Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.14 n.1 Mérida Jan./Mar. 2023 Epub Mar 24, 2023
https://doi.org/10.22319/rmcp.v14i1.6129
Artículos
Genetic variability in aerial biomass and its components in alfalfa under irrigation and drought
a Colegio de Postgraduados. Postgrado en Recursos Genéticos y Productividad. Carretera México-Texcoco km. 36.5, Montecillo, Texcoco, Estado de México, México.
Drought decreases the yield of aerial biomass (BM) and its components, and the quality of forage in alfalfa. The genetic variation in BM and its components was studied in 10 varieties of alfalfa under irrigation (I) and drought (D) in a greenhouse. A randomized complete block experimental design was used, with four repetitions in I and four in D. The experimental unit was an individual plant in a PVC pipe. Sowing was carried out on March 15, 2017, and transplanting in the pipes, 20 days after sowing. The fertilization dose 60-140-00 was applied at 44, 240 and 420 dat (days after transplanting). D reduced (P≤0.01) BM, leaf dry matter yield (LDMY), number of stems (NS) and radiation use efficiency (RUE). The plants in D did not recover their productive capacity after experiencing the water deficit, even after the recovery irrigation. D also decreased (P≤0.01) the phenotypic variance for BM and its components; the additive variance was greater (P≤0.01) than the dominance variance for all traits in I and D. The BM, L:S ratio, plant height (PH), NS and RUE had higher (P≤0.01) heritability in I and D. The Genex, Atlixco, Júpiter and Milenia varieties were the most productive (P≤0.01) in D and could be used for forage production in water-scarce areas or as parental lines for forage yield improvement in selection programs.
Key words Greenhouse; Heritability; Principal component analysis; Variance components
La sequía disminuye el rendimiento de biomasa aérea (BM) y sus componentes, y la calidad del forraje en alfalfa. Se estudió la variación genética en BM y sus componentes en 10 variedades de alfalfa bajo riego (R) y sequía (S) en invernadero. Se utilizó un diseño experimental de bloques completos al azar, con cuatro repeticiones en R y cuatro en S. La unidad experimental fue una planta individual en un tubo de PVC. La siembra se realizó el 15 de marzo de 2017 y el trasplante en los tubos, 20 días después de la siembra. Se aplicó la dosis de fertilización 60-140-00 a 44, 240 y 420 ddt (días después del trasplante). La S redujo (P≤0.01) la BM, el rendimiento de materia seca de hojas (RMSH), número de tallos (NT) y la eficiencia en el uso de la radiación (EUR). Las plantas en S no recuperaron su capacidad productiva después de experimentar el déficit hídrico, aún después del riego de recuperación. La S también disminuyó (P≤0.01) la varianza fenotípica para la BM y sus componentes; la varianza aditiva fue mayor (P≤0.01) que la varianza de dominancia para todos los caracteres en R y S. La BM, relación H:T, altura de planta (AP), NT y EUR tuvieron mayor (P≤0.01) heredabilidad en R y S. Las variedades Genex, Atlixco, Júpiter y Milenia fueron las más productivas (P≤0.01) en S, y podrían utilizarse para la producción de forraje en áreas con escasez de agua o como líneas parentales, para el mejoramiento del rendimiento de forraje en los programas de selección.
Palabras clave Análisis de componentes principales; Componentes de varianza; Heredabilidad; Invernadero
Introduction
In Mexico, alfalfa (Medicago sativa L.) for forage is grown mainly under irrigation conditions and consumes large volumes of water. In regions with irrigation systems, a plant canopy of alfalfa can consume an amount of water of 10 mm day-1 at its peak of maximum development1. In these growing conditions, the fall in the amount of precipitation over long periods of time decreases the water storage capacity in the subsoil and, therefore, the availability of irrigation. Likewise, when drought extends, the scarcity of water for irrigation is more severe and alfalfa crops may experience some degree of water stress, which can be reflected in a significant decrease in yield and forage quality2.
In the near future, the water resource will be less available for the production of alfalfa forage, due to the occurrence of frequent periods of drought, climate change and greater demands caused by the increase in the human population3. One way to meet the demand in alfalfa forage production will be through the obtaining of new varieties with drought tolerance, high capacity of osmotic adjustment and gas exchange, high water use efficiency (e.g., more dry matter per unit of transpired or evapotranspirated water) and productive capacity3. Alfalfa is considered a drought-resistant species, but its aerial biomass yield can fluctuate considerably under water deficit conditions; under these conditions, alfalfa has some agronomic advantages compared to other annual crops, as it has a root system that allows it to explore deeper soil layers to absorb water and tolerate drought to a greater degree; in addition to reducing the stomatal conductance and minimizing the transpiration rate4.
The most common reaction to a soil water deficit is the increase in the ratio of dry weight of root biomass/dry weight of aerial biomass, as a result of a greater reduction in the growth of aerial organs than in the growth of roots under drought. The increase in the root/aerial part ratio implies greater increases in root density with respect to aerial biomass, which is consequently reflected in a better capacity to maintain the water status of the plant under a given evapotranspiratory demand5. Drought also reduces the yield of aerial biomass and its components, relative rates of growth, transpiration and elongation of the stem, chlorophyll content, relative water content, and dry weight and diameter of the root6, and concentration of crude protein and water-soluble carbohydrates7.
On the other hand, drought-resistant alfalfa varieties exhibit high concentration of water-soluble carbohydrates in storage organs under conditions of severe water stress. This situation is combined with a water conservation strategy that implies less evapotranspiration in the initial phases of drought stress, due to a limited development of the root system that results in more available moisture, for its use under severe conditions of water stress8. Biomass accumulation rates in plant roots and aerial organs were higher in 2-yr-old grasslands and aerial biomass accumulation was higher and maintained the best soil moisture conditions in 4-yr-old grasslands, once the crop reached the maximum development of the root system and cover of the soil surface9. Drought-tolerant germplasm shows a lower degree of wilting under initial conditions of water deficit, more plants with the green plant canopy under severe water stress conditions and more stems per plant under stress conditions or favorable moisture conditions3. Despite the existence of a wide genetic variability in morphological and physiological traits associated with drought resistance, it is difficult to achieve the combination of adaptive traits to specific environments in the same variety with wide adaptation to environments vulnerable to drought8.
