Bovine viral diarrhea (BVD) remains one of the most common endemic diseases of cattle and other ruminant populations worldwide. Furthermore, BVD has a significant economic impact on the cattle industry due to its negative effects on cattle reproduction and health conditions1,2. BVD is caused by a positive-sense single-stranded RNA genome virus termed bovine viral diarrhea virus (BVDV), belonging to the Flaviviridae family within the Pestivirus genus. BVDV is currently divided into three species: Pestivirus A (Bovine viral diarrhea virus 1, BVDV-1), Pestivirus B (Bovine viral diarrhea virus 2, BVDV-2), and Pestivirus H (HoBi-like pestivirus), which are segregated into subgenotypes3. Pestivirus A is subdivided into up to 21 subgenotypes (1a to 1u), Pestivirus B, and Pestivirus H into four subgenotypes each (a to d)4. Further, BVDV strains are classified in cytopathic (CP) and non-cytopathic (NCP) biotypes according to their effect on replication and morphological changes induced in cell culture. This classification is relevant because cytopathogenicity in vitro is not related to cytopathogenicity in vivo. Thus, NCP strains are predominant in the field, involved in most natural infection cases and persistent infections. In contrast, CP strains are rare and isolated almost exclusively from a fatal form of BVD named mucosal disease (MD)5.
BVDV infection is characterized by clinical manifestations, including respiratory, gastrointestinal, and reproductive disorders. However, reproductive failures such as abortions, mummification, stillbirth, congenital defects, and the birth of persistently infected animals (PI) are considered of major economic importance6.
PI animals are generated as a result of transplacental infection with NCP BVDV strain during the first 125 d of gestation. Such animals acquire immunologic tolerance towards the infecting BVDV strain and develop persistent infection; hence, a PI calf will not induce an immune response by antibodies or T-cells against the virus7. Additionally, PI cattle shed the virus in body secretions like nasal and oral discharges, milk, urine, feces, and semen throughout their entire lives. Therefore, they are considered a permanent source of viral infection and play an essential role in BVD pathogenesis and epidemiology8.
Calves born as PI appear normal and sometimes as weak animals but are characterized by reduced growth rates, immunosuppression, and high death rate2. Moreover, PI has increased morbidity and mortality rates owing to susceptibility to other diseases and may eventually die from pneumonia or MD. Most PI calves succumb to MD, usually between 6 to 24-mo old9,10. Nevertheless, older PI cattle of 3, 5, and 7-yr old have been previously reported, implying a broader viral dissemination period2,11,12.
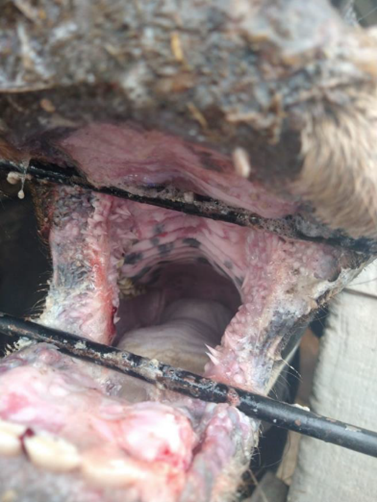
MD is a sporadic fatal condition restricted to PI cattle that occurs when the PI causative NCP BVDV mutates into CP as a result of a recombination event or when the PI animal is co-infected with an antigenically homologous related strain of CP BVDV13,14. Therefore, both biotypes can be consistently found in animals with MD15,16. The outcome of MD is death occurring within two weeks after the onset of the clinical signs. Erosions and extensive ulceration of the gastrointestinal tract are the main lesions found17. Conversely, late MD onset after several months has also been described18. Other clinical signs include anorexia, fever, dehydration, diarrhea, dermatitis, necrosis of lymphoid tissue, poor condition, and death19.
This case report describes the onset of MD in a two-year bull with severe clinical signs suggesting the description of PI cattle from Mexico for the first time.
