Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.13 no.4 Mérida oct./dic. 2022 Epub 11-Nov-2022
https://doi.org/10.22319/rmcp.v13i4.5959
Technical notes
Bacterial evaluation of Zacazonapan artisanal cheese matured under non-controlled conditions in two production periods
a Universidad Autónoma del Estado de México. Instituto de Ciencias Agropecuarias y Rurales. Campus UAEM El Cerrillo, El Cerrillo Piedras Blancas. 50295, Toluca, Estado de México, México.
b Universidad Autónoma del Estado de México. Centro Universitario UAEM Temascaltepec. Temascaltepec, Estado de México, México.
c Universidad Autónoma del Estado de México. Facultad de Medicina Veterinaria y Zootecnia. Campus UAEM El Cerrillo, Toluca, Estado de México, México.
Traditional Zacazonapan cheeses have unique organoleptic characteristics and are characterized by being linked to the territory of origin. In the maturation process, there are many interactive variables that are responsible for physical, chemical, biological and structural changes. In order to evaluate the bacteriological evolution of artisanal cheeses during their maturation under non-controlled conditions in two production periods, samples of raw milk and cheese were collected at 0, 30, 60, 120 and 150 d of maturation. The presence of molds and yeasts (MaY), mesophilic aerobic bacteria (MAB), Staphylococcus spp. (Staph), total coliforms (TC), fecal coliforms (FC), Salmonella spp. (Salm) and Listeria spp. (List) was determined. The average microbial load was 9.68, 9.38, 8.55 and 8.10 log10 CFU/g of cheese for MaY, MAB, Staph and TC respectively, as well as 2.68 log10 MPN/g of cheese for FC. Salm was not detected but List was. The microbiological evolution of Zacazonapan matured cheese had counts that exceed the maximum levels of the Official Mexican Standard 243 SSA1 2010.
Key words Environmental maturation; Aging; Microbiological evolution; Raw milk
Los quesos tradicionales Zacazonapan tienen características organolépticas únicas y se caracterizan por estar vinculados al territorio de origen. En el proceso de maduración se tienen numerosas variables interactivas que son responsables de los cambios físicos, químicos, biológicos y estructurales. Con el objetivo de evaluar la evolución bacteriológica de quesos artesanales durante su maduración bajo condiciones no controladas en dos épocas de producción, se colectaron muestras de leche cruda y de queso a los 0, 30, 60, 120 y 150 días de maduración. Se determinó la presencia de mohos y levaduras (MyL), bacterias mesófilas aerobias (BMA), Staphylococcus spp. (Staph), coliformes totales (CT), coliformes fecales (CF), Salmonella spp. (Salm) y Listeria spp. (List). La carga microbiana promedio fue 9.68, 9.38, 8.55 y 8.10 log10 UFC/g de queso para MyH, BMA, Staph y CT respectivamente, así como 2.68 log10 NMP/g de queso para CF. No se detectó Salm pero si List. La evolución microbiológica del queso madurado Zacazonapan presentó conteos que superan los niveles máximos de la Norma Oficial Mexicana 243 SSA1 2010.
Palabras clave Maduración ambiental; añejamiento; evolución microbiológica; leche cruda
Traditional cheeses are produced from a complex system that gives rise to unique organoleptic characteristics and are characterized by strong links with their territory of origin1. In the process, there are several interactive variables that are responsible for physical, chemical, biological and structural changes. Their quality depends on environmental factors, and on interactions between inoculated microorganisms and curd substrates that result from variations in the quality of raw milk and processing conditions2. Lactic microflora is of particular interest because the biochemical activities of these organisms are involved in cheese making and may play a role in the development of organoleptic characteristics during maturation3; however, due to their process of preparation and use of raw milk, they can generate outbreaks of food poisoning4.
Zacazonapan cheese is handmade with raw milk from Creole cattle from the southern region of the State of Mexico in the rainy season (July-November). In this season, the feeding of the cattle is based on grazing and the cheeses are consumed after four months of maturation at environmental temperature and relative humidity. This cheese has also been described as Zacazonapan aged cheese5.
Knowing the evolution of the main microbial groups during the maturation of this cheese and the final microbiological quality can suggest modifications to the maturation process that improve the quality without losing any of its characteristics. Therefore, the objective of the study was to evaluate the bacteriological evolution of artisanal cheeses during their maturation under non-controlled conditions in two production periods.