The genetic improvement of drought resistance and the yield of aerial biomass and its components requires special attention to traits with high heritability, general combining ability, additive genetic effects, maternal genetic effects, low genotype*environment interaction and ease of selection. In the analysis of the genetic variation of a population of the same species, additive genetic variance is the most important because it is the main determinant of the genetic properties observable in the population and of the response to selection10. The additive variance is the only one that can be estimated directly from the observations made in the population and can be used in the estimation of heritability, which represents the reliability of the phenotypic value as an indication of the reproductive value, which determines its influence on the next generation10. The similarity observed in the heritability values, for the traits measured in the plant under irrigation and drought, can be used as an indication of the effectiveness in the selection of new progenies, regardless of the selection environment10. Broad-sense heritability (H
2
) measures the contribution of the genotype to the total phenotypic variance (
Selection for drought resistance can be achieved by increasing water use efficiency, drought severity index, mean productivity, harmonic mean, geometric mean, stress tolerance index, modified stress tolerance index, superiority index and abiotic tolerance index in water deficit conditions12. Selection for morphological components of aerial biomass yield can be achieved by including the number of secondary stems and crown diameter per plant in the selection criteria13. Other components of aerial biomass yield with moderate to high heritability that could be successfully used in selection to increase yield are absolute growth rate, radiation use efficiency, number of stems, L:S ratio and plant height, in addition to the presence of maternal genetic effects favorable to aerial biomass yield14. The selection of new varieties with drought resistance and high yield of aerial biomass and its components can be achieved by identifying the genetic traits with greater heritability and contribution to the productivity of the genotype. The objective of the present research was to study the genetic variability in the production of aerial biomass and its components, in commercial varieties of alfalfa under irrigation and drought in greenhouse conditions.
Material and methods
An experiment was carried out under irrigation and drought conditions in a greenhouse with a metal structure and transparent glass without whitewashing, and with a mechanical ventilation system in the College of Postgraduates, Montecillo, Texcoco, State of Mexico (19° 29’ N, 98° 53’ W and altitude of 2,250 masl) in the 2017-2019 period. The locality is characterized by having a subhumid temperate climate with long cool summer (Cb (wo) (w) (i´)g), average annual rainfall of 637 mm and winter rainfall of less than 5 %; average annual temperature with fluctuations from 12 to 18 °C and thermal oscillation between 5 and 7 °C15. The genetic material used included the following commercial varieties of alfalfa: San Miguel, Oaxaca, Atlixco, Aragón, Victoria, Genex, Júpiter, Milenia, San Isidro and Cuf 101, with germination percentage greater than 95 %. A randomized complete block experimental design was used, with four repetitions and two soil moisture treatments (irrigation and drought). The experimental unit was an individual plant transplanted in a cylindrical polyethylene bag inside a PVC pipe 1 m high and 4” in diameter, to favor the expression of the genetic potential of the morphological characteristics of the variety. The sowing was carried out on March 15, 2017, by placing five seeds of each variety in individual cells of seedbed boxes. At 20 days after sowing (das), the most vigorous seedling of each cell was selected and transplanted individually into the PVC pipes. The PVC pipes were filled with dry soil of sandy-loamy texture, bulk density of 1.12 T m-3 and pH of 7.3; 18.8 and 0.22 % of organic matter and total nitrogen; 176.3 mg kg-1 and 2,420 mg kg-1 of phosphorus and potassium; 54.6 Cmol(+) kg-1 and 0.53 dS m-1 of cation exchange capacity and electrical conductivity; and 52 and 38.2 % of field capacity (FC) and permanent wilting percentage (PWP) (Central University Laboratory, Chapingo Autonomous University, Chapingo, Mexico, 2016). The fertilization dose 60-140-00 was applied at 44 days after transplantation (dat), using urea and calcium triple superphosphate as sources of nitrogen and phosphorus, diluted in the irrigation water; a second and third fertilization was done at 240 and 420 dat with the same dose of fertilizer. Two treatments of soil moisture were used: irrigation, where the soil water content remained close to FC from the date of transplantation (20 das) to 406 dat (I1) and from 406 dat until the end of the experiment (798 dat) (I2), and drought, where the application of water to plants was suspended in a first period for 61 d [345 to 406 dat; March to May 2018; (D1)] and a second period for 68 days [620-688 dat; November 2018 to February 2019; (D2)]. Recovery irrigation (RI) was applied to the plants at the end of the treatments of D1 (406 dat, RI1) and D2 (688 dat, RI2).
Cuts were made in the aerial part of the plant every 5 wk in the autumn-winter period and every four weeks in the spring-summer period, at a height of 5 cm above ground level. In each cut, the plant height (PH, cm) was measured from the soil surface to the last leaf exposed on the highest stem with a ruler graduated to 5 mm; in addition, the total number of stems (NS) was counted and the leaf:stem ratio (L:S) was determined in a subsample of four secondary stems, by dividing the leaf dry weight (LDW) by the stem dry weight (SDW), obtained after a drying period of 48 h at a temperature of 65 °C (L:S = LDW/SDW). The total dry matter yield (TDMY, g) or aerial biomass (BM) was calculated by adding the dry weight of leaves and secondary stems of the subsample used to determine the L:S ratio, and the dry weight of the leaves and secondary stems of the remaining sample of the plant. The leaf dry matter yield (LDMY, g) was represented by the dry weight of leaves. The radiation use efficiency (RUE, g d DM MJ-1) was calculated by dividing the TDMY by the solar radiation accumulated daily (data obtained from the meteorological station of the Chapingo Autonomous University) during the period between subsequent cuts16. The maximum and minimum air temperature in the greenhouse was recorded daily with a maximum and minimum mercury column thermometer, Taylor brand model 5458P, placed next to the plants at a height of 2 m above floor level. The maximum temperature during the study ranged from 19 to 40 °C and the minimum from -4 to 15 °C, with an average of 32 and 8.5 °C. The water content in the soil was determined by the gravimetric method every third day with a Tor-Rey electronic balance, PCR Series model. In irrigation, the water content of the soil was kept close to FC, by adding water in each weighing during the experiment, while in drought, the plants were treated in the same way as in irrigation, except in the periods in which the application of water was suspended [345 to 406 (D1) and 620-688 (D2) dat] and only the decrease in soil weight in each PVC pipe (data not shown) was recorded.
The phenotypic variance (
Where,
Yijk is the value of the response variable;
μ is the overall mean;
DCi is the effect of the date of cut;
R(DC)ij is the effect of repetitions within the date of cut;
Gk is the effect of genotypes;
G*DCik is the effect of the interaction between genotypes and dates of cut;
Eijk is the experimental error.