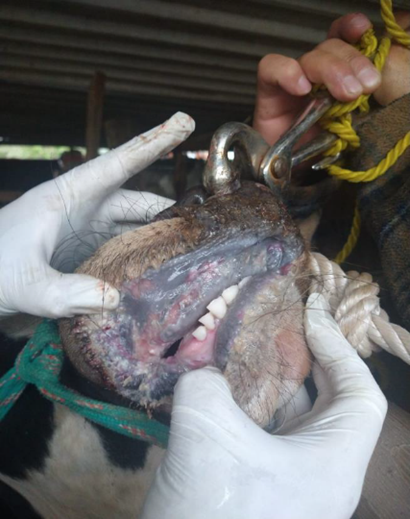
On June 2021, a 2-year-old bull was reported with 15 d course of clinical signs including anorexia, depression, ptyalism, severe hemorrhagic watery diarrhea, dehydration, nasal discharge, and deep and extensive ulceration in muzzle, nares, lips, gums, and hard palate (Figures 1, 2, and 3). The affected animal belonged to a traditional backyard farm located in Texcoco, State of Mexico, Mexico. The farm kept four bovines, four horses, six dogs, and three pigs, apparently healthy at the report. No similar clinical manifestations were registered in the neighboring farms prior to the event. According to the owner, no animal mobilization among nearby farms, and new animals were introduced.

Figure 1 Two years old bull with mucosal disease presentation showing erosive lesions in nasal discharge, extensive ulceration in muzzle and nares
Scab samples from skin lesions, whole blood, and feces samples were obtained and submitted for diagnosis to the Immunology, Cellular and Molecular Biology Laboratory from the Comisión México-Estados Unidos para la prevención de fiebre Aftosa y Otras enfermedades exóticas de los Animales (CPA). The case report was identified with the number CPA-0861-21. Main vesicular cattle diseases were considered for differential diagnosis, including foot and mouth disease (FMD), vesicular stomatitis (VS), malignant catarrhal fever disease (MCF), and BVD using RT-PCR, PCR, ELISA, and virus isolation. Negative results were obtained on viral isolation in cell culture, RT-PCR, and ELISA for FMD and VS. Similarly, the MCF virus was not detected by PCR in surveyed samples.
Conversely, BVDV was isolated from scab samples, and positive amplification was obtained from whole blood samples using RT-PCR. Consequently, the BVDV isolate was submitted to the Molecular Biology Laboratory of the Centro Nacional de Servicios de Diagnóstico en Salud Animal (CENASA) for partial sequencing. The 5'UTR, Npro, and E2 BVDV sequences obtained were deposited in GenBank under accession numbers OM812936, OM812937, and OM812938, respectively. Moreover, phylogenetic analysis was performed based on 5'UTR, Npro, and E2 regions. Partial 5'UTR (360 bp), Npro (504 bp), and E2 (1482 bp) sequences obtained in this study were compared to BVDV reference strains to characterize BVDV isolate. The evolutionary history was inferred using the Maximum likelihood method with a Kimura 2-parameter substitution model20 for 5'UTR and Npro sequences, and a Tamura 3-parameter substitution model21 for the E2 sequences was conducted in commercial software MEGA7 using 1000 bootstrap replicates each (Figure. 4). A discrete gamma distribution with two categories was used to model evolutionary rate differences among sites, with some sites being evolutionary invariable for Npro and E2 sequences.

