Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias pecuarias
versão On-line ISSN 2448-6698versão impressa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.14 no.1 Mérida Jan./Mar. 2023 Epub 24-Mar-2023
https://doi.org/10.22319/rmcp.v14i1.6273
Articles
Use of Wharton's jelly-derived mesenchymal stromal cells for the treatment of equine recurrent uveitis: a pilot study
a Universidad Nacional Autónoma de México. Facultad de Medicina Veterinaria y Zootecnia. Departamento de Medicina, Cirugía y Zootecnia para Équidos. Ciudad de México, México.
b Universidad Nacional Autónoma de México. Facultad de Medicina Veterinaria y Zootecnia. Posgrado en Ciencias de la Producción y de la Salud Animal. Ciudad de México, México.
c Universidad Nacional Autónoma de México. Facultad de Medicina Veterinaria y Zootecnia. Departamento de Microbiología e Inmunología. Ciudad de México, México.
d Comisión México-Estados Unidos para la prevención de fiebre Aftosa y otras enfermedades exóticas de los animales. Ciudad de México, México.
Equine recurrent uveitis (ERU) is a disease that affects 2 to 25 % of equines worldwide, 56% of which go blind; therefore, it is considered the most common cause of blindness in horses. ERU is a spontaneous immune-mediated condition characterized by recurrent intraocular inflammatory events. Currently, there is no treatment for horses with this disease. Mesenchymal stromal cells (MSCs) derived from various tissues, such as Wharton's jelly (WJ), have demonstrated their ability to modulate the immune response by negatively regulating the inflammatory process. The objective of this pilot study was to evaluate the effect of using MSCs derived from WJ as a treatment for ERU. The WJ was obtained and processed according to previously described methodologies for obtaining EMF. The horses involved in this study received a dose of 5x106 MSCs in the subpalpebral area. The research evaluated the concentration of interleukins (IL: IL-1(, IL-2, IL-10, IFN-(, and TNF() in tear samples obtained before treatment inoculation, 30 min after the inoculation, and 7 days post inoculation. No significant changes in IL concentration were observed suggesting a decrease in pro-inflammatory ILs. However, horses with ERU treated with MSCs exhibited a positive response to therapy, evidenced by a decrease in signs of ERU. The results obtained suggest that treatment of ERU with WJ-derived MSCs is a safe alternative with promising results.
Keywords Wharton's Jelly; Mesenchymal Stromal Cells; Equine recurrent uveitis; Therapeutics
La uveítis recurrente equina (URE) es una enfermedad que afecta del 2 al 25 % de los equinos a nivel mundial, de los cuales el 56 % se quedan ciegos; por lo tanto, es considerada la causa más común de ceguera en caballos. La URE es un padecimiento espontáneo inmunomediado caracterizado por eventos recurrentes de inflamación intraocular. Actualmente, no existe tratamiento para los caballos con esta enfermedad. Las células estromales mesenquimales (CEM) derivadas de diversos tejidos, como la gelatina de Wharton (GW), han demostrado su capacidad de modular la respuesta inmune al regular negativamente el proceso inflamatorio. El objetivo del presente estudio piloto fue el evaluar el efecto del uso de CEM derivadas de GW como tratamiento para la URE. La GW se obtuvo y procesó con base en metodologías previamente descritas para la obtención de CEM. Los caballos involucrados en este estudio recibieron una dosis de 5x106 CEM en la zona subpalpebral. Se evaluó la concentración de interleucinas (IL) (IL-1(, IL-2, IL-10, IFN-( y TNF() en muestras de lágrima obtenidas antes de la inoculación del tratamiento, 30 min después y 7 días post inoculación. No se observaron cambios significativos en la concentración de IL que sugieran la disminución de IL proinflamatorias. Sin embargo, los caballos con URE tratados con CEM mostraron una respuesta positiva a la terapia, evidenciada por la disminución en la signología de la URE. Los resultados obtenidos sugieren que el tratamiento de la URE con CEM derivadas de GW es una alternativa segura con resultados prometedores.
Palabras clave Gelatina de Wharton; Células estromales mesenquimales; Uveítis recurrente equina; Terapéutica
Introduction
Mesenchymal stromal cells (MSCs) are characterized by their ability to differentiate into various cell lineages; therefore, they may be involved in the regeneration of damaged tissues. Another important characteristic of MSCs is that they have anti-inflammatory properties and regulate the immune response by producing a set of immunomodulatory factors such as interleukin 6 (IL-6), prostaglandin E2 (PEG2), and nitric oxide1,2. The secretion of these factors inhibits the proliferation of activated T lymphocytes, reduces the secretion of proinflammatory cytokines, and increases the population of regulatory T lymphocytes (Tregs)2,3,4.