Estimates of phenotypic variance and its components were made under the assumption of Hardy-Weinberg equilibrium, linkage equilibrium and absence of epistasis17,19. The values of phenotypic variance (
Where,
Narrow-sense heritability (h 2) was calculated according to the following equation:
h
2 = (
The dominance variance (
Where,
Narrow-sense heritability (h 2) was calculated under the assumption that the varieties used are a random and representative sample of the genetic variability of alfalfa and considering that this is an allogamous species17. Thus, the component of variance obtained from the mathematical expectation of the mean square of the factor of varieties is an estimator of the additive variance21.
The data obtained were analyzed with the GLM22 procedure, version for Windows 10, with a completely randomized design in factorial arrangement. The means of soil moisture treatments, genotypes and genotypes within soil moisture treatments were compared with the honest minimum significant difference (HMSD, P<0.05) according to the following model:
Where,
Y ij is the value of the response variable;
μ is the overall mean;
T i represents soil moisture treatments;
Gj represents genotypes;
T*Gjj represents the interaction between soil moisture treatments and genotypes;
Eij is the experimental error23.
Results and discussion
The soil moisture treatments were different (P≤0.01) in total dry matter yield and leaf dry matter yield in the cuts made between 406 and 798 dat; differences (P≤0.01) in the L:S ratio at 406, 434, 462, 490 and 686 dat; differences (P≤0.01) in plant height at 406, 434, 462, 686, 742, 770 and 798 dat; and differences (P≤0.01) in number of stems and radiation use efficiency between 406 and 798 dat (Table 1). The varieties showed differences (P≤0.01) in total dry matter yield, L:S ratio, plant height and radiation use efficiency in all cuts made between 112 and 798 dat; differences (P≤0.01) in leaf dry matter yield and number of stems in all cuts, except for cuts made at 245, 406, 434, 553 and 588, and 140 dat. The interaction of soil moisture treatments*varieties showed differences (P≤0.01) in total dry matter yield at 112, 140, 210, 406 and 746 dat and differences (P≤0.05) at 175, 315, 434 and 770 dat; differences (P≤0.01) in leaf dry matter yield at 112, 140 and 210 dat, and differences (P≤0.05) at 175, 742 and 770 dat; differences (P≤0.01) in the L:S ratio at 112, 140, 175, 210, 245, 280, 315, 406, 434, 490, 686, 770 and 798 dat, differences (P≤0.05) at 588 dat; differences (P≤0.01) in plant height at 112, 245, 280, 490, 742 and 798 dat, and differences (P≤0.05) at 112, 210, 315 and 406 dat; differences (P≤0.01) in number of stems at 175, 315 and 434 dat, and differences (P≤0.05) at 140, 245, 462, 518 and 686 dat; and differences (P≤0.01) in radiation use efficiency at 140, 210, and 742 dat, and differences (P≤0.05) at 112, 175, 315, 434, and 770 dat.
Table 1: Factors of variation, degrees of freedom (DF) and significance of total dry matter yield (TDMY) and leaf dry matter yield (LDMY), leaf:stem ratio (L:S), plant height (PH), number of stems (NS) and radiation use efficiency (RUE) in irrigation (I1) and drought (D1) (112-434 dat), and in I2 and D2 (462-798 dat)
| Characteristic | DF | 112 | 140 | 175 | 210 | 245 | 280 | 315 | 406 | 434 | 462 | 490 | 518 | 553 | 588 | 686 | 742 | 770 | 798 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDMY (g DM plant-1) | |||||||||||||||||||
| A | 1 | ns | ns | ns | ns | ns | ns | ns | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| B | 9 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| A*B | 9 | ** | ** | * | ** | ns | ns | * | ** | * | ns | ns | ns | ns | ns | ns | ** | * | ns |
| LDMY (g DM plant-1) | |||||||||||||||||||
| A | 1 | ns | ns | ns | ns | ns | ns | ns | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| B | 9 | ** | ** | ** | ** | ns | ** | ** | ns | ns | ** | * | ** | ns | ns | * | ** | ** | ** |
| A*B | 9 | ** | ** | * | ** | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | * | ns |
| L:S ratio | |||||||||||||||||||
| A | 1 | ns | ns | ns | ns | ns | ns | ns | ** | ** | ** | ** | ns | ns | ns | ** | ns | ns | ns |
| B | 9 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| A*B | 9 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ns | ** | ns | ns | * | ** | ns | ** | ** |
| PH (cm) | |||||||||||||||||||
| A | 1 | ns | ns | ns | ns | ns | ns | ns | ** | ** | ** | ns | ns | ns | ns | ** | ** | ** | ** |
| B | 9 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| A*B | 9 | ** | * | ns | * | ** | ** | * | * | ns | ns | ** | ns | ns | ns | ns | ** | ns | ** |
| NS | |||||||||||||||||||
| A | 1 | ns | ns | ns | ns | ns | ns | ns | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| B | 9 | ** | ns | ** | ** | ** | ** | ** | * | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| A*B | 9 | ns | * | ** | ns | * | ns | ** | ns | ** | * | ns | * | ns | ns | * | ns | ns | ns |
| RUE (g DM MJ-1) | |||||||||||||||||||
| A | 1 | ns | ns | ns | ns | ns | ns | ns | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| B | 9 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| A*B | 9 | * | ** | * | ** | ns | ns | * | ns | * | ns | ns | ns | ns | ns | ns | ** | * | ns |
A=Soil moisture treatments (Irrigation=I 1 and I 2 , and Drought=D 1 and D 2 ); B=Genotypes; A*B Interaction of soil moisture treatments*genotypes; *(P≤0.05); **(P≤0.01); ns (not significant). D 1 (345-406 dat) and D 2 (620-688 dat).
