Introduction
Insulin-like growth factor I (IGF-1) is the most important known growth factor for cartilage repair in horses, because it stimulates proteoglycan synthesis, and therefore extra cellular matrix (ECM), and promotes mitosis of chondrocytes. It has an important growth-promoting activity not only in articular cartilage, but in several tissues, mainly in muscle, bones and brain. It activates the mitogen-activated protein kinase (MAPK) pathway, having several effects in promoting: cell survival, growth, proliferation, protection to hypoxia, inflammation regulation in muscle injuries and in bone growth plates1-4). It has also a key role in brain development, along with estradiol, regulating a variety of developmental and neuroplastic events5. In structure, it is very similar to insulin. When insulin is instilled in a joint, IGF-1 expression in synovial fluid is enhanced6.
Injuries of the articular cartilage are normally repaired by substitution with fibrocartilage, which leads to loss of function of the joint resulting in osteoarthritis (OA)7. Lameness is the most frequent reason for which equine practitioners are required from horse owners, and OA represents more than 60 % of all lameness cases in sport horses8. The main problem in OA is inflammation, conditioning an imbalance between catabolism and anabolism in the articular cartilage. In this particular tissue, the only cellular component is constituted by chondrocytes, which are responsible of ECM synthesis, in order to maintain adequate cartilage function.
There is evidence regarding exogenous efficacy of IGF-1 in vitro, which enhances proteoglycan synthesis by stimulated chondrocytes. Other therapies, such as chondrocyte transplantation from mature and neonatal chondrocytes, gene therapy strategies to upregulate IGF-1 expression by transfected chondrocytes, require general anesthesia, a surgical procedure and therefore specialized equipment and personnel9.
On a pilot study conducted by the authors in which 13 synovial fluid samples obtained from different joints (distal interphalangeal joints, metacarpophalangeal joints, shoulder joints, tarsometatarsal joints, and stifles) from horses with associated lameness AAEP (American Association of Equine Practitioners) grade: 2/5, no radiographic changes but a positive response on 1-minute flexion test. By ELISA, a significant increase of IGF-1 and a positive correlation between total protein and IGF-1 levels in synovial fluid (data not shown) were found. In this study it was obtained synovial fluid samples from 21 horses with different degrees of OA (confirmed by intraarticular block and radiographic changes) in the metacarpo-phalangeal joint (MCPJ), where IGF-1 and total protein correlated positively in horses with acute OA, and negatively in horses with chronic OA and marked bone remodeling. In horses with mild of non-radiogrphic changes (acute OA), IGF-1 correlated negatively with interleukin-6 (IL-6) and tumoral necrosis factor alpha (TNF(). Interestingly, were able to find by western blot, at least two functional isoforms of IGF-1 expressed in synovial fluid, one present only in control horses, and the other in lame horses.
To our knowledge, there is no information regarding IGF-1 fluctuations on naturally occurring OA. There are no in vivo studies regarding IGF-1 levels during OA. Perhaps this paper can help practitioners to understand the role of IGF-1 for this particular condition and could be used as a baseline for further studies on IGF-1 concentration and its possible use as an alternative treatment.
Material and methods
Synovial fluid samples were obtained from Warmblood and Thoroughbred horses (n=11) from two different disciplines: showjumpers (Warmblood) (n= 8), and race horses (Thoroughbred) (n= 3) with a mean age of 10.5 yr old and a mean weight of 520 kg. Control (Ctrl) samples were obtained from two geldings, Warmblood horses of 5 and 7 yr old. No more control horses were available for the study, since they all were sound, it was not easy to obtain consent from the owners to sample their joints. A complete lameness evaluation and radiographic assessment were performed in all control horses in order to be included in this study. None of them showed signs of front limb lameness and were negative to passive and active flexion tests (30 sec). Additionally, none of them presented any radiographic changes associated with joint pathology in the metacarpophalangeal joint.
Lameness evaluation
A clinical evaluation was performed on all horses included in this study, in order to find evidence of lameness associated with the metacarpo-phalangeal joint of the front limbs. Evaluation consisted on static observation, palpation and passive flexion response; dynamic evaluation of walk and trot on a straight line and lunged on hard and soft surface. All included horses demonstrated a 2 and 3/5 lameness (AAEP), with a positive flexion test (1 min). Additionally, all horses were positive to low-4-point block (lateral and medial palmar nerves and lateral and medial metacarpal nerves), using 2 and 1.5 ml respectively of 2 % mepivacaine (Carbocaine, Zoetis Inc.) and further intra-articular block of the metacarpal-phalangeal joint, using a volume of 6 ml of 2 % Mepivacaine (Carbocaine, Zoetis Inc.) as previously described10. Any horse negative to these blocks, was excluded from the study.