In equines, MSCs can be obtained from bone marrow, adipose tissue, amniotic membrane, umbilical cord blood, and foal umbilical cord tissue known as Wharton's jelly (WJ)5. WJ is the primitive mucous connective tissue of the umbilical cord that lies between the amniotic epithelium and the umbilical vessels; it consists of a hyaluronic acid and chondroitin sulfate-based substance with a high concentration of MSCs6. Due to their molecular characteristics, such as the absence of the expression of histocompatibility molecules I and II, these cells offer a unique advantage for autologous and allogeneic application7. In particular, WJ is considered an important source of MSCs, both in humans and in other species, with great potential in the therapeutics of various inflammatory and immune-mediated conditions, such as equine recurrent uveitis (ERU).
ERU, also known as moon blindness, is a disease recognized as the leading cause of blindness in horses. It has been reported to be prevalent in 2 to 25 % of equines in the USA8. It is characterized by recurrent episodes of intraocular inflammation or low levels of persistent inflammation, predominantly in the iris, ciliary body, and choroid9. This disease has an acute presentation that includes signs such as miosis, decreased intraocular pressure, and iris adhesions, while its chronic presentation results in the development of cataracts, glaucoma, and blindness.
The triggering or etiological factors of ERU remain unknown; however, it has been reported that genetic components as well as Leptospira interrogans infections may be involved in the development of this condition8,10. Subsequently, the signs that occur in ERU are the result of T lymphocyte activation, specifically Th1 and Th17, causing destruction of the uveal tract of the eye11,12,13. Currently, there is no cure for ERU; therefore, treatment focuses on decreasing inflammation with the goal of preserving vision, limiting the recurrence of episodes, and reducing pain with anti-inflammatory and mydriatic drugs14.
It has been shown that the use of MSCs in immune diseases of dogs, cats, and horses can induce the switch of proinflammatory T lymphocyte subsets to regulatory T lymphocytes15,16,17. Therefore, the use of WJ-derived MSCs in the treatment of ERU is a promising alternative. This article describes the procurement, culture, characterization and differentiation of MSCs derived from umbilical cord WJ of foals at foaling and their preliminary use in horses with ERU.
Material and methods
MSC procurement and cultivation
WJ MSCs were collected from 26 foals of full English blood mares (EBM) aged 5 to 20 yr. The umbilical cords were collected was performed following the delivery of the placenta. They were handled and processed under sterile conditions at the Tissue Engineering, Cell Therapy, and Regenerative Medicine Unit of the National Rehabilitation Institute of Mexico.
In short, a 15 to 20 cm fragment of each cord, still wrapped in amnion, was taken, followed by two washes with iodine solution interspersed with washes of sterile physiological saline solution (PSS). The sections were then cut to approximately 5 cm and stored at 4 ºC in phosphate buffered solution (PBS) with penicillin (10,000 U/ml), amphotericin B (25 µg/ml) and streptomycin (10,000 µg/ml) for processing in the laboratory. Subsequently, the WG was separated from the umbilical cord tissue and deposited in a Petri dish with PBS where cuts were made to facilitate enzymatic digestion. The latter was carried out in 10 ml of Dulbecco's modified Eagle's medium (DMEM) solution with collagenase (0.8 mg/ml) in incubation at 37 ºC for 1 hr. After the incubation time had elapsed, the cells were centrifuged at 700 xg for 7 min at 37 ºC, the supernatant was decanted and the cells were resuspended in DMEM supplemented with 10 % fetal bovine serum (FBS) and 1% penicillin, amphotericin B and streptomycin (10,000 U/ml; 25 µg/ml; 10,000 µg/ml). The primary culture was maintained in 25 cm2 cell culture bottles incubated with 5 % CO2 at 37 ºC, and three passages were performed once 80 % confluence was reached.
EMF characterization
Cells obtained before the third passage were subjected to surface phenotype evaluation in order to corroborate their mesenchymal profile by flow cytometry. It was used 2.5 x 105 cells contained in round-bottom polystyrene tubes resuspended in 1ml PBS. For cell labeling, cells were incubated for 1h with specific primary antibodies for detection of CD90 (FITC Mouse Anti-Human CD90 Clone 5E10 555595), CD73 (APC Mouse Anti-Human CD73 Clone AD2560847), CD105 (PE Mouse Anti-Human CD105 Clone 266 560839), CD45 (FITC Mouse Anti-Human CD45 Clone G44-26 555478), CD34 (PE Mouse Anti-Human CD166 Clone 34 559263), CD14 (PerCP Mouse Anti-Human CD14 Clone MφP9 340585), and MHC-II (APC Mouse Anti-Human HLA-DR Clone G46-6 559866). Cells were then washed twice and analyzed using the FACS-Calibur Becton and Dickinson flow cytometer.