The work was carried out in seven cheese factories in the municipality of Zacazonapan (19° 07’ 27” N, 100° 02’ 57” W and 1,470 m asl). Its average annual temperature and precipitation is 23.0 °C and 1,041.8 mm respectively. The study was carried out at the end of the cheese production season (from November 2010 to April 2011), which was called a batch of “dry season cheese” due to the environmental conditions in which it is matured. In the middle of the following year’s production season (September 2011 to February 2012), the batch was called “rainy season cheese”. Before the cheese was made, a milk sample was taken from each production site according to the Official Mexican Standard (NOM, for its acronym in Spanish) 109 (handling and collection of samples)6. The samples were placed in closed sterile containers and transported at 4 °C for their analysis in the microbiology laboratory the next day. Two pieces of fresh cheese weighing approximately 2.0 kg were acquired in both sampling seasons and taken to a cheese factory in the area, where they were left to mature for 150 d under normal conditions of temperature and relative humidity. Using a cork borer, two samples of 50.0 g were collected from each piece of cheese at 0, 30, 60, 120 and 150 d of maturation. Campeche wax was used to seal the holes in the place where the sample was taken. From each sample of cheese, the presence of molds and yeasts (MaY), mesophilic aerobic bacteria (MAB), Staphylococcus spp. (Staph), total coliforms (TC), fecal coliforms (FC), Salmonella spp. (Salm) and Listeria spp. (List) was determined. To determine MaY, 10 g of the central part of the cheese + 10 ml of milk from each sample obtained were homogenized in 90 ml of 0.1 % peptone water (in duplicate) and decimal dilutions were prepared from this first dilution, obtaining dilutions 10-2 to 10-7. Subsequently, they were incubated for 24 h at a temperature of 25 °C7.
To determine MAB, Staph, TC, FC, Salm and List, 25 g from the central part of the cheese + 25 ml of milk from each sample were collected and homogenized in 225 ml of 0.1 % peptone water (in duplicate). Decimal dilutions up to 10-7 were made from this solution, then incubated for 24 h at a temperature of 35 °C7.
According to NOMs, the presence of MaY was determined by plate counting with potato dextrose agar after incubation at 25 °C for 48 h8. For MAB, tryptone-yeast extract agar was used, incubating at 35 °C for 48 h9. TCs were determined in plate by violet red bile agar after incubation at 35 °C for 24 h10. The presence of FC was determined by the most probable number technique, using lauryl sulfate tryptose broth, after incubating at 35 °C for 24 h11. The determination of Staph with Baird-Parker agar and the addition of egg yolk tellurite, incubated at 37 °C for 48 h after growth12. Analysis for Salm in Salmonella-Shigella agar incubated for 48 h at 35 °C13 and List incubated at 35 °C for 48 h in Oxford agar14. The data obtained were normalized by log10, an experimental design of completely randomized blocks was used and analyzed with the command of the general linear model of minitab V.1415. When significant differences were observed (P<0.05), the Tukey test was applied.
The evolution of temperature and precipitation in the study region is shown in Figure 1. The maturation temperature of dry season cheeses starts at 24.8 °C in November and ends with 31.8 °C in April; rainfall is almost zero (<12 mm/mo), typical characteristics of the dry season (Figure 1a). In rainy season cheeses, the maturation temperature begins with 22.1 °C in September and ends with 22.7 °C in February, rainfall was 240 mm at the beginning of maturation and 36 mm at the end (Figure 1b).