Synovial fluid collection
All synovial fluid samples were obtained from the metacarpal-phalangeal joint MCPJ joints of lame horses, using an aseptic technique on the palmaro-lateral approach as previously described and 5 d after the intraarticular (IA) block10. Samples were obtained from healthy horses and were used as controls (n= 4).
Radiographic evaluation
All selected horses were radiographically evaluated from the MCPJ, using four standard views (dorso-palmar, latero-medial, dorso-lateral palmaro-medial, and dorso-medial palmaro-lateral) in order to assess the radiologic condition of all horses. Three different grades of radiologic changes were determined associated with the clinical condition of the horse (Table 1).
Table 1 Grade of severity and its relation on clinical and radiographic findings on horses included in this study
| Grade | Radiographic and clinical findings |
|---|---|
| I | Non to minor changes associated with joint pain and lameness: Irregularity and loss of normal homogeneity of the sagittal ridge of MTCIII. |
| II | Moderate changes associated with joint pain and lameness: Osselets (osteophytes) on P1 and MTCIII. |
| III | Severe changes associated with severe lameness and decrease of motion range: suprachondilar or subchondral lysis, osteophytes and new bone formation with periostic reaction and loss of articular space. |
(Modified from: Verwilghen D, et al. 2009)11.
Protein concentration determination
The concentration of total protein from all synovial fluid samples was obtained by using the BCA Protein Assay Kit, (Pierce BCA Protein Assay Kit cat. 23225), according with the manufacturer’s instructions. For each sample the final concentration was 100 (g/50 (L for the ELISA procedure.
IGF-1 concentration analysis
Determination of IGF-1 concentration in synovial fluid samples of control and osteoarthritic horses was made with 50 (L using a commercial ELISA kit (Horse IGF1 ELISA kit, #MBS017382, MyBio-Source®) following the manufacturer’s instructions.
Interleukin 6 (IL-6) concentration
A quantitative determination of IL-6 in synovial fluid of all samples was performed using a commercial ELISA kit (Horse interleukin-6 ELISA kit, cat. #: CSB-E16634Hs), which is a sandwich immunoassay technique, where the plates are coated with a specific horse IL-6 antibody, then, a specific biotin-conjugated antibody for IL-6 and then avidin conjugated horseradish peroxidase (HRP) are added. Protocol is performed following manufacturer’s instructions.
Tumoral necrosis factor alpha (TNF() concentration
Determination of TNF( concentration in synovial fluid samples of control and osteoarthritic horses was made with 100 (l using a commercial ELISA kit (Equine TNF( ELISA kit, cat #: ESS0017 Invitrogen) following the manufacturer’s instructions.
Western Blot analysis
Equal amounts of protein (100 µg per lane), were subjected to a 16 % SDS-PAGE (90V for 30 min and 120 V for 3.5 h). Precision Plus Protein Dual Color Standards marker was used, containing ten prestained recombinant proteins (10 to 250 kD), including eight blue-stained bands and two pink reference bands (25 and 75 kD). After electrophoresis, gels were transferred using a semi-dry transfer system (271mA for 15 min) to PVDF (0.45uM) (Bio-Rad) membranes, which were blocked using 4% skim milk diluted in PBS (pH 7.4) and incubated on a shaker at 37 oC, 120 rpm for 2 h. After blocking, membranes were washed 3x (for 5 min each) using PBS containing 0.05% Tween-20. As a primary antibody, a goat polyclonal anti-IGF-1 (1:1000) (Sta. Cruz #Sc-1422) was used, incubated on a shaker first at 37 oC, 120 rpm for 2 h, and left overnight at 4 oC; membranes were washed again as previously described and as a secondary antibody, a polyclonal anti-goat IgG (1:5000) (Millipore #AP180B) was used, and incubated on a shaker at 37 oC, 120 rpm for 2 h and a final wash of the membranes was performed. Proteins were detected by using an enhanced chemiluminescence method and visualized using a high-resolution Imaging System (Bio-Rad ChemiDoc). Membranes were incubated to a 1:1 dilution of luminol and peroxidase (Merck Millipore, Luminata # WBLUF0500), and exposed at various times, where the optimum time of exposure was 35 seconds.