EMF differentiation
WJ MSCs were grown in 12-well plates at a density of 5x104 using DMEM supplemented with 5% SFB and 1% antibiotic, under the same culture conditions as previously mentioned. After 48 h, the culture medium was replaced by adipogenic, osteoblastic, and chondrogenic medium as described below.
For the adipose lineage induction, after 48 h of incubation the cell culture medium was replaced by differentiation medium formulated with DMEM supplemented with 0.5% SFB, dexamethasone (1 mM), 3-isobutylmethylxanthine (0.5 mM), insulin (10 %), indomethacin (50 mM), as well as penicillin (10,000 U/ml), amphotericin B (25 µg/ml), and streptomycin (10,000 µg/ml). Medium changes were performed every third day for 21 d. Finally, cell differentiation was evaluated using Nile red staining.
MSC differentiation into osteoblastic lineage was carried out using DMEM culture medium supplemented with SFB 1%, and with dexamethasone (100 nM), ascorbic acid (0.05 mM), 10 mM/L -glycerophosphate and BMP-7 (10 ng/ml). Likewise, the medium was changed every third day for 21 d, and osteogenic differentiation was evaluated by Von Kossa staining.
The chondrogenic lineage was obtained by using DMEM culture medium added with insulin (10 %), ascorbic acid (1 mg/ml), transforming β growth factor (TGF-β) (10 ng/ml), sodium pyruvate (1 %), and bone morphogenic protein 2 (BMP-2) (100 ng/ml). The evaluation of the differentiation was carried out with Alcian blue staining.
Horses
A total of 15 EBM horses aged 2 to 7 yr were included for this study. Twelve clinically healthy horses were used as a control group. As part of the experimental group, 3 horses with at least one episode of ERU with characteristic signs such as miosis, iris hyperpigmentation, blepharospasm, corneal edema, aqueous flame, hypopyon, hyphema, epiphora, photophobia, fibrin in the anterior chamber, conjunctival hyperemia, and scleral injection were considered.
The horses used in this study underwent a strict general physical and complete ophthalmological examination consisting of threat reflex assessment, pupillary response, consensual reflex, Schirmer's test, corneal sensibility, flourescein stain, Jones test, rose Bengal stain and fundus observation.
Tear sample collection and EMF inoculation
Once the control and experimental groups were formed, the horses were sedated using intravenous xylazine at a dose of 0.3 a 0.5 mg/kg. Subsequently, 100 µl of tears were collected using a sterile capillary tube without additives; the sample was placed in sterile vials and stored at -80 ºC until use.
Using an insulin syringe, the inoculum, PBS and 5x106 MSCs were collected for six horses of the control group, as well as for the three horses with ERU of the experimental group; both in a volume of up to 200 (l. This part of the procedure was carried out under sterile conditions. Prior to inoculum application, the area was aseptically cleaned with alcohol, avoiding direct contact with the eye. A 25 G needle was inserted into the subpalpebral area and the syringe was connected to inoculate the contents. The second and third tear samples were taken 30 min and one week post inoculation, respectively.
Evaluation of interleukins in tear samples
The evaluation of interleukins IL-1, IL-2, IL-10, IFN- and TNF in tear samples obtained before and after the treatment was performed by multiplex enzyme-linked immunoassay (ELISA), in order to determine whether there are changes in the pattern of interleukins detected.
For the multiplex ELISA test, the Equine Cytokine/Chemokine Magnetic Bead Panel Milliplex MAP Kit was used, following the manufacturer's instructions on the Luminex equipment (Bio-Plex 200, Bio-Rad Laboratories, EU). Briefly, 200 (l of wash buffer was added to each of the 96 wells of the plate, the plate was covered and incubated in agitation for 10 min at 20 ºC; the contents of the wells were then discarded, removing the excess by turning the plate over and tapping it on a bed of absorbent towels. Subsequently, 25 (l of the standard and cores were added to the corresponding wells. 25 (l of assay buffer were added to the sample wells. Subsequently, 25 (l of matrix solution were added to all wells, and 25 (l of tear sample were added to the corresponding wells. Finally, 25 (l of the bead mixture were added to each of the wells of the plate, which was incubated for 18 h under agitation at 4 ºC covered with aluminum foil.
After the incubation time had elapsed, the entire contents of the plate were discarded, and the plate was washed three times. Subsequently, 25 (l of interleukin detection antibodies were added to all wells, the plate was sealed and incubated in agitation at room temperature for 1 h. Then 25 (l of streptavidin-phycoerythrin were added to all wells, the plate was sealed and incubated in agitation at room temperature for 30 min. Once this step was completed, the contents were again decanted, and three washes were performed. To each of the wells, 150 (l of "Seath Fluid" were added and agitated for 5 min at room temperature. Finally, the plaque was read to estimate the concentration of interleukins detected in the tear samples corresponding to the three sampling times.
