent fiber (NDF) differed between cultivars (P<0.0001), mainly in response to treatment (Figure 2); an interaction (P<0.0001) was apparent in cultivar C. Among the untreated stover treatments, cultivar A had a NDF content 9 % lower (P<0.05) than that of cultivar B and 5 % lower than that of cultivar C; cultivar B had the overall highest (P<0.05) NDF content. Neutral detergent fiber (NDF) content generally increased (P<0.05) in response to sterilization and G. ludicum colonization, an effect most notable in the landraces (cultivars A and C).
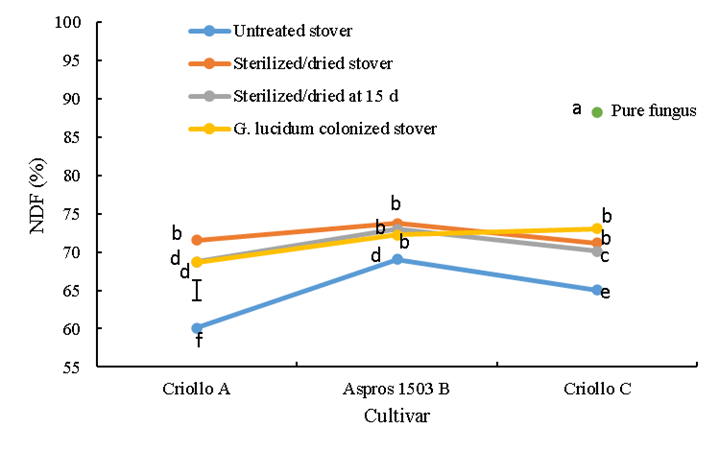
Bars represent honestly significant difference in the comparison of means.
abc Different lowercase letters on bar points indicate significant difference (P≤0.05).
Figure 2 Neutral detergent fiber (NDF) concentration of the three evaluated corn stovers in four treatments, including colonization with the white rot fungus Ganoderma lucidum
The G. lucidum-colonized stovers had higher (P<0.05) NDF concentrations than the other treatments. Among the sterilized/dried treatments , cultivar A had a lower concentration (68.6 %) than cultivars B and C, which did not differ (P>0.05). The G. lucidum-colonized stovers exhibited higher NDF (P<0.05) than the untreated stovers; 8.6 % higher in cultivar A, 3.2 % higher in B and 8.1 % higher in C. Of note is that the colonized cultivar B stover had a NDF that did not differ from those of the sterilized/dried treatments. Also, among the cultivar C stovers NDF did not differ between the colonized and sterilized/dried treatments, but both differed (P<0.05) from the sterilized/dried at 15 d treatment. In contrast, in cultivar A NDF in the colonized treatment did not differ from the sterilized/dried at 15 d treatment but did differ from the sterilized/dried treatment (Figure 2). The pure G. lucidum had NDF content (88.2 %) much higher (P<0.05) than the in the stover treatments (Figure 2).
Acid detergent fiber (ADF) differed among the cultivars (P<0.0001), with a clear effect from treatment (P<0.0001)(Figure 3), in addition to an interaction effect (P<0.0001). The untreated stovers did not differ in terms of ADF and all had the lowest (29.0 to 29.9 %) values among the treatments. The sterilized/dried stovers had higher (P<0.05) ADF concentrations than the untreated stovers; 5.1% higher than cultivar A, 2.8 % higher than B and 5.7 % higher than C. In both the sterilized/dried and sterilized/dried at 15 d treatments, ADF differed minimally (3 %) between cultivars B and C. The sterilized/dried at 15 d treatment had a higher (P<0.05) ADF content than the untreated stovers; 2.9 % higher in cultivar A, 2.2 % higher in B and 4.4 % higher in C. When comparing the sterilized/dried treatment with the sterilized/dried at 15 d treatment, difference (P<0.05) was observed only in cultivar A (2.2 %). In the sterilized/dried treatment, ADF in cultivar C was 2.4 % higher (P<0.05) than in cultivar A (Figure 3).