The comparison of the total dry matter yield and its components in irrigation vs. drought showed that the water deficit of the soil in D1 and D2 reduced (P≤0.01) the total dry matter yield and leaf dry matter yield, number of stems and radiation use efficiency from 406 to 798 dat; plants under drought did not recover their productive capacity after experiencing the water deficit in D1 and D2 with respect to plants under irrigation (I1 and I2), even after recovery irrigations (RI1 and RI2) (Figure 1). The L:S ratio in plants under drought was higher (P≤0.01) than in irrigation (I1 and I2), and these differences between irrigation and drought were more noticeable during the application of drought (D1 and D2). The plant height in D1 and D2 was lower (P≤0.01) than in irrigation (I1 and I2) and subsequently recovered its growth capacity with respect to its behavior in irrigation. The survival of alfalfa through periods of water deficit in field conditions depends on the length and intensity of the drought, the genotype, the type of soil (water capacity of the soil and depth of the root system) and the environment (salinity and temperature); its survival to short periods (2-3 weeks) without irrigation is reflected in its high recovery capacity when receiving irrigation again and producing normal yields in subsequent years24. The greater recovery capacity of alfalfa when receiving water after experiencing periods of water deficit24 may be due to the fact that plants that grow in field conditions have greater access to moisture and nutrients in the soil profile, unlike plants that grow in greenhouse conditions in pots or PVC pipes, where plant roots grow in an environment limited in soil volume, moisture and nutrients; this is reflected in a reduction in the accumulation of aerial biomass due to a decrease in stomatal conductance, transpiration and assimilation3. The high values in the L:S ratio in drought could be due to a lower partition of assimilates to the stem with respect to the leaf; plants subjected to water stress show some morphological changes in response to water deficit, by reducing the loss or increasing the absorption of water to maintain the water status of the tissue25. Plant height was the only morphological characteristic that showed recovery capacity after water application (RI1 and RI2), reaching values similar to those observed in plants under irrigation; soil water deficit affects different morphological characteristics of plants, such as plant height, stem diameter, number, size and area of leaves, dry matter production, assimilate partitioning, flower and fruit production, and physiological maturity25.
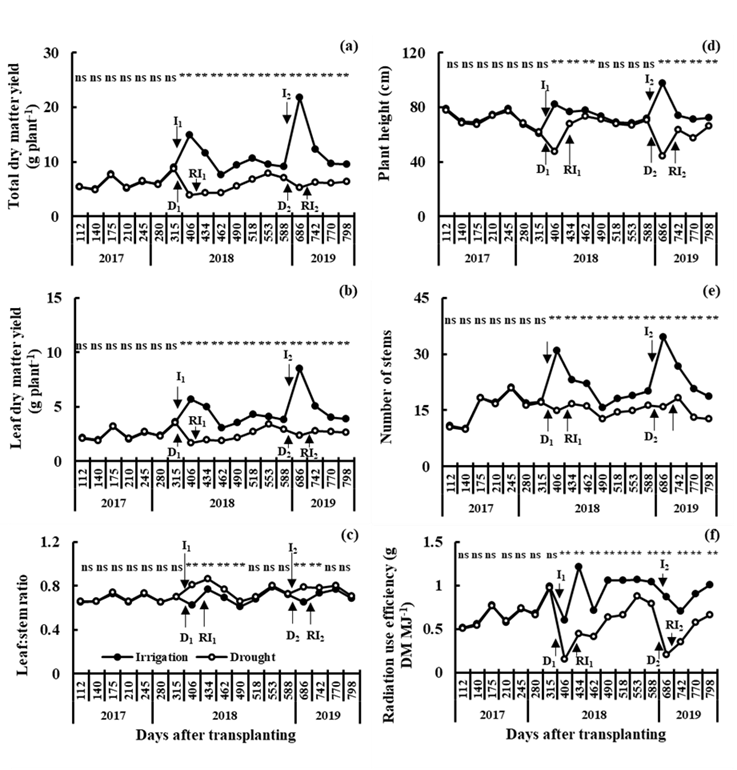
Montecillo, Texcoco, State of Mexico [RR1=Recovery irrigation in I1 (RI1); RR2=Recovery irrigation in I2 (RI1); *(P≤0.05); **(P≤0.01); ns (not significant)].
Figure 1 Yield of total dry matter (a) and leaf dry matter (b), leaf:stem ratio (c), plant height (d), number of stems (e) and radiation use efficiency (f) in 18 cuts in irrigation (R1=I1 and R2=I2) and drought (S1=D1 and S2=D2), average of 10 varieties of alfalfa
On the other hand, in irrigation (I1 and I2), a wide variability (P≤0.01) was observed between genotypes for total dry matter yield (Figures 2a and 3a), L:S ratio (Figures 2c and 3c), plant height (Figures 2d and 3d) and radiation use efficiency (Figures 2f and 3f) all cuts in I1 (112 to 434 dat) and I2 (462 to 798 dat). The Genex, Atlixco, Júpiter, Oaxaca, San Miguel and Milenia varieties produced more (P≤0.01) total dry matter yield than the other varieties in all cuts in I1 (Figure 2a), and only the Genex, Atlixco, Júpiter and Milenia varieties showed high (P≤0.01) total dry matter yield in I2 (Figure 3a). The high total dry matter yield in the Genex, Atlixco, Júpiter, Oaxaca, San Miguel and Milenia varieties (Figure 2a) was accompanied by high (P≤0.01) leaf dry matter yield (Figure 2b), plant height (Figure 2d), number of stems (Figure 2e) and radiation use efficiency (Figure 2f) in I1. The high (P≤0.01) total dry matter yield of the Genex, Atlixco, Júpiter and Milenia varieties (Figure 3a) was also accompanied by high (P≤0.01) leaf dry matter yield (Figure 3b), plant height (Figure 3d), number of stems (Figure 3e) and radiation use efficiency (Figure 3f) in I2. The Victoria, Aragón and San Isidro (Figure 2c), and Aragón and San Isidro (Figure 3c) varieties showed a higher (P≤0.01) L:S ratio than the other varieties in I1 and I2. In a study with 11 alfalfa cultivars under greenhouse irrigation conditions, it was determined that BCB, ALF and AFR varieties showed higher yields of total dry matter, root dry matter, stem elongation rate, relative water content and root diameter than the other alfalfa varieties6. The varieties F 1412-02, F 1535-03, Roxana and F 2007-08, and F 1414-02, F 1711-05, F 1715-05 and F 2010-08 stood out from a group of 74 genotypes under greenhouse irrigation conditions, producing higher total dry matter yield, plant height and number of stems than the rest of the varieties4.