Statistical analysis
For the analysis of the results of the interleukin concentration, nonparametric statistics were used according to the results obtained from the homogeneity of variance (analysis of residuals) and normal distribution (Shapiro-Wilk test) tests included in the Prism 8.0 statistical program (GraphPad, Software Inc., EEUU).
In particular, the Mann Whitney U test was used to compare the concentration of cytokines between the media used (PBS and MSC) in the control group. Subsequently, this test was repeated to compare the concentration of cytokines between the control and experimental groups. Additionally, the Kruskal Wallis test followed by Dunn's multiple comparison test was performed to determine possible changes in cytokine concentration at the different times evaluated (baseline, after 30 min, and after 7 d) in both groups. In all cases, a value of P<0.05 was considered significant.
Results
MSC procurement and cultivation
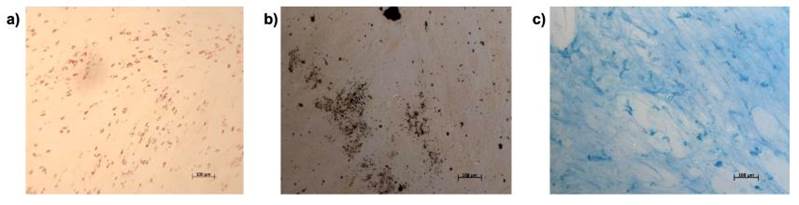
Primary cultures of MSCs obtained from WJ initially showed a rounded morphology and clustered in clusters of up to 100 m. Once attached, after 120 h they acquired fibroblastoid morphology (Figure 1).

a) Rounded morphology (white arrow) of MSCs and formation of cell clusters (black arrow); b) adherence and fibroblastoid morphology of MSCs (black arrow); c) 80 % confluence of the EMF monolayer with characteristic morphology (black arrow); c) 80 % confluence of the EMF monolayer with characteristic morphology.
Figure 1 Primary culture of mesenchymal stromal cells obtained from Wharton's jelly
Characterization of EMF
The identification of positive MSC surface markers was carried out by flow cytometry. CD90, CD73, and CD105 markers should be expressed, while negative markers are CD14, CD34, CD45, and MHC-II. Figure 2 shows the phenotype characterization of MSCs obtained from WJ. The lack of expression of CD45, CD34, and CD14 markers is shown; so is the expression of the positive markers CD73 and CD90. On the other hand, the MScs samples evaluated showed a reduction in the expression of the CD105 marker.
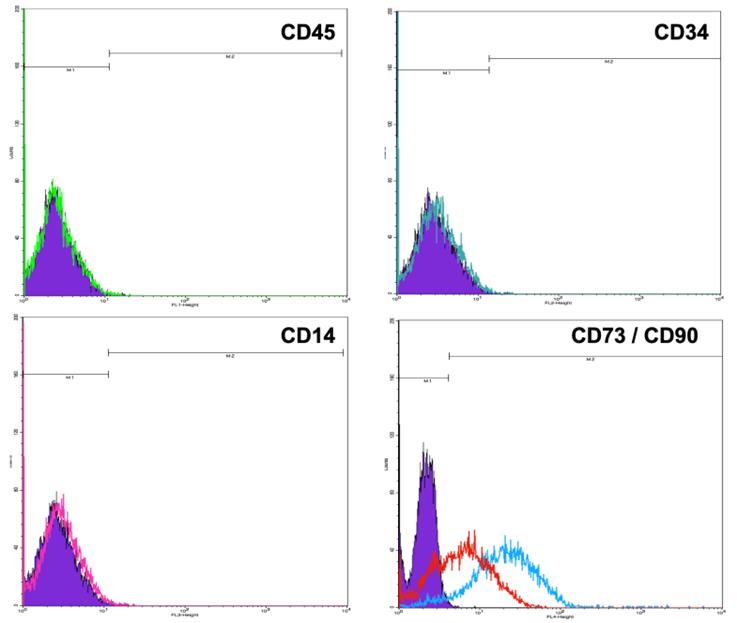
Flow cytometry analysis of cultured WJ-derived MSC protein expression labeled with anti CD45 (green), CD34 (turquoise), CD14 (pink), CD73 (red), and CD90 (blue) antibodies. The histogram in purple indicates the intensity of the fluorescence of MSCs labeled with the control antibody. Open histograms indicate positive reactivity with the indicated antibody.