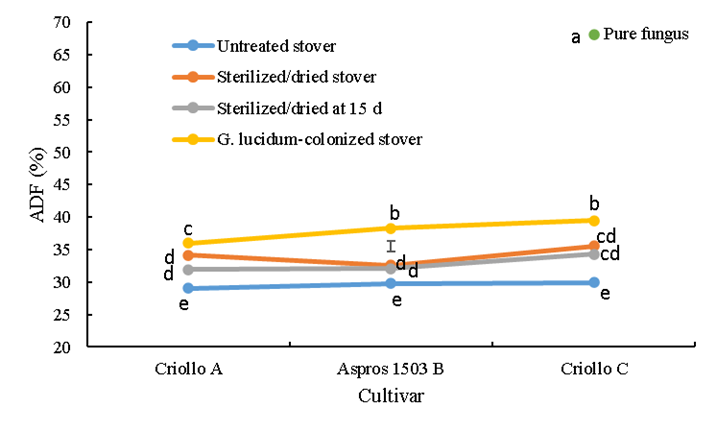
Bars represent honestly significant difference in the comparison of means.
abc Different lowercase letters on bar points indicate significant difference (P≤0.05).
Figure 3 Acid detergent fiber (ADF) concentration of the three evaluated corn stovers in four treatments, including colonization with the white rot fungus Ganoderma lucidum
When colonized with G. lucidum, all three stovers exhibited higher (P<0.05) ADF concentration than the untreated stovers; 7.0 % higher in cultivar A, 8.5 % in B and 9.6 % in C. When compared to the sterilized/dried treatment, the colonized cultivar A stover had 1.9 % more ADF, cultivar B had 5.7 % more and cultivar C had 3.9 % more (P<0.05). The colonized stover also had higher (P<0.05) ADF than the sterilized/dried at 15 d treatment: 4.1 % higher in cultivar A, 6.3 % higher in B and 5.2 % higher in C. Among the colonized stovers, only cultivar C had higher (P<0.05) ADF than cultivar A (3.5 %). The pure G. lucidum contained 68 % ADF, far more than all the stover treatments (P<0.05) (Figure 3).
Lignin content differed (P<0.0001) between the three cultivars, with a notable effect (P<0.0001) from treatment and a slight but significant (P<0.0001) interaction (Figure 4). The untreated stovers had the lowest (P<0.05) lignin values (2.1-2.5 %), with only a 0.4 % difference (P<0.05) between cultivars C and B (Figure 4). The sterilized/dried stovers had higher (P<0.05) lignin contents than the untreated stovers; 1.0 % more in cultivar A, 1.4 % more in B and 0.7 % more in C. Lignin content did not differ between the sterilized/dried stovers. This variable was also higher (P<0.05) in the sterilized/dried at 15 d treatment than in the untreated stovers (0.9 % higher in cultivar A, and 1.5 higher in B), although it did not differ (P>0.05) in cultivar C (0.4 %). Indeed, in the sterilized/dried at 15 d treatment lignin content in cultivar C was 0.5 % lower than in cultivar A and 0.8 % lower than in cultivar B (Figure 4).

Bars represent honestly significant difference in the comparison of means.
abc Different lowercase letters on bar points indicate significant difference (P≤0.05).
Figure 4 Lignin content of the three evaluated corn stovers in four treatments, including colonization with the white rot fungus Ganoderma lucidum
Lignin content was highest (P<0.05) overall in the G. lucidum-colonized treatment in all three cultivars. Compared to the untreated stovers, colonization with G. lucidum increased lignin content by 1.9 % in cultivar A, 2.5 % in B and 1.6 % in C. The colonized stover also had higher lignin content than both the sterilized treatments: about 1% higher for cultivars A and B (both treatments); 0.9 % higher than the cultivar C sterilized/dried treatment, and 1.3 % higher than the cultivar C sterilized/dried at 15 d treatment (Figure 4). Within the G. lucidum-colonized treatment, lignin content was 0.5 % lower in cultivar C than in cultivar B. The highest overall lignin content (6.4 %) was in the pure G. lucidum (Figure 4).
Crude protein (CP) content differed (P<0.0001) between cultivars, which was affected (P<0.0001) by treatment and exhibited an interaction effect (P<0.0001) (Figure 5). This variable was lowest (P<0.05) in untreated cultivars A (1.1 %) and B (2.0 %); however, untreated cultivar C had 3.0 % CP, higher (P<0.05) than the other two cultivars. Most of the sterilized stovers had CP content higher than the corresponding untreated stover; again, the exception was cultivar C for which this variable was lower (P<0.05) in the sterilized/dried treatment than in the untreated stover. The G. lucidum-colonized stovers all had higher (P<0.05) CP content than the untreated and the sterilized stovers: 7.2 % in cultivar A, 4.2 % in B and 3.8 % in C. The pure G. lucidum had the highest (P<0.05) CP content (9.4 %) overall (Figure 5).