Results
A total of 45 horses were examined, from which only 11 horses (22 samples) were included in this study, and 2 horses (4 samples) as controls. All horses varied from each other in degrees of lameness and radiographic changes, and all responded positively to the digital flexion test, low-4-point block and intraarticular (IA) block of the fetlock joint. Six joints were scored as grade I, five joints were scored as grade II and 8 joints were grade III (Figure 1).
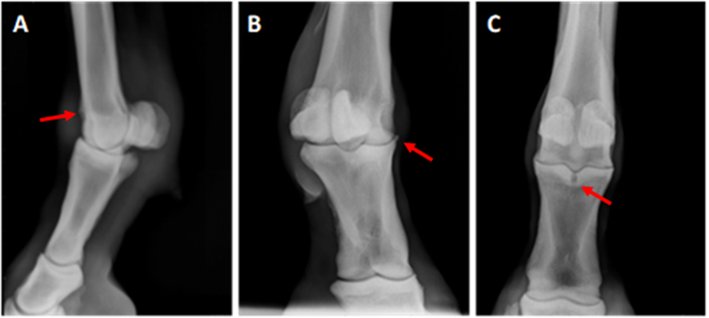
Grade 1 (A) Lateromedial view with a mild irregularity of the proximal-dorsal aspect of the sagittal ridge (arrow); Grade II (B) Dorsolateral palmaromedial oblique view with a visible osteophyte on the proximal dorso-medial aspect of P1 (arrow); and Grade III (C) Dorsopalmar view where a subchondral bone cyst in the proximal aspect of first phalanx in the sagittal groove with areas of bone sclerosis (arrow).
Figure 1 Representative radiographs from horses scored with various grades
IGF-1 concentration
All horses with joint associated pain, lameness, and less radiographic changes, demonstrated a significant increase of IGF-1 concentration (P<0.05) (Figure 2). All samples were repeated by pairs and read three times in a 5, 10 and 15-min period with no difference between measurements (data not shown) and the values of linear regression and standard curve were: P<0.001; r2=0.9931.
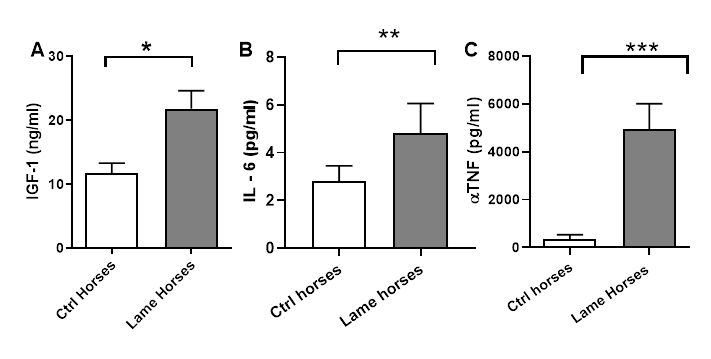
Figure 2 A) IGF-1 determination between control (sound) and lame horses, demonstrating a significant difference (P<0.05)*. B) Concentrations of IL-6 between controls and lame horses, showed a significant difference as well (P<0.01)**. C) TNF( concentrations between controls and lame horses showing significant differences in concentration (P<0.001)***.
IL-6 and TNF( analysis
Concentrations of IL-6 between controls and lame horses, showed a significant difference as well (P<0.01). TNF( concentrations between controls and lame horses showed even more significant differences in terms of concentration (P<0.001), being higher on lame horses with more severe changes in the affected joints (Grade III). A Pearson’s correlation analysis was performed demonstrating a positive correlation between total protein and IGF-1 concentrations (r= 1), which was seen in grade I and II horses, whereas in grade III this correlation is negatively or inversely proportional. In other words, the worse changes a joint had (as seen in grade III horses), the less IGF-1 concentration in synovial fluid was observed.
Western blot analysis
Horses with less radiographic changes demonstrated a higher IGF-1 concentration concordantly with the ELISA results for IGF-1 (Grade I and II). Horses with more severe radiographic changes and a chronic state of the pathologic condition (Grade III), were the ones with the lowest IGF-1 concentrations in both ELISA and WB analysis. Interestingly, with this analysis it was able to identify in all samples, two different bands, one of ~12 kDa which was seen only in control (normal) horses with no joint pathology, and another of ~7.5 kDa seen in all lame horses (Figure 3).