Figure 2 Phenotype of cultured MSCs
MSC differentiation
MSCs were treated with three formulations of culture medium to evaluate their differentiation into adipose, osteoblastic, and chondrogenic cell lineages. Differentiation was assessed by cell staining (Figure 3); shown are representative images of a) adipocytes stained with Nile red to detect fatty acid vacuoles within cells; b) osteoblasts stained with Von Kossa stain to identify calcium deposits; c) chondrocytes detected with Alcian blue stain, mucopolysaccharide staining is visualized in the extracellular matrix of this tissue.
Evaluation of horses with ERU
Horse 1. Warmblood, 12-yr-old castrated male Retinto. He presented signs of uveitis and started treatment with betamethasone, cyclosporine A, and artificial tears. Subsequently, the MSC treatment protocol was started, and only the left eye was injected; throughout the week, no clinical changes were observed.
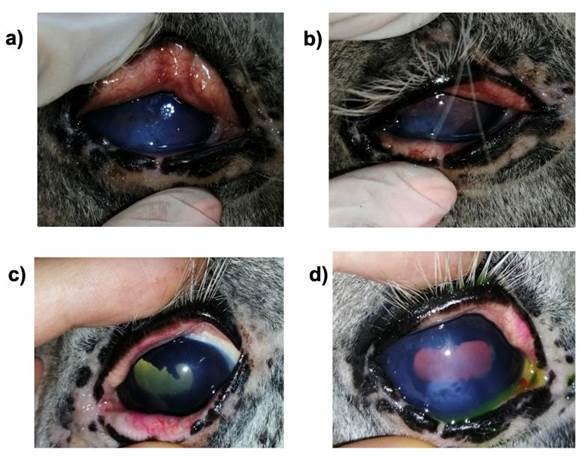
Horse 2. Friesian, 15-yr-old whole male. He exhibited acute uveitis in the left eye, and had been previously treated with prednisolone and cyclosporine A. He showed signs such as pain, edema, vascularization, epiphora, and blepharospasm. (Figure 4). One week after the MSC treatment, he exhibited improvement from the clinical point of view; all the previous signs decreased slightly, and he showed a better mood.
a) shows blepharospasm and epiphora, b) shows edema and vascularization; palpebral margins are green due to previous fluorescein staining. c) shows a decrease in blepharospasm of the left eye d) shows decreased edema.
Figure 4 Horse 2
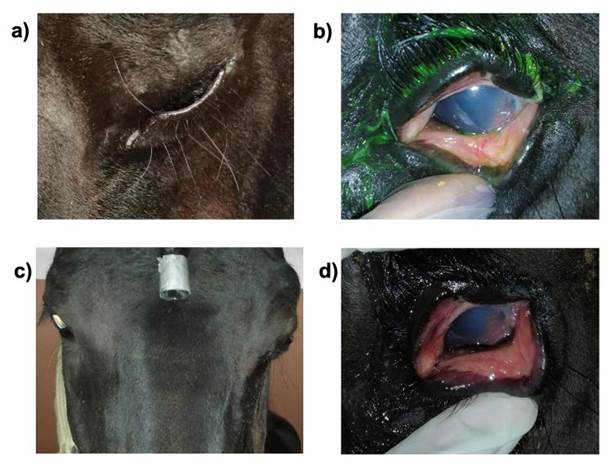
Horse 3. Apaloosa, entire male, 21 yr old. He exhibited an acute condition, but received no treatment. Both eyes had miosis, epiphora, corneal edema, and neovascularization, scleral and conjunctival injection, both eyes were treated with MSCs and maintained for one week with a topical mydriatic. At 7 d both eyes were mydriatic, without pain, minor epiphora, edema, and conjunctival and scleral injection. Three days after the second visit, he started medical treatment, but he exhibited even less edema and was much more comfortable (Figure 5).
Evaluation of interleukins in tear simples
Comparison between the media used (PBS and MSC) for the control group showed no differences in any of the interleukins measured. However, when comparing the concentration of these at different times (basal, after 30 min and after 7 d), it was found that the concentration of IL-1⍺ with the use of PBS as vehicle, showed statistically significant differences between the basal measurement and the one performed 7 d later (Table 1).