Bars represent honestly significant difference in the comparison of means.
abc Different lowercase letters on bar points indicate significant difference (P≤0.05).
Figure 5 Crude protein (CP) content of the three evaluated corn stovers in four treatments, including colonization with the white rot fungus Ganoderma lucidum
Discussion
The sterilized stovers lost hydrosoluble substances. This coincides with previous reports of losses of carbohydrates, soluble proteins, organic and inorganic acids, and minerals caused by sterilization with high-pressure steam5,6. This would explain the lower fiber content in the unsterilized stovers, since the absence of leaching, and consequent nutrient loss, would have allowed them to maintain a higher soluble substances content by weight (mainly cellular contents). Sterilizing by autoclave would therefore have caused a dilution effect in stover NDF content.
The three evaluated cultivars varied in terms of cell wall and cellular contents. Cultivar A had the lowest amount of cell wall (perhaps due to thinner walls or a different chemical composition), while the hybrid (cultivar B) was more fibrous, a possible genetic differentiation related to plant architecture (i.e. erect leaves). Hybrid corns have been developed with erect leaves to increase sowing density, capture more solar radiation and therefore increase yield22,23. This trait also implies increased leaf venation, and changes in venation pattern and sclerenchyma24, which result in higher fiber content. Inter-cultivar differences in cell wall fiber content, even among hybrids, is widely reported and confirms diversity between cultivars15,25.
The stovers colonized with G. lucidum contained more fiber than the untreated stovers, as shown in NDF values. This fungus was also found to be quite fibrous. Several reports indicate that in G. lucidum fibrous compounds are synthesized in the mycelium and contain high concentrations of polysaccharides such as mannose, xylose, arabinose, galactose, glucose and rhamnose9,10,11,12, as well as chitin13. This suggests that in the colonized stover NDF values were higher due to the combination of the fungus’s insoluble compound content and the stover fiber content. Some cell wall degradation by the fungus was expected, but the mycelial development time and evaluated colonization time used in the present study may have been insufficient to permit significant lignin solubilization. For example, when inoculated onto a mixture of maple, chestnut and blackberry with other ingredients, G. lucidum did not degrade lignin until three to five months post inoculation8. Only 15 d colonization was allowed in the present study, which is apparently not enough time to detect degradation of cell wall components. It is possible that the fungus began by metabolizing stover cell content, which could explain the lack of any significant decrease in NDF content. This is supported by previous studies of fungus-inoculated corn stovers. Various maize hybrid stovers inoculated with fungi such as Sporotrichum pulverulentum, Bjerkandera adusta and Trametes trogii, were found to have higher concentrations of the β-glucosidase and exoglucanase enzymes during the first fifteen days’ incubation15. This indicates a preference for cell parts containing reducing sugars, including those easily available in cell contents. Several fungi, including brown-rot fungi (Serpula lacrymans, Coniophora puteana and Gloeophyllum trabeum, among others) consume large amounts of fermentable sugars26, which are very accessible in the cell content. White-rot fungi are also known to consume a higher proportion of non-structural sugars during initial colonization27.
Comparing the present results obtained with G. lucidum with previous research is challenging because many studies have utilized substrates composed of 80 % corn stover mixed with other grain-derived ingredients28. In addition, fungus growth times are not mentioned in some studies. However, decreases in NDF of up to 5 % have been reported in an inoculated substrate (59.76 %) versus an uninoculated control (64.94 %)28; this is a larger decrease than observed in the present results.
The principal difference between the evaluated cultivars was NDF content, since both ADF and lignin content differed little between them (Figures 3 and 4). Therefore, the main differences between cultivars were due to the contents of hemicelluloses and other soluble substances in the neutral detergent fiber. Sterilization increased fiber content in the treated corn stovers, and inoculation raised it higher in response to higher ADF and lignin contents.
Acid detergent fiber (ADF) levels in untreated cultivar A stover were lower than in the sterilized/dried treatment, implying that hydrolysis may have occurred in the cell wall, which contributed to the interaction effect. The cultivar C stover had the highest lignin content, which did not change significantly after sterilization and drying after 15 d, and probably contributed to the interaction effect. These data suggest that the evaluated cultivars differ in terms of cell wall composition.