1: Protein marker (marking 10 kDa); 2: Samples from a control horse and a horse with OA; 3: Control horse; 4 & 5: Two different samples from horses with OA.
Figure 3 Representative photograph of Western blot analysis for IGF-1, demonstrating a difference in molecular weight between synovial fluid samples indicating the existence of two different isoforms present in normal joints and during an inflammatory process
Discussion
Sport horses are exposed to excessive loads to their joints and soft tissue structures. The joint that can udergo traumatic OA depends on the discipline in which the horse performs. There’s evidence regarding interventions such as joint injections on acute phases of the disease that can help modify its course and prevent further damage while the horse is still performing12.
Impact loads due to exercise are responsible of damaging articular cartilage by first cracking the surface, and depending on the force applied and the time it is being applied, the depth and therfore the severity of the development of the disease (OA) are produced. Characterization of mechanical consequences of impact injuries to articular cartilage has been proven to develop damage, by continuously and directly stressing the joint structures13.
When inflammation occurs, chondrocytes migrate to the lesion site in an attempt to regenerate the defect by forming groups of cells or clusters with the ability to synthesize ECM de novo. Since the cellular component (chondrocytes) of the articular cartilage is only 1-2 % of the whole tissue, they are unable to repair the damaged area, because their ability to synthesize ECM is surpassed by the matrix metalloprotease (MMP) activity which degrades the already damaged ECM aggravating the condition by increasing necrosis and activating local inflammation by releasing intracellular components which act as damage associated molecular patterns (DAMPs) and proinflammatory cytokines such as prostaglandins (PGs), nitrous oxide (NO), interleukin-1 (IL-1), interleukin-6 (IL-6), tumoral necrosis factor alpha (TNF() and substance P. Particularly, TNF( inhibits IGF-1 expression by increasing ECM catabolism, and blocking AKT pathway via activating JNK pathway. If the cartilage defect reaches subchondral bone, the cartilage repairs forming a low-quality articular cartilage called fibrocartilage2,4,7.
Factors that contribute to the inflammation cascade other than citokines, include extracellular vesicles, which play an important role on promoting joint inflammation and are also involved on apoptosis and ECM degradation. These vesicles are exosomes, microvesicles and apoptotic vesicles, which are all released to the articular cavity (into the synovial fluid), and have intimate relation with cell-cell communication during the inflammatory process7,8,14.
The aim of this study was to compare IGF-1 concentration in synovial fluid from sound (control) horses and horses with different degrees of lameness and joint pathology (OA) in the MCPJ. It was hypothesized that the more severe and chronic conditions of the joint, the highest IGF-1 levels in synovial fluid would be found, because of the joint’s high demand for repairing the defect was higher than in horses with mild changes. The rationale behind the hypothesis was: to our knowledge, there is still no data available regarding IGF-1 concentrations and its correlation with a particular clinical condition in horses, so it was conducted a pilot study, where a total of 13 synovial fluid samples were collected from different joints of different horses. All of these horses were high performance show jumpers with a positive flexion test from the sampled joints (no radiographic evaluation was conducted on any of these horses). What was found, it was a significant increase of total protein, with a positive correlation (Pearson’s correlation, P=0.0229) on IGF-1 levels in synovial fluid when compared to control samples (synovial fluid obtained from sound horses). This gave sufficient information to hypothesize that horses with more severe clinical signs and more chronic joint pathology would have higher IGF-1 levels when compared to control horses.
Interestingly, with the results obtained, this hypothesis was refuted. It was encountered that the horses with more severe radiographic changes and thus, the more chronic inflammatory conditions within the joint, were the ones that demonstrated a decrease on IGF-1 concentrations, very similar to what control horses had.
Similar results were seen on studies in which experimentally induced lesions on articular cartilage in horses, make an acute peak of mRNA expression of igf-1, and at 4 weeks tend to decrease. When IGF-1 decreases, TGF-( predominates and it is responsible of new bone formation and activation of quiescent lymphocytes to Th177.