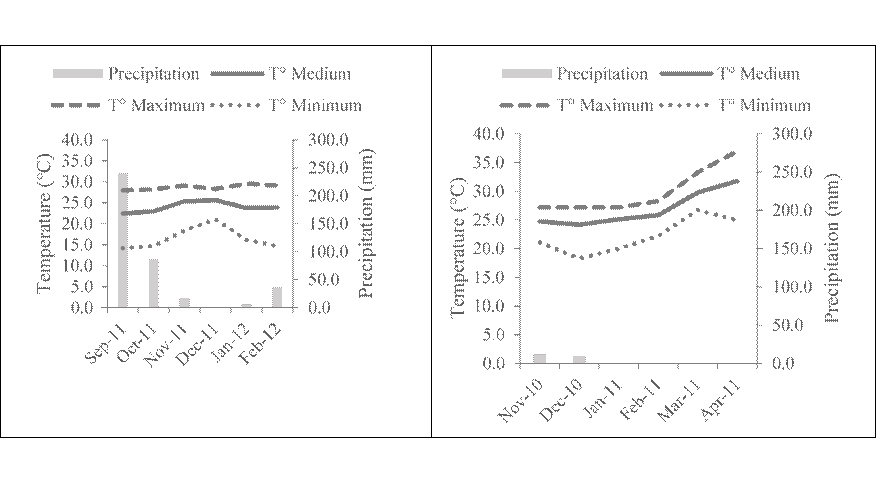
a) Dry season cheese b) Rainy season cheese
Figure 1 Precipitation and temperature during the environmental maturation of Zacazonapan cheese
Relative humidity, temperature and time are important factors during cheese maturation16. The maturation temperatures observed in this work favored the development of microorganisms and rainfall affected the final texture of the cheeses; the absence of rains produced drier cheeses, which showed cracked crusts, and the presence of rains produced softer cheeses with wet crusts, which prevented the cheeses from bursting due to the production of gas. However, these cheeses had holes and putrefactive areas inside, a phenomenon known as late swelling17.
The counts made on the milk used for cheese making (Table 1) were 7.03, 7.02 and 4.9 (log10 CFU/ml of milk) for MaY, MAB and TC. The use of poor microbiological quality milk is a common practice during the production of artisanal raw milk cheeses and is attributed to poor milking practices. Similarly, the absence of a cold chain and poor transport conditions leads to the counts of MaY, MAB and TC18.
Table 1 Microbiological quality of milk and Zacazonapan cheese during maturation
| Milk / Cheese | MaY 1 | MAB 1 | Staph 1 | TC 1 | FC 2 |
|---|---|---|---|---|---|
| Milk | 7.03 | 7.02 | ND | 4.9 | ND |
| Cheese maturation days | |||||
| 0 | 9.250b | 9.499 a | 9.090ab | 9.468 a | 3.040 a |
| 30 | 9.632b | 9.882 a | 9.827a | 9.854 a | 3.040 a |
| 60 | 9.628b | 9.403 a | 7.133b | 7.276 b | 3.040 a |
| 120 | 9.908ab | 8.858 b | 8.438b | 6.680 b | 2.340 b |
| 150 | 10.102a | 8.900 b | 7.797b | 7.147 b | 1.767 c |
| Average | 9.704 | 9.308 | 8.457 | 8.085 | 2.645 |
| Cheese production season | |||||
| Dry | 9.722 | 8.912 b | 7.992 b | 8.189 | 3.040 a |
| Rainy | 9.648 | 9.865 a | 9.123 a | 8.022 | 2.195 b |
| Average | 9.685 | 9.388 | 8.558 | 8.106 | 2.618 |
| SEM | 0.21 | 0.26 | 0.529 | 0.947 | 0.264 |
MaY= molds and yeasts; MAB= mesophilic aerobic bacteria; Staph= Staphylococcus spp. TC= total coliforms; FC= fecal coliforms.
1= log10 colony-forming units / g of cheese or ml of milk.
2= log10 most probable number / g of cheese.
ac= different letters within a column indicate differences (P<0.05). SEM= standard error of the mean. ND: Not determined
In both batches of cheese, it is observed that, in the first days of maturation, there is an increase in microbial counts (d 30), which decrease throughout the maturation process due to the biochemical and microbiological processes that occur inside the cheese, such as the reduction of water content, the concentration of solids, the increase in acidity and a reduction in pH caused by the action of lactic bacteria. This causes a microbial competition for nutrients19.
The group of MaY (Table 1) had significant differences (P<0.05) between days of maturation. This is due to the physical retention of microorganisms in rennet and microbial multiplication during milk curdling and whey draining18. The reduction of MaY from day 60 is attributed to the fact that, as the maturation progresses, the center of the cheese becomes compressed, and the oxygen needed to microbial reproduction reduces20.
The MaY counts observed in this study are consistent with those found in similar cheeses. For example, the Tepeque aerated cheese had counts of 7.6 log10 CFU/g of cheese in the dry season and 7.7 log10 CFU/g of cheese in the rainy season; and when the Tepeque cheese was taken to maturity, it had 6.2 log10 CFU/g in the dry season and 6.4 log10 CFU/g in cheese matured in the rainy season21.