Both ADF and lignin contents were highest in the stovers colonized with G. lucidum. Some edible fungi can degrade lignin in forages via enzymes and other compounds2, although this was not observed in the G. lucidum-colonized corn stovers evaluated here. One possible explanation is that this fungus contains chitin13 and traces of lignin9-12, which would have caused the colonized stovers to exhibit higher values for these two variables. This contrasts with a previous study in which inoculation of corn stover (14.9 % initial lignin content) with G. lucidum reduced lignin values by 6 %, although fungus growth times and stover preparation methods are not specified29.
In vitro dry matter digestibility (IVDMD) was higher in the sterilized/dried and sterilized/dried at 15 d treatments than in the untreated and G. lucidum-colonized stovers. The stovers in both of the sterilized treatments exhibited higher values for the three evaluated fibers, but the pressure, temperature, and possibly the water vapor, involved in the sterilization technique increased their digestibility. Pressurized steam treatment of corn stover has been reported to improve digestibility by reducing polymerization, breaking down bonds between hemicellulose, cellulose and lignin, hydrolyzing hemicellulose, increasing porosity, and changing the cellulose crystalline structure30.
In cultivar C, sterilization followed by humid storage for 15 d prior to drying resulted in more hydrolysis than with sterilization followed immediately by drying, which contributed to the interaction effect. This may have occurred due to differences in composition of the synthesized hemicellulose compounds. Sterilization of substrates at temperatures greater than 100 °C and in moisture for more than 45 min degrades macromolecule structure and function due to denaturation and hydrolysis31,32. Temperatures higher than 85 °C partially break hydrogen bonds in the lignin-cellulose complex, solubilizing simple sugars33, which may explain the increased digestibility in the sterilized treatments.
In the untreated stovers, digestibility was lower in cultivars B and C than in A, highlighting inter-cultivar differences in digestibility15. The overall lowest digestibility values were observed in the G. lucidum-colonized stovers because fungus mycelium components such as chitin, lignin and structural polysaccharides apparently negatively influenced digestibility9-12.
Colonization with G. lucidum increased CP content in all three evaluated corn stovers. Several proteins have been isolated from G. lucidum mycelia, including LZ-8, which contains 110 amino acids34. A polysaccharide-protein complex has also been found in G. lucidum which contains various essential amino acids35. These amino acids may have increased CP content in the colonized stovers. Inoculation with other fungi, such as strains of Ceriporiopsis subvermispora, Lentinula edodes, Pleurotus eryngii or Pleurotus ostreatus, has also been reported to increase CP content by approximately 30%6; in the present study this was notable in cultivar Criollo A.
Compared to the untreated stovers, both the sterilized/dried and sterilized/dried at 15 d treatments had higher CP values. This probably occurred due to hydrolysis of cell wall compounds, since substrates sterilized at temperatures above 100 °C with moisture are known to lose macromolecule structure and function31,32. The cultivar C stover in the sterilized/dried at 15 d treatment was the one exception in that CP content decreased, possibly because its components were more soluble, which contributed to the interaction effect. Further study is needed to better understand the causes of this loss.
Conclusions and implications
The three untreated stovers differed in terms of NDF and CP contents, but not in ADF and lignin. Digestibility among the untreated stovers was highest in cultivar A. Steam sterilization increased NDF and ADF content. It also raised lignin content, but not in cultivar C, which exhibited the most pronounced negative interaction. Stover crude protein content differed in response to sterilization, increasing it in one, leaving it unchanged in others, and even lowering it another. Digestibility generally increased after sterilization, indicating the strong effect of high temperature and pressure. Colonization with G. lucidum for 15 d did not improve digestibility, but rather lowered it by increasing fiber concentrations. In contrast, CP content increased after G. lucidum colonization (7.2 % for cultivar A, 4.2 % for B and 3.8 % for C), raising it above CP contents in both the untreated and sterilized treatments. Colonization with the white-rot fungus Ganoderma lucidum increased crude protein content in the three evaluated corn stovers but did not affect in vitro digestibility. Longer fungus growth times would be required to detect changes in the corn stover cell wall, mainly via solubilization of lignin and other elements, and any consequent improvement in digestibility.