Table 1 Measurement of interleukins by median and range technique (<Quantification level)
| Control group(n=12) | Experimental group(n=3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal | 30 minutes | 7 days | Basal | 30 minutes | 7 days | |||||||
| Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | |
| IL-1α | 43.27 | 38.71 - 53.20 | 41.62 | 36.95 - 51.00 | 38.71* | 36.17 - 46.36 | 40.39 | 38.31 - 45.32 | 41.26 | 35.19 - 43.78 | 38.31 | 36.17 - 39.06 |
| IFN-γ | 33.94 | 7.450 - 175.3 | 18.52 | 3.900 - 186.6 | 28.3 | 3.900 - 341.5 | 93.12 | 25.22 - 97.09 | 45.18 | 11.17 - 95.31 | 108.3 | 14.78 - 329.7 |
| IL-2 | 1.323 | 0.4534 - 10.75 | 0.781 | 0.1316 -14.56 | 0.8325 | 0.3679 - 30.42 | 1.383 | 0.5992 - 1.729 | 0.8926 | 0.4229 - 1.611 | 1.516 | 0.9307 - 2.082 |
| IL-10 | 19.52 | 6.709 - 70.25 | 19.43 | 7.694 - 46.30 | 17.84 | 6.709 - 52.23 | 18.59 | 10.34 - 19.46 | 12.43 | 7.857 - 14.38 | 14.89 | 11.49 - 28.56 |
| TNF-α | 14.63 | 2.200 - 56.80 | 2.998 | 2.200 - 52.03 | 25.61 | 2.200 - 70.88 | 11.53 | 2.200 - 19.22 | 2.797 | 2.200 - 15.64 | 13.86 | 8.015 -17.99 |
IL-1⍺ (*) detected 7 d post-treatment showed a statistically significant decrease compared to the baseline measurement (P=0.0356). Values are reported in pg/ml.
Since no statistically significant differences were found between the two media applied to the control group, the measurements were considered within a single group and contrasted with the data obtained in the experimental group. In this regard, no statistically significant differences were found in the concentration of ILs at any of the three times when they were used when comparing the control group with the treated experimental group.
Discussion
In the field of regenerative medicine, interest in MSC research has increased over the last decade. These cells, also known as mesenchymal progenitor cells, have the ability to promote tissue regeneration, modulate the immune response, and regulate the inflammatory process18. They are also considered to be cell populations with the ability to self-renew and differentiate into various types of connective tissue cells19. Consequently, they have the potential to act at sites of inflammation by synthesizing interleukins that participate in the modulation of this process20.
Specifically, previous studies have described the immunomodulatory effect of MSCs in horses; additionally, it has been shown that equine-derived MSCs, compared to those of other species, have a greater capacity to inhibit the proliferation of activated T lymphocytes and to decrease the production of IFN-( and TNF(1,3. Likewise, it has been reported that the use of equine MSCs as a treatment induces lymphocyte apoptosis and reduces IL-2 receptor (CD25) expression in lymphocytes T CD4+.
In equine medicine, they are currently used, above all, to treat diseases of the locomotor system, skin wounds, equine metabolic syndrome, asthma, laminitis, neurological, and ophthalmological problems21. Within the latter, ERU is considered the leading cause of blindness in equines and is described as an autoimmune inflammatory disease with characteristics similar to human uveitis22. Horses suffering from ERU are characterized by an inflammatory phenotype of Th1 lymphocytes (CD4+ IFN-); therefore, the described data suggest that the use of MScs is a suitable alternative in the treatment of ERU, as well as other immune-mediated diseases23. Together, ocular therapeutic benefits have been documented in equine and other species24,25,26.
In particular, it has been proven that the application of MSCs in rabbits with corneal surface damage accelerates the corneal healing process, reduces oxidative stress, and suppresses the production of proinflammatory interleukins, resulting in a decrease in corneal opacity and neovascularization of the affected area27. In rats, the use of MSCs in the treatment of corneal burns and reconstruction of the corneal surface has yielded positive results25.
On the other hand, its use in horses as a treatment for immune-mediated keratitis has yielded promising results; it was observed that 3 out of 4 horses submitted to this therapy had positive results evidenced by the decrease in opacity, irregularity, and vascularization on the corneal surface; in addition to maintaining the corneal disease stable for up to one year after MSC treatment. Therefore, it is considered a new alternative immunomodulatory therapy for this condition24. Likewise, in another study on equine immune-mediated keratitis, a satisfactory response to MSC inoculation in the ophthalmic artery and subconjunctival topical application three times a day for three weeks were reported28.
In the present study, was evaluated parameters that would indicate the benefits of subpalpebral administration of EMF in the treatment of ERU. Once the treatment with WJ-derived MSC was applied in horses with ERU, tear samples were analyzed to evaluate the pattern of interleukins present in the sample before and after treatment (30 min and 7 d after). However, no significant changes in interleukin concentrations were observed at the different times evaluated where a decrease in proinflammatory interleukins and an increase in anti-inflammatory interleukins were expected, as that horses with ERU are said to maintain a high concentration of IL-10, IL-1(, IFN-(, IL-6, and IL-17 in tears23.