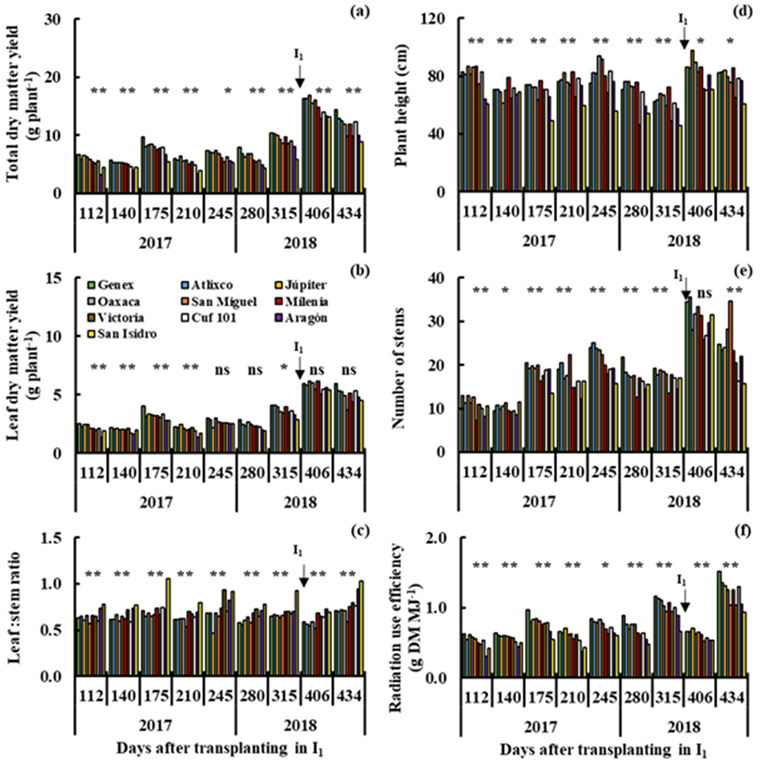
R1= Irrigation in the cutting period from 112 to 406 dat (I1).
Figure 2 Yield of total dry matter (a) and leaf dry matter (b), leaf:stem ratio (c), plant height (d), number of stems (e) and radiation use efficiency (f) in nine cuts in irrigation (I1), for 10 varieties of alfalfa
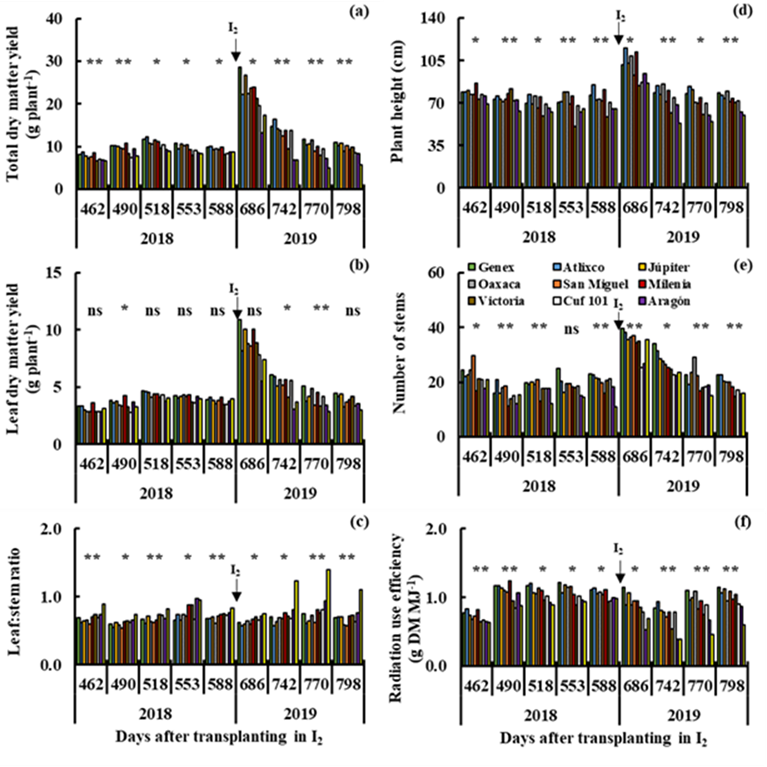
R2= Irrigation in the cutting period from 462 to 798 dat (I2).
Figure 3 Yield of total dry matter (a) and leaf dry matter (b), leaf:stem ratio (c), plant height (d), number of stems (e) and radiation use efficiency (f) in nine cuts in irrigation (I2), for 10 varieties of alfalfa
In drought, a wide variability (P≤0.01) was also observed between genotypes for total dry matter yield (Figures 4a and 5a), L:S ratio (Figures 4c and 5c), plant height (Figures 4d and 5d) and radiation use efficiency (Figures 4f and 5f) in all cuts in D1 (112 to 406 dat) and D2 (462 to 798 dat). The Genex, Atlixco, Júpiter, Oaxaca, San Miguel and Milenia varieties produced higher (P≤0.01) total dry matter yield than the other varieties in all cuts in D1 (Figure 4a), and only the Genex, Atlixco, Júpiter and Milenia varieties showed high (P≤0.01) total dry matter yield in D2 (Figure 5a). The high total dry matter yield of the Atlixco, Júpiter, Oaxaca, San Miguel and Milenia varieties (Figure 4a) was accompanied by higher (P≤0.01) leaf dry matter yield (Figure 4b), plant height (Figure 4d), number of stems (Figure 4e) and radiation use efficiency (Figure 4f) in I1. In I2, the highest (P≤0.01) total dry matter yield of the Genex, Atlixco, Júpiter and Milenia varieties (Figure 5a) was also accompanied by high (P≤0.01) leaf dry matter yield (Figure 5b), plant height (Figure 5d), number of stems (Figure 5e) and radiation use efficiency (Figure 5f). The Milenia, Victoria, Cuf-101, Aragón and San Isidro (Figure 4c), and Victoria, Aragón and San Isidro (Figure 5c) varieties showed a higher (P≤0.01) L:S ratio than the other varieties in I1 and I2. Other studies in different varieties of alfalfa under greenhouse drought detected genotypes that reduce less stem elongation, relative growth rate and aerial biomass with respect to irrigation, in addition to maintaining greater root growth capacity, relative water content, chlorophyll content and water use efficiency6. The Gold Queen variety produced higher yield of dry matter and water-soluble carbohydrates and was more drought-resistant than the Suntory variety under field conditions; drought decreased crude protein content and increased fiber fraction in response to water deficiency in the two alfalfa varieties7. The Amerist (USA), Sardi10 and Siriver (Australia), and Melissa (France) genotypes showed greater drought tolerance than other alfalfa varieties, because they produced thinner leaves, accumulated more proline and potassium, and maintained greater efficiency in the use of water in conditions of water deficiencies26. The Aragon and San Isidro varieties consistently showed high average values for the L:S ratio in irrigation and drought; this morphological characteristic of the plant is highly appreciated as an estimator of forage quality and can be used to improve yield, and dry matter quality in lines, half-sib families or clones in large populations, considering its high values of narrow-sense heritability (h 2 =0.75)27.