Equine Insulin-like growth factor 1 (IGF-1) has been widely studied, there are several studies where its importance in cell proliferation, growth and survival, repair and extracellular matrix production is well documented, although there are not enough studies regarding the different isoforms and their functionality15. It is known that the mRNA undergoes post-transcriptional modifications (alternate splicing) which generates different isoforms along with post-translation modifications. IGF-1 propeptides are encoded by multiple alternately spliced transcripts including C-terminal extension peptides called E-peptides, and N-terminal signal peptides. When an immature protein has signal peptide, mature peptide and E peptide is called pre-proIGF-1, and when the signal peptide is eliminated leaving only the mature peptide and the E peptide, is called pro-IGF-1. These E-peptides control the bioavailabilty of mature IGF-1, by binding to the ECM due to their highly positive charge, preventing its systemic circulation and therefore, its local use. They also modulate mature IGF-1 re-entry to the cell in a murine muscle cell-line1.
In humans, three different IGF-1 isoforms have been identified (IGF-1Ea, IGF-1Eb and IGF-1Ec, also known as mecano-growth factor or MGF), and have been proposed to have various functions in muscle repair16.
Nixon, et al17 described the igf1 gene consisting of 5 exons with 4 intron sequences, which undergo both post-transcriptional and post-traslational modifications, where the translated proteins resulting from alternate splicing of exon 4 form a smaller propeptide (105 aminoacids) transcript named Pre-proIGF-1A consisting of signal peptide (encoded by exons 1 and 2), mature peptide (encoded by exons 2 and 3), and a C-terminal E-peptide encoded by exons 3 and 5); and when exon 4 is not alternately spliced, a larger transcript is translated forming Pre-proIGF1B (111 aminoacids)17. To our knowledge, this was the last research paper published regarding post-transcriptional and post-traslational modifications and alternate splicing of IGF-1 mRNA in horses. It was conducted a bioinformatic analysis of igf1 gene undergoing different types of alternate splicing, which according to Le, et al18 are: exon skipping, intron retention, mutually exclusive exons and alternative 5’ donor or 3’ acceptor sites. This analysis revealed that IGF-1 mRNA consisted of not 5, but 4 exons and 3 introns, which transcipts form 4 isoforms: variant 1 (exons 1-3), variant 2 (exons 2 and 3), variant 3 (exons 1-3, a 93 pb intron retention, and exon 4) and variant 4 (exons 2-4)18.
The Western blot analysis demonstrated the presence of at least two different functional isoforms of IGF-1, where the one seen in all normal horses is heavier (~12 kDa) than the one seen in all horses with different degrees of OA (7.5 kDa). Probably the lighter one is the mature form of IGF-1, although aminoacid sequencing techniques must be carried out in order to confirm this statement. With this result, can be presumed that the expression of these two different functional isoforms depends on inflammation.
This could lead to a new line of research which can focus on determine by advanced sequencing techniques the exact isoforms of IGF-1 and to target overexpression of the isoform which is not present when there is an inflammatory process of the joint, and its role on repairing cartilage defects.
Articular cartilage does not regenerate by itself, since is the only connective tissue in mammals that does not have either blood and lymphatic vessels, or nerves19. Therefore, it is virtually impossible to regenerate after an injury, so it is repaired via substitution with fibrous tissue, which generates a low quality fibrous cartilage called fibrocartilage. There have been several treatments to improve cartilage regeneration, in humans, osteochondral allograft transplantation has proven to be effective in function improvement and overall repair with graft survivorship of up to 80 % of the patients who had undergone previous surgical treatment: Microfracture, cartilage debridement, forage, abrasion chondroplasty, osteochondral and periosteal grafts, cartilage flap reattachment, among others7,17,20.
Local anestethics and steroids have been used widely by practitioners in the field, for diagnostic and therapeutic reasons respectively. However, excessive use of these components, have been proven to damage articular cartilage. Intraarticular injection using local anesthetics and steroids have make a growing concern about inducing potential toxicity to chondrocytes and synoviocytes. Sherman et al21, conducted an interesting comparisson of lidocaine, bupivacaine, betamethasone acetate, methylprednisolone acetate, and triamcinolone acetonide in a canine model. They found that in vitro, 1 and 0.5 % lidocaine, 0.2 and 0.25 % bupivacaine, betamethasone acetate and methylprednisolone acetate were severely chondrotoxic and synoviotoxic when compared with 0.625 % bupivacaine and triamcinolone21.
Conclusions and implications
For this reason, treatmentwise, the main goal is to have alternatives that could be used in the field by clinicians, that can provide an alternative other than steroids that can also enhance cartilage repair without the need of getting the horse under general anesthesia and still have an effect that lead to horses having a long lasting sport career. This paper provides important information that can serve as a base for further research regarding IGF-1 isoforms and their role in cartilage repair.











 texto en
texto en 