Acknowledgements
The Consejo Nacional de Ciencia y Tecnología (CONACYT) granted a doctoral sholarship to LSPM.
REFERENCES
1. Dejene M, Divon RM, Walsh KB, McNeill D, Seyoum S, Duncan AJ. High-cut harvesting of maize stover and genotype choice can provide improved feed for ruminants and stubble for conservation agriculture. Agron J 2021;114(1):187-200. [ Links ]
2. Arora D, Sharma R. Enhancement in in vitro digestibility of wheat straw obtained from different geographical regions during solid state fermentation by white rot fungi. BioResources 2009;4(3):909-920. [ Links ]
3. Salmones D, Mata G, Waliszewski K. Comparative culturing of Pleurotus spp. on coffee pulp and wheat straw: biomass production and substrate biodegradation. Bioresource Technol 2005;96(5):537-544. [ Links ]
4. Tao L, Zhang L, Tu Y, Zhang F, Si W, Ma T, et al. Improving the in situ ruminal degradability of maize stalk using fungal inoculants in dorper × thin-tailed Han crossbred ewes. Small Ruminant Res 2016;144:119-125. [ Links ]
5. He Y, Dijkstra J, Sonnenberg A, Mouthier T, Kabel M, Hendriks W, et al. The nutritional value of the lower maize stem cannot be improved by ensiling nor by a fungal treatment. Anim Feed Sci Tech 2019;247:92-102. [ Links ]
6. Tuyen V, Phuong H, Cone J, Baars J, Sonnenberg A, Hendriks W. Effect of fungal treatments of fibrous agricultural by-products on chemical composition and in vitro rumen fermentation and methane production. Bioresource Technol 2013;129:256-263. [ Links ]
7. Atuhaire A, Kabi F, Okello S, Mugerwa S. Optimizing bio-physical conditions and pre-treatment options for breaking lignin barrier of maize stover feed using white rot fungi. Anim Nutr 2016;2(4):361-369. [ Links ]
8. Zhou S, Zhang J, Ma F, Tang C, Tang Q, Zhang X. Investigation of lignocellulolytic enzymes during different growth phases of Ganoderma lucidum strain G0119 using genomic, transcriptomic and secretomic analyses. Plos One 2018;13(5):1-20. [ Links ]
9. Evsenko M, Shashkov A, Avtonomova A, Krasnopolskaya L, Usov A. Polysaccharides of basidiomycetes. Alkali-Soluble polysaccharides from the mycelium of white rot fungus Ganoderma lucidum (Curt.: Fr.) P. Karst. Biochemistry (Mosc.) 2009;74(5):533-542. [ Links ]
10. Zhou H, Liu G, Huang F, Wu X, Yang H. Improved production, purification and bioactivity of a polysaccharide from submerged cultured Ganoderma lucidum. Arch Pharm Res 2014;37(12):1530-1537. [ Links ]
11. Hu Y, Ahmed S, Li J, Luo B, Gao Z, Zhang Q, et al. Improved ganoderic acids production in Ganoderma lucidum by wood decaying components. Scientific Reports 2017;7(46623):1-10. [ Links ]
12. Ma Y, He H, Wu J, Wang C, Chao K, Huang Q. Assessment of polysaccharides from mycelia of genus Ganoderma by Mid-Infrared and Near-Infrared Spectroscopy. Sci Rep 2017;8(10):1-10. [ Links ]
13. Mesa N, Ospina S, Escobar D, Rojas D, Zapata P, Ossa C. Isolation of chitosan from Ganoderma lucidum mushroom for biomedical applications. J Mater Science Mater Med 2015;26(135):1-9. [ Links ]
14. van Kuijk S, Sonnenberg A, Baars J, Hendriks W, Cone J. Fungal treated lignocellulosic biomass as ruminant feed ingredient. Biotechnol Adv 2015;33(1):191-202. [ Links ]
15. Tirado-González DN, Jáuregui-Rincón J, Tirado-Estrada GG, Martínez-Hernández PA, Guevara-Luna F, Miranda-Romero LA, Production of cellulases and xylanases by white-rot fungi cultured in corn stover media for ruminant feed applications. Anim Feed Sci Tech 2016;221:147-156. [ Links ]
16. ANKOM Technology. Operator´s Manual ANKOM 200/220 Fiber analyzer. ANKOM Technology: Macedon, New York, USA. 2017. [ Links ]