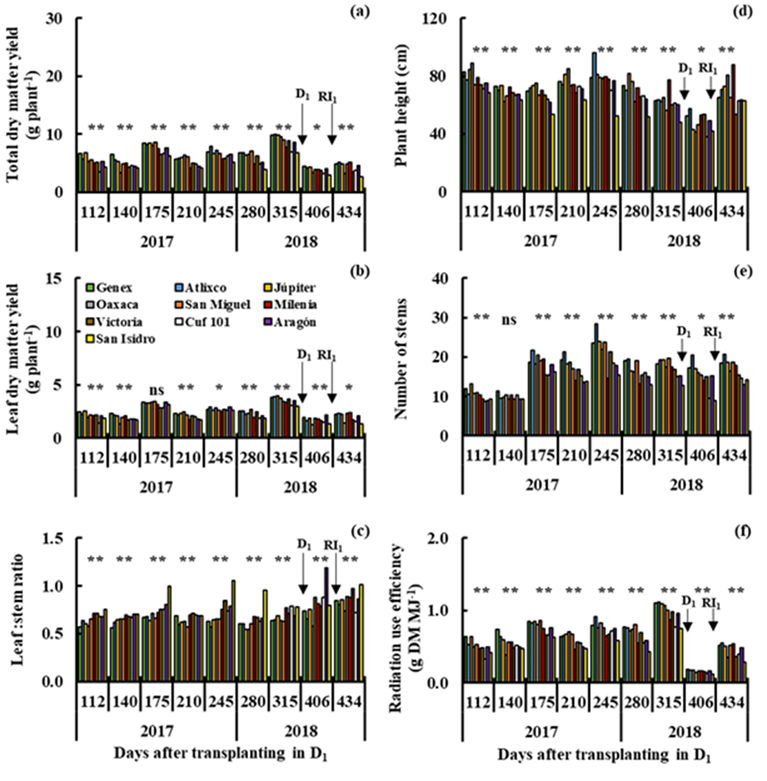
S1= Drought in the cutting period from 112 to 406 dat (D1).
Figure 4 Yield of total dry matter (a) and leaf dry matter (b), leaf:stem ratio (c), plant height (d), number of stems (e) and radiation use efficiency (f) in nine cuts in drought (D1), for 10 varieties of alfalfa
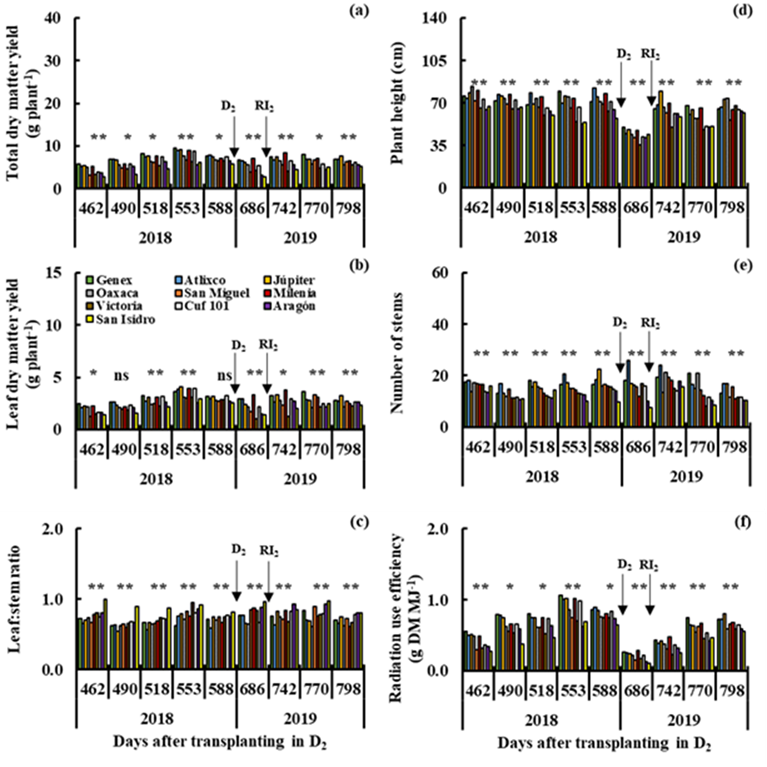
S2= Drought in the cutting period from 462 to 798 dat (D2).
Figure 5 Yield of total dry matter (a) and leaf dry matter (b), leaf:stem ratio (c), plant height (d), number of stems (e) and radiation use efficiency (f) in nine cuts in drought (D2), for 10 varieties of alfalfa
The phenotypic variance for total dry matter yield and leaf dry matter yield, L:S ratio, plant height, number of stems and radiation use efficiency in irrigation (I1 and I2) was higher (P≤0.05) than in drought (D1 and D2). The phenotypic variance for the total dry matter yield and its components was greater (P≤0.05) than the other components of variance in irrigation and drought. However, environmental variance contributed more (P≤0.05) to phenotypic variance than genetic variance in both irrigation and drought. The additive genetic variance was greater (P≤0.05) than the dominance genetic variance for all traits measured in plants in irrigation and drought. The variance of the interaction was lower than the phenotypic, environmental and additive genetic variances, for all the traits measured in the plants in irrigation and drought (Table 2). In autotetraploid alfalfa, similar results were obtained when estimating the components of variance; the dominance variance was much lower than the additive variance for the yield of dry matter and its components28. The additive variance was significantly greater than zero and the genetic variance for dry matter yield was mainly additive in an F1 population of alfalfa under controlled growth conditions29. Heritability (h 2 ) was low for leaf dry matter yield to moderate for total dry matter yield, L:S ratio, plant height, number of stems, and radiation use efficiency in irrigation and drought (Table 2). These heritability values are similar to those obtained for aerial biomass and plant height in annual alfalfa (Medicago sativa subsp. falcata) under field conditions28 and could be useful in improving the yield of alfalfa dry matter with the support of genomic selection27.