Phylogenetic inference was conducted in MEGA 7 according to maximum likelihood method. Analysis was supported by 1000 bootstraps replicates. Reference sequences are identified by GenBank accession number. Mexican nucleotide sequences are highlighted with symbol "⧫"
Figure 4 Phylogenetic tree based on 5'UTR region (a), Npro (b), and E2 (c) sequences
BVD continues to be a significant concern to the cattle industry, with substantial economic impact mainly associated with reproductive disorders2. Depending on the stage of pregnancy at the time of infection, transplacental infections with BVDV NCP strains may result in the birth of immunotolerant PI calves. These animals are consistently viremic, BVDV spreads through most organs in the animal, but no apparent lesions are developed22. Consequently, PI cattle sustain lifelong viral replication and excretion in all body secretions23. Thus, PI animals represents the main transmission and maintenance source of BVDV within and between herds. Moreover, NCP BVDV can also be transmitted from acutely infected cattle and by fomites such as contaminated surgical and handling material, rectal examination, bovine sera used in embryo transfer, and vaccine production, infected semen, and contaminated vaccines24-27.
Further, BVDV infections directly impact PI animals' fertility, i.e., PI bulls can produce semen of acceptable quality. However, they are associated with poor fertility related to spermatozomal abnormalities and low motility28. Likewise, BVDV infections alter ovarian function by causing hypoplasia and reduced ovulations in PI cows29. Nevertheless, bulls and PI cows can still sire normal PI offspring, which may recirculate BVDV in susceptible dams30.
Continual exposure of healthy animals to BVDV from a PI animal may lead to the perpetuation of BVDV infections31; thus, herd infertility, immunosuppression, and generation of new PI calves may arise32. Furthermore, acute NCP infections compromises herd fertility by producing retarded and reducing follicle growth33) and diffuse interstitial ovaritis34, and conception failure by preventing embryo implantation22. In addition, embryonic death before d 79 of gestation in pregnant cows or congenital malformations between days 79 and 150 can also occur35.
In areas where adequate BVDV control measures are implemented, the estimated prevalence of PI animals is around 1%-2 %1; however, no report of MD outbreaks nor presentation in the Mexican bovine population has been previously described. In addition, the current proportion of PI's calves in the country remains unknown. Recently, limited information regarding the BVDV genetic characterization and prevalence in Mexico has begun to be surveyed36.
In the present study, it was described a case of MD by BVDV-1b affecting a beef bull in which ulcerative lesions in the gastrointestinal tract were predominant. BVDV-1b is currently defined as the most common strain found in the field; thus, it is considered the predominant subgenotype worldwide, followed by 1a and 1c4. BVDV-1b is also described as the most prevalent strain in PI calves37. Similar to these studies, the genetic characterization of the virus isolated from the evaluated bull in this study, reveals the identification of BVDV subgenotype 1b. The latter correlates to a previous study where BVDV-1b was described as an endemic virus circulating in Mexican cattle, together with 1a, 1c, and 2a38. Despite these initial efforts to report BVDV cases, BVD remains a non-regulated disease hence no control strategies nor prevention measures are officially implemented.
Consequently, vaccination protocols are based on voluntary procedures, and monitoring and biosafety measures are applied depending on cattle producers' BVD knowledge. The evaluated bull from this clinical case belongs to a farm where scarce sanitary measures and no vaccination practices against BVDV are applied. BVDV positive tests and clinical presentation suggest an MD case developed in a PI bull of 2 yr old.
The latter has important implications for BVD control in the nation. These results confirm the presence of BVDV-1b circulating in Mexican cattle, similar to the findings reported by Gómez-Romero et al38. Clinical presentation from the case highlights the severe outcome of MD and the relevance of underdiagnoses of PI animals and, therefore, BVDV epidemiological status. Furthermore, national BVD case reports will impulse the development of control strategies that allow producers to detect BVDV and remove PI calves from the herd. Moreover, when vaccination is applied, the choice of a specific vaccine should be evaluated for protection provided against circulating BVDV. In Mexico, the recent addition of BVDV-1b as vaccine antigen has been included in one commercial vaccine; however, vaccination alone is not adequate for the BVD control programs. The finding of BVDV-1b in a non-vaccinated bull demonstrates the crucial role of biosecurity and disease surveillance to mitigate the effects of BVDV infections in cattle populations.











 texto en
texto en