During the maturation of Kurdish cheese, fresh cheese starts with 5.6 log10 CFU/g of cheese, after 20 d it presents 5.95 log10 CFU/g of cheese, until reaching 9.28 log10 CFU/g of cheese at 40 d, then the load begins to decrease, reaching 9.06 log10 CFU/g of cheese on d 6022. The same population dynamics were observed in the present study and are related to the depletion of lactose content due to the simultaneous use of lactic acid bacteria18,20,22.
NOM 243 SSA1, which indicates the sanitary provisions and specifications for milk and milk products23, does not specify the determination of MAB as quality indicator microorganisms in cheeses, because this group includes lactic acid bacteria, which are desirable microorganisms in cheese maturation24, which, due to their long-term proteolytic and lipolytic activities, contribute to the development of flavor and aroma18,20,22, providing the typical characteristics to the product of a region. In this study, MAB had significant differences (P<0.05) between days of maturation and between production periods. In this regard, the counts of the dry season cheese were 8.91 log10 CFU/g of cheese and that of the rainy season was 9.86 log10 CFU/g of cheese (Table 1). Microbiological counts increased in the first 30 d of maturation due to the presence of inherent lactic acid bacteria in the milk or rennet used to make the cheese, obtained from young calves from the region.
The decrease in MAB counts from d 60 is attributed to the fact that the growth of these organisms during cheese maturation is controlled by some physicochemical factors such as water activity, salt concentration, pH, organic acids, temperature during maturation, oxidation-reduction potential and presence of nitrates25.
The population dynamics of MAB in this study are consistent with those of other cheeses. In artisanal pore cheese three days after being made, MAB were present with a concentration of 6.77 log10 CFU/g of cheese and after 12 d of being made, they increased to 7.44 log10 CFU/g of cheese16. In the Tepeque aerated cheese, concentrations of 7.9 log10 CFU/g of cheese were reported in the dry season and 7.6 log10 CFU/g of cheese in the rainy season. In Tepeque cheese taken to maturity, the concentration in both the cheese produced in the dry season and in the rainy season was 6.1 log10 CFU/g of cheese21. In the study carried out by Hernández et al26 on the Zacazonapan aged cheese, the cheeses were matured at a temperature of 24 °C and at a relative humidity of 65 % and at 27 d of maturation, MAB were present at a concentration of 1.5 to 7.8 log10 CFU/g of cheese.
The group of Staph (Table 1) had differences (P<0.05) between days of maturation and between production periods. The average of the counts made to the dry season cheese was 7.99 log10 CFU/g of cheese and that of the rainy season was 9.12 log10 CFU/g of cheese. The presence of staphylococci and the appearance of enterotoxins in foods are important parameters in the evaluation of food safety27. Staphylococcus aureus is often found in raw milk and in the environment of cheese plants (equipment and staff), is salt tolerant and can grow under a wide range of conditions; low acid production can allow staphylococci to grow and produce enterotoxins16,27.
In artisanal pore cheese, it was found that S. aureus was present with an average concentration of 5.91 log10 CFU/g of cheese in three-day old cheese and 6.29 log10 CFU/g of cheese with 12 d of maturation16. In Tepeque aerated cheese, the microbial load was 7.9 and 7.7 log10 CFU/g of cheese in cheese from the dry season and the rainy season, respectively21. In Kurdish cheese, the concentration was 3.06 log10 CFU/g of cheese in fresh cheese and 1.12 log10 CFU/g of cheese at 40 d of maturation22. Results similar to those found in this study indicate serious failures in the hygienic-sanitary conditions of cheese factories28 and, consequently, the consumption of this cheese can cause staphylococcal poisoning, representing a danger to the consumer29.
Significant differences (P<0.05) were observed in the TC content between days of maturation, decreasing throughout the process. The average of both batches of cheese was 8.10 log10 CFU/g of cheese (Table 1). TCs are a good indicator of hygienic quality, their presence is undesirable because it causes structural defects in the cheese and are an indicator of fecal contamination and reflect lack of hygiene during the preparation or handling of the product and warn of the possible presence of other pathogens30. During salting, there is a slow process of natural dehydration of cheeses, favoring the survival of these bacteria for longer16.
The bacterial load of TC in cheeses at day zero (Table 1) was similar to that reported in fresh cheeses28,29. The minimum load observed in this study was 6.68 log10 CFU/g of cheese at 120 d, similar to matured Tepeque cheese21. However, it exceeds the counts reported in aerated or controlled maturation cheeses, such as artisanal pore cheese16, Zacazonapan aged cheese26 and Corrientes cheese31, which makes clear the importance of controlling the maturation conditions of this type of cheese.