These results may be associated with the route of administration, where MSC inoculation via the subpalpebral route shows less efficacy in resolving the condition, probably because it reaches the site of action (eye) in an insufficient proportion, in an inadequate dosage and frequency of treatment application, and at an inadequate stage of the disease, therefore having a low capacity to affect the inflammatory process at that level, since most reports indicate a significant improvement when the treatment is applied at the acute phase of the disease23. On the other hand, the absence of proinflammatory IL detection in tear samples may be due to the peak concentration occurring at different periods of the disease than those evaluated in this study.
These findings are useful when choosing the route of administration for MSC treatment. Although success stories of the use of the subconjunctival route have been described, there is a need to evaluate and compare additional routes of administration that may provide better results for short- and long-term efficacy. In contrast, intravenous administration of MSCs has been reported to be completely safe; however, it is not known whether or not it yields better results29. Therefore, further studies are needed to establish the necessary conditions for the treatment of ERU.
Subsequently, in the three patients with ERU who were part of the present pilot study, the effect of treatment with subpalpebral MSCs was evaluated. No clinical changes indicating improvement were observed in horse 1. In contrast, horse 2 showed improvement of the clinical signs presented (pain, edema, vascularization, epiphora, and blepharospasm), including improvement in mood 7 d after post-treatment. In the case of horse 3, there was improvement in some clinical signs such as less epiphora, reduction of edema and conjunctival and scleral injection, as well as improvement in mood.
Two of the three treated horses showed improvement and decrease in clinical signs of ERU 7 days post treatment. The positive results obtained when applying equine-derived WJ MSCs highlight the importance of further studies to establish a uniform treatment and the development of an efficient MSC application protocol to achieve better results. For example, treatment options promoting a long-lasting response and treatment efficacy are enhanced by a specific number of, or multiple applications with a standardized MSC dose, as well as by co-application with local immunosuppressive therapy30.
Conclusions and implications
This pilot study describes the experimental use of WJ-derived MSCs for the treatment of ERU. Although there were certain limitations, e.g. in the number of animals analyzed that might allow us to reach firm conclusions, the obtainment of positive results in the respective clinical presentations without generating adverse effects reaffirms the use of MSCs as a viable alternative to the treatment of ERU. Despite its promising results, controlled studies of MSC treatment must be carried out in order to demonstrate and confirm the benefits of the MSC treatment for ERU.
REFERENCES
1. Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, Borjesson DL. Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells. Cell Med 2012;4(1):1-11. doi: 10.3727/215517912X647217. [ Links ]
2. Martínez‐Montiel MDP, Gómez‐Gómez GJ, Flores AI. Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol 2014;20:1211‐1227. [ Links ]
3. Carrade Holt DD, Wood JA, Granick JL, Walker NJ, Clark KC, Borjesson DL. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev 2014;23:1258‐1265. [ Links ]
4. Le Blanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunol Lett 2015;168:140‐146. [ Links ]
5. Iacono E, Rossi B, Merlo B. Stem cells from foetal adnexa and fluid in domestic animals: an update on their features and clinical application. Reprod Dom Anim 2015; 50:353-64. doi: 10.1111/rda.12499. [ Links ]
6. Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev 2016;2:155-162. [ Links ]
7. Iacono E, Pascucci L, Rossi B, Bazzucchi C, Lanci A, Ceccoli M, et al. Ultrastructural characteristics and immune profile of equine MSCs from fetal adnexa. Reproduction 2017;154:509-519. doi: 10.1530/REP-17-0032. [ Links ]
8. Gilger BC, Hollingsworth SR. Diseases of the uvea, uveitis, and recurrent uveitis. In: Gilger BC, editor. Equine ophthalmology. Hoboken, NJ: John Wiley & Sons, Inc. 2016: 369-415. doi: 10.1002/9781119047919.ch8. [ Links ]
9. Gilger BC, Deeg C. Chapter 8‐Equine recurrent uveitis. Gilger BC editor. Equine Ophthalmology, 2nd ed. Saint Louis, MO: W.B. Saunders; 2011:317‐349. [ Links ]