Table 2 Estimated genetic parameters for total dry matter yield (TDMY) and leaf dry matter yield (LDMY), leaf:stem ratio (L:S), plant height (PH), number of stems (NS) and radiation use efficiency (RUE) in irrigation (I1 and I2), and drought (D1 and D2), average of 10 varieties of alfalfa
| Genetic parameters | TDMY | LDMY | L:S | PH | NS | RUE |
|---|---|---|---|---|---|---|
| Irrigation I1 and I2 | ||||||
| Phenotypic variance ( |
3.6 (0.7) | 0.5 (0.1) | 0.01 (0.001) | 86.4 (7.6) | 16.0 (1.6) | 0.021 (0.001) |
| Genotypic variance ( |
||||||
| additive ( |
1.2 (0.4) | 0.1 (0.05 | 0.005 (0.0003) | 31.6 (1.2) | 4.5 (0.8) | 0.01 (0.001) |
| dominance ( |
0.3 | 0.02 | 0.001 | 7.9 | 1.1 | 0.002 |
| interaction ( |
0.7 | 0.06 | 0.002 | 12.3 | 2.5 | 0.002 |
| Environmental variance ( |
1.7 (0.4) | 0.4 (0.08) | 0.004 (0.0008) | 42.6 (6.9) | 9.0 (1.6) | 0.01 (0.001) |
| Heritability (h 2 ) | 0.3 (0.04) | 0.2 (0.04) | 0.4 (0.04) | 0.4 (0.03) | 0.3 (0.04) | 0.4 (0.04) |
| Drought D1 and D2 | ||||||
| Phenotypic variance ( |
1.5 (0.2) | 0.2 (0.03) | 0.01 (0.001) | 61.6 (5.8) | 11.5 (0.8) | 0.015 (0.007) |
| Genotypic variance ( |
||||||
| additive ( |
0.5 (0.02) | 0.04 (0.004) | 0.004 (0.0003) | 20.4 (2.0) | 4.1 (0.3) | 0.046 (0.005) |
| dominance ( |
0.1 | 0.01 | 0.001 | 5.1 | 1.0 | 0.001 |
| interaction ( |
0.2 | 0.04 | 0.004 | 13.7 | 1.8 | 0.002 |
| Environmental variance ( |
0.8 (0.2) | 0.1 (0.03) | 0.001 (0.0003) | 27.5 (4.9) | 5.5 (0.8) | 0.008 (0.2) |
| Heritability (h 2 ) | 0.3 (0.04) | 0.2 (0.04) | 0.4 (0.03) | 0.3 (0.04) | 0.4 (0.04) | 0.3 (0.04) |
The analysis of principal components (PC1 and PC2) identified two components that explain the largest proportion of the total variation (75.8 %) shown in the experiment. PC1 explained 56.2% of the variation and had a positive correlation with total dry matter yield (r= 0.52), leaf dry matter yield (0.50), number of stems (r= 0.42), radiation use efficiency (r= 0.40) and plant height (r= 0.34), and negative correlation with L:S ratio (r= -0.19). PC2 explained only 19.6 % of the observed variability and had a positive correlation with the L:S ratio (r= 0.78) and leaf dry matter yield (r=0.31), and negative correlation with plant height (r= -0.49) (Figure 6). Additionally, total dry matter yield was positively related to the number of stems and leaf dry matter yield, and negatively related to plant height; plant height was negatively related to L:S ratio. The variability observed for yield of dry matter and its components in the present study was similar to that observed in a group of 27 populations and cultivars of alfalfa under field conditions, where PC1 contributed 58.2 % of the total variability and showed positive association with dry and green matter yield, vigor, growth habit, regeneration of the plant and width of the central leaflet30. Other results in irrigated and rainfed alfalfa in the field showed a PC1 with 54.3 % of the total variability and positive association with the diameter of lateral roots and number of lateral or branched roots31. It is interesting to note the similarity in the values observed for PC1 and the variability between genotypes in these studies, and the traits of the plant that had the greatest positive association with this component, especially with dry matter yield.
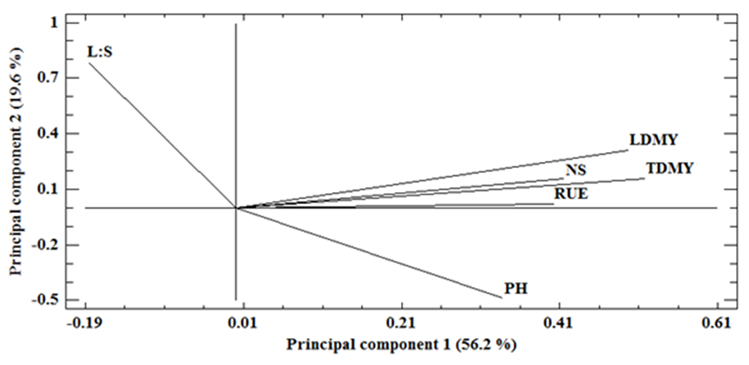
Figure 6 Biplot plane of dry matter yield vs. total dry matter yield (RMST), leaf dry matter yield (RMSH), L:S ratio (H:T), number of stems (NT), plant height (AP) and radiation use efficiency (EUR) in irrigation (I1 and I2) and drought (D1 and D2), on average of 10 varieties of alfalfa in greenhouse conditions
Conclusions and implications
The drought decreased the total dry matter yield and its components, and plants under soil water deficit conditions did not recover their productive capacity after experiencing the water deficiencies of the soil, even after recovery irrigation. In contrast, the L:S ratio was higher in plants in drought than in irrigation and plant height was the only component of yield that regained its growth capacity after recovery irrigation. Soil water deficit also reduced phenotypic variance for total dry matter yield and its components; environmental variance was greater than genetic variance in irrigation and drought. Additive variance was greater than dominance variance for all traits measured in irrigation and drought. Total dry matter yield, L:S ratio, plant height, number of stems, and radiation use efficiency had higher heritability in irrigation and drought. Leaf dry matter yield, number of stems, radiation use efficiency and plant height were positively related to total dry matter yield. The most productive varieties could be used for forage production in water-scarce areas and/or as parental lines for forage yield improvement in selection programs. Future research work on this topic requires confirmation under field conditions.