17. Jones D, Hayward M. The effect of pepsin pretreatment of herbage on the prediction of dry matter digestibility from solubility in fungal cellulase solution. J Sci Food Agric 1975;26:711-718. [ Links ]
18. Clarke T, Flin PC, McGowan AA. Low cost pepsin-cellulase assays for prediction of digestibility of herbage. Grass Forage Sci 1982;37(2):147-150. [ Links ]
19. A.O.A.C. Association of Official Analytical Chemists. Official Methods of Analysis. 12th edition. A.O.A.C. Washington DC. USA 1975. [ Links ]
20. Federer, W. Experimental design: theory and application. 6th ed. New York. Macmillan. Oxford & IBH: 1979;544. [ Links ]
21. Statistical Analysis System (SAS). User’s Guide. Statistics, version 9.0. SAS Institute Inc. Cary, North Caroline, USA 2002. [ Links ]
22. Chen X, Xu D, Liu Z, Yu T, Mei X, Cai Y. Identification of QTL for leaf angle and leaf space above ear position across different environments and generations in maize (Zea mays). Euphytica 2015;204(2):395-405. [ Links ]
23. Perez R, Fournier C, Cabrera-Bosquet L, Artzet S, Pradal C, Brichet N, et al. Changes in the vertical distribution of leaf area enhanced light interception efficiency in maize over generations of selection. Plant Cell Environ 2019;42:2105-2119. [ Links ]
24. Ford D, Cocke A, Horton L, Fellner M, Van Volkenburgh. Estimation, variation and importance of leaf curvature in Zea mays hybrids. Agr Forest Meteorol 2018;148(10):1598-1610. [ Links ]
25. Wolf DP, Coors JG, Albrecht KA, Undersander DJ, Carter PR. Forage quality of maize genotypes selected for extreme fiber concentrations. Crop Sci 1993;33:1353-1359. [ Links ]
26. Narasyanaswami N, Deehran P, Verma S, Kumar S. Biological pretreatment of lignocellulosic biomass for enzymatic saccharification. In: Fang Z, editor. Pretreatment techniques for biofuels and biorefineries, green energy and technology. Berlin Heildelberg: Springer-Verlag; 2013:3-34. [ Links ]
27. Karunanandaa K, Varga GA. Colonization of crop residues by white-rot fungi: cell wall monosaccharides, phenolic acids, ruminal fermentation characteristics and digestibility of cell wall fiber components in vitro. Anim Feed Sci Tech 1996;63:273-288. [ Links ]
28. Luna MP, Leal-Lara H, García-Pérez Á, Corona L, Romero-Pérez A, Márquez-Mota CC. Evaluation of 21 fungal strains as pretreat ment of corn stover: chemical composition and in vitro digestibility. J Animal Sci 2019;97(Suppl S3):444. [ Links ]
29. Asgher M, Shahid M, Kamal S, Iqbal HMN. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J Mol Catal B: Enzym 2014;101:56-66. [ Links ]
30. Zhao S, Li G, Zheng N, Wang J, Yu Z. Steam explosion enhances digestibility and fermentation of corn stover by facilitating ruminal microbial colonization. Bioresource Technol 2018;253:244-251. [ Links ]
31. Madigan M, Martinko J, Stahl D, Clark D. Brock biology of microorganisms. 13th ed. California, USA: Prentice Hall International; 2012. [ Links ]
32. Albertó E. Cultivo intensivo de los hongos comestibles: cómo cultivar champiñones, gírgolas, shiitake y otras especies. 1a ed. Buenos Aires: Hemisferio Sur; 2008. [ Links ]
33. Stölzer S, Grabbe K. Mechanisms of substrate selectivity in the cultivation of edible fungi. Mushroom Sci 1991;13:141-145. [ Links ]
34. Paterson R. Ganoderma A therapeutic fungal biofactory. J Phytochem 2006;67(18):1985-2001. [ Links ]
35. You Y, Lin Z. Protective effects of Ganoderma lucidum polysaccharides peptide on injury of macrophages induced by reactive oxygen species. Acta Pharmacol Sin 2000;23(9):787-791. [ Links ]
Received: June 07, 2021; Accepted: November 07, 2022











 texto em
texto em 