10. Sauvage AC, Monclin SJ, Elansary M, Hansen P, Grauwels MF. Detection of intraocular Leptospira spp. by real-time polymerase chain reaction in horses with recurrent uveitis in Belgium. Equine Vet J 2019;51:299-303. [ Links ]
11. Deeg CA. Ocular immunology in equine recurrent uveitis. Vet Ophthalmol 2008;11(Suppl 1):61‐65. [ Links ]
12. Deeg CA, Ehrenhofer M, Thurau SR, Reese S, Wildner G, Kaspers B. Immunopathology of recurrent uveitis in spontaneously diseased horses. Exp Eye Res 2002;75:127‐133. [ Links ]
13. Gilger BC, Malok E, Cutter KV, Stewart T, Horohov DW, Allen JB. Characterization of T‐lymphocytes in the anterior uvea of eyes with chronic equine recurrent uveitis. Vet Immunol Immunopathol 1999;71:17‐28. [ Links ]
14. Gilger BC, Michau TM. Equine recurrent uveitis: new methods of management. Vet Clin North Am Equine Pract 2004;20:417-27. doi: 10.1016/j.cveq.2004.04.010. [ Links ]
15. Kol A, Walker NJ, Nordstrom M, Borjesson DL. Th17 pathway as a target for multipotent stromal cell therapy in dogs: Implications for translational research. PLoS One 2016;11:e0148568. [ Links ]
16. Arzi B, Mills-Ko E, Verstraete FJM, Kol A, Walker NJ, Badgley MR, et al. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl Med 2016;5:75-86. [ Links ]
17. Holt DDC, Wood JA, Granick JL, Walker NJ, Clark KC, Borjesson DL. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev 2014;23:1258-1265. [ Links ]
18. Stewart MC, Stewart AA. Mesenchymal stem cells: characteristics, sources, and mechanisms of action. Vet Clin North Am Equine Pract 2011;27(2):243-61. doi: 10.1016/j.cveq.2011.06.004. [ Links ]
19. Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med 2014;12:260. [ Links ]
20. Meirelles LS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009;20(5-6):419-27. doi: 10.1016/j.cytogfr.2009.10.002. [ Links ]
21. Cequier A, Sanz C, Rodellar C, Barrachina L. The usefulness of mesenchymal stem cells beyond the musculoskeletal system in horses. Animals (Basel). 2021;11(4):931. doi:10.3390/ani11040931. [ Links ]
22. Malalana F, Stylianides A, McGowan C. Equine recurrent uveitis: Human and equine perspectives. Vet J 2015;206(1):222-9. doi: 10.1016/j.tvjl.2015.06.017. [ Links ]
23. Saldinger LK, Nelson SG, Bellone RR, Lassaline M, Mack M, Walker NJ, Borjesson DL. Horses with equine recurrent uveitis have an activated CD4+ T-cell phenotype that can be modulated by mesenchymal stem cells in vitro. Vet Ophthalmol 2020;23(1):160-170. doi: 10.1111/vop.12704. [ Links ]
24. Davis AB, Schnabel LV, Gilger BC. Subconjunctival bone marrow-derived mesenchymal stem cell therapy as a novel treatment alternative for equine immune-mediated keratitis: A case series. Vet Ophthalmol 2019;22(5):674-682. doi: 10.1111/vop.12641. [ Links ]
25. Jiang TS, Cai L, Ji WY, Hui YN, Wang YS, Hu D, Zhu J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis 2010;14;16:1304-16. [ Links ]
26. Dodi PL. Immune-mediated keratoconjunctivitis sicca in dogs: current perspectives on management. Vet Med (Auckl) 2015;30;6:341-347. doi: 10.2147/VMRR.S66705. [ Links ]
27. Cejkova J, Trosan P, Cejka C, Lencova A, Zajicova A, Javorkova E, Kubinova S, Sykova E, Holan V. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res 2013;116:312-23. doi: 10.1016/j.exer.2013.10.002. [ Links ]
28. Marfe G, Massaro-Giordano M, Ranalli M, Cozzoli E, Di Stefano C, Malafoglia V, Polettini M, Gambacurta A. Blood derived stem cells: an ameliorative therapy in veterinary ophthalmology. J Cell Physiol 2012;227(3):1250-1256. doi: 10.1002/jcp.22953. [ Links ]
29. Kol A, Wood JA, Carrade HDD, Gillette JA, Bohannon-Worsley LK, Puchalski SM, et al. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res Ther 2015;6(1):73. doi: 10.1186/s13287-015-0050-0. [ Links ]
30. Zhang J, Huang X, Wang H, Liu X, Zhang T, Wang Y, Hu D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther 2015;6:234. doi: 10.1186/s13287-015-0240-9. [ Links ]
FinancingFunding for the study was granted by Project No. IN228919 of the Support Program for Research and Technological Innovation Projects (Programa de Apoyo a Proyectos de Investigación e Innovación tecnológica, PAPIIT), “Use of allogenous stem cells for the treatment of equine recurrent uveítis” ("Uso de células troncales alógenas para el tratamiento de uveítis recurrente equina") of the Faculty of Veterinary Medicine and Animal Husbandry-Universidad Nacional Autónoma de México (Facultad de Medicina Veterinaria y Zootecnia-Universidad Nacional Autónoma de México).
Received: June 27, 2022; Accepted: August 01, 2022











 texto em
texto em