Literatura citada
1. Guitjens JC. Alfalfa. In: Stewart BA, Nielsen DR, editors. Irrigation of agricultural crops. American Society of Agronomy, Inc. Madison, Wisconsin USA; Monograph Number 30 in the series of Agronomy; 1990:537-596. [ Links ]
2. Lauriault L, Marsalis M, Contreras-Govea F, Angadi S. Managing alfalfa during drought. Cooperative Extension Service, College of Agricultural and Environmental Sciences, New Mexico State University. Las Cruces, New Mexico; 2009(Circular 646):4. [ Links ]
3. Luna-Guerrero MJ, López-Castañeda C, Quero-Carrillo AR, Herrera-Haro JG, Ortega-Cerrilla ME, Martínez-Hernández PA. Water relations and gas exchange in lucerne under drought conditions. Rev Mex Cienc Agríc 2020; Special Publication Number 24:81-92. [ Links ]
4. Petcu E, Schitea M, Drăgan L, Bǎbeanu N. Physiological response of several alfalfa genotypes to drought stress. Rom Agric Res 2019;36:107-118. [ Links ]
5. Blum A. Crop responses to drought and the interpretation of adaptation. Plant Grow Reg 1996;20:135-148. [ Links ]
6. Anower MR, Boe A, Auger D, Mott IW, Peel MD, Xu L, Kanchupati P, Wu Y. Comparative drought response in eleven diverse alfalfa accessions. J Agron Crop Sci 2017;203:1-13. [ Links ]
7. Liu Y, Wu Q, Ge G, Han G, Jia Y. Influence of drought stress on alfalfa yields and nutritional composition. BMC Plant Biology 2018;18(13):1-9. doi:10.1186/s12870-017-1226-9. [ Links ]
8. Annicchiarico P, Pecetti L, Tava A. Physiological and morphological traits associated with adaptation of lucerne (Medicago sativa) to severely drought-stressed and to irrigated environments. Ann Appl Biol 2013; 162:27-40. doi:10.1111/j.1744-7348.2012.00576.x. [ Links ]
9. Huang Z, Liu Y, Cui Z, Fang Y, He H, Liu BR, Wu GL. Soil water storage deficit of alfalfa (Medicago sativa) grasslands along ages in arid area (China). Field Crop Res 2018;221:1-6. [ Links ]
10. Falconer DS. Introducción a la genética cuantitativa. México: Cía. Editorial Continental, SA de CV: 1984. [ Links ]
11. Hill J, Becker HC, Tigerstedt PMA. Quantitative and ecological aspects of plant breeding. London: Chapman & Hall; 1998. [ Links ]
12. Bellague D, Hammedi-Bouzina MM, Abdelguerfi A. Measuring the performance of perennial alfalfa with drought tolerance indices. Chil J Agric Res 2016;76(3):273-284. doi:10.4067/ S0718-58392016000300003. [ Links ]
13. Márquez-Ortiz JJ, Lamb JFS, Johnson LD, Barnes DK, Stucker RE. Heritability of crown traits in alfalfa. Crop Sci 1999;39:38-43. [ Links ]
14. Luna-Guerrero MJ, López-Castañeda C, Hernández-Garay A. Genetic improvement of aerial alfalfa biomass and its components: half-sib family selection. Rev Mex Cienc Pecu 2020;11(4):1126-1141. doi.org/10.22319/rmcp.v11i4.5344. [ Links ]
15. García E. Modificaciones al sistema de clasificación climática de Köppen. Serie Libros Núm. 6, Instituto de Geografía, UNAM. México, DF; 2004. [ Links ]
16. Luna-Guerrero MJ, López-Castañeda C, Hernández-Garay A, Martínez-Hernández PA, Ortega-Cerrilla ME. Evaluación del rendimiento de materia seca y sus componentes en germoplasma de alfalfa (Medicago sativa L.). Rev Mex Cienc Pecu 2018;9(3):486-505. doi.org/10.22319/rmcp.v9i3.4440. [ Links ]
17. Molina-Galán JD. Introducción a la genética de poblaciones y cuantitativa (algunas implicaciones en genotecnia). México, DF: AGT Editor, SA; 1992. [ Links ]
18. Márquez-Sánchez F, Sahagún-Castellanos J. Estimation of genetic variances with maternal half-sib families. Maydica 1994;39(3):197-201. [ Links ]
19. Melendres-Martínez JI, Valdivia-Bernal R, Lemus-Flores C, Medina-Torres R, García-López M, Ortiz-Caton M, et al. Estimación de parámetros genéticos de maíz bajo mejoramiento por selección recíproca recurrente. Rev Mex Cienc Agríc 2018;9(7):1327-1337. [ Links ]
20. Galicia-Juárez M. Varianza genética y mapeo molecular de rendimiento y calidad nutricional en familias de medios hermanos en Medicago sativa [tesis maestría]. Texcoco, México: Colegio de Postgraduados; 2012. [ Links ]
21. Hill J, Becker HC, Tigerstedt PMA. Quantitative and ecological aspects of plant breeding. London: Chapman & Hall ; 1998. [ Links ]
22. SAS (Statistical Analysis System), Version 9.4 para Windows. SAS Institute Inc., Cary, NC, USA; 2012. [ Links ]
23. Hinkelmann K, Kempthorne O. Design and analysis of experiments. Volume 1: Introduction to experimental design. USA: A John Wiley and Sons, Inc; 2008. [ Links ]
24. Orloff S, Putnam D, Bali K. Drought strategies for alfalfa. Agriculture and Natural Resources, UC, USA. Publication 8522; 2015:1-9 (http://anrcatalog.ucanr.edu/). [ Links ]
25. Anjum SA, Ashraf U, Zohaib A, Tanveer M, Naeem M, Ali I, Tabassum T, Nazir U. Growth and developmental responses of crop plants under drought stress: a review. Zemdirbyste-Agriculture 2017;104(3):267-276. doi:10.13080/z-a.2017.104.034. [ Links ]
26. Benabderrahim MA, Hamza H, Haddad M, Ferchichi A. Assessing the drought tolerance variability in Mediterranean alfalfa (Medicago sativa L.) genotypes under arid conditions. Plant Biosystems 2015; 149 (2):395-403. doi.org/10.1080/11263504.2013.850121. [ Links ]
27. Annicchiarico P. Alfalfa forage yield and leaf/stem ratio: narrow-sense heritability, genetic correlation, and parent selection procedures. Euphytica 2015;205(2):409-420. doi:10.1007/s10681-015 1399-y. [ Links ]
28. Riday H, Brummer EC. Narrow sense heritability and additive genetic correlations in alfalfa subsp. falcata. J Iowa Academy Sci 2007;114(1-4):28-34. [ Links ]
29. Bowley SR, Christie BR. Inheritance of dry matter yield in a heterozygous population of alfalfa. Can J Plant Sci 1981;61:313-318. [ Links ]
30. Tucak M, Popović S, Ćupić T, Šimić G, Gantner R, Meglić V. Evaluation of alfalfa germplasm collection by multivariate analysis based on phenotypic traits. Rom Agric Res 2009;26:47-52. [ Links ]
31. Odorizzi A, Basigalup D, Arolfo V, Balzarini M. Análisis de la variabilidad de caracteres de raíz en poblaciones de alfalfa (Medicago sativa L.) con alto número de raíces laterales. AgriSci 2008;25(2):65-73. [ Links ]
Received: December 22, 2021; Accepted: June 22, 2022











 text in
text in 