The counts for FC (Table 1) showed significant differences (P<0.05) between production seasons, being higher in dry season cheeses (3.04 log10 MPN/g of cheese). The results of this study were lower than those found in Mexican tropical cream cheese made with unpasteurized milk from the region of Tonalá, Chiapas24 and their presence in cheese indicates contamination by feces32. The decrease in counts after 120 d of maturation in rainy season cheeses is due to the action of lactic acid bacteria27.
The results of the analyses carried out for Salm and List during the maturation of the cheeses (Table 2) indicate that Salm was only detected in the cheeses of d 0 (fresh cheeses) and List was present throughout the maturation.
Table 2 Salmonella spp. and Listeria spp. during the maturation of Zacazonapan cheese
| Microbial group | Batch | Days of maturation | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | 150 | ||
| Salm | Dry season | P | ||||
| Rainy season | P | |||||
| List | Dry season | P | P | P | P | P |
| Rainy season | P | P | P | P | P | |
Salm= Salmonella spp.; List= Listeria spp.; P= Present in 25 g of sample.
The presence of Salm in cheeses is related to the preparation of products with unpasteurized milk and it has been detected in fresh cheeses29, in the first days of maturation22,27,32. In cheeses with 7 d of maturation, Salm was not detected, and this is attributed to the accumulation of lactic acid and the decrease in pH (< 4.7)27.
The presence of List in cheeses (Table 2) suggests contamination when using milk contaminated by cows that suffer from subclinical mastitis33. Bacteria of the genus List have been detected in equipment used in the manufacture of cheeses34, in aerated and matured cheeses21,28. Although the species was not determined, the risk to consumer health that List represents must be considered, since it can produce important alterations to the central nervous system and even death34.
The results obtained in this research; the bacterial count of the studied groups exceeds what is allowed by NOM 243 SSA123. However, there is a worldwide trend of consuming artisanal products that are sought after for their taste and quality linked to the place of origin. Microbial diversity as well as interactions between populations are the main factors that contribute to the taste of traditional cheeses16,20.
It is concluded that the maturation for 150 d of both batches of cheeses, as is traditionally done, is insufficient to inhibit the development of pathogenic microorganisms, although the rainy season cheese had a lower microbial load. The presence of bacteria of the genus List and Salm indicated a greater health risk, and it is not suitable for consumption. It is necessary to carry out actions throughout the cheese process, and it is suggested to implement good manufacturing practices for the production of cheese, and that a space of the cheese factory be adapted as a maturation chamber.
Acknowledgements and conflicts of interests
Work funded by the projects FE016/2009 (COMECYT) and 3101/2011 (Autonomous University of the State of Mexico). The authors thank the National Council for Science and Technology (CONACYT, for its acronym in Spanish) for the grant to the first author in his postgraduate studies and the cheese producers of the municipality of Zacazonapan for the support provided for carrying out this research. The authors of this paper declare that there is no conflict of interest of any kind.
REFERENCES
1. Cardoso VM, Dias RS, Soares BM, Clementino LA, Araújo CP, Rosa CA. The influence of ripening period length and season on the microbiological parameters of a traditional Brazilian cheese. Braz J Microbiol 2013;44:743-749. [ Links ]
2. Sicard M, Perrot N, Leclercq-Perlat MN, Baudrit C, Corrieu G. Toward the integration of expert knowledge and instrumental data to control food processes: Application to Camembert-type cheese ripening. J Dairy Sci 2011;94:1-13. [ Links ]
3. Licitra G, Carpino S. The microfloras and sensory profiles of selected protected designation of origin Italian cheeses. Microbiol Spectr 2014;2(1):1-12. [ Links ]
4. Soto VZ, Pérez LL, Estrada AD. Bacterias causantes de enfermedades transmitidas por alimentos: una mirada en Colombia. Salud Uninorte Barranq 2016;32(1):105-122. [ Links ]
5. Hernández MC, Hernández MA, Villegas de Gante AZ, Aguirre ME. El proceso socio-técnico de producción de Queso Añejo de Zacazonapan, Estado de México. Rev Mex Cienc Pecu 2011;2:161-176. [ Links ]
6. Norma Oficial Mexicana NOM-109-SSA1-1994. Bienes y servicios. Procedimientos para la toma, manejo y transporte de muestras de alimentos para su análisis microbiológico. México: Diario Oficial de la Federación, 4 de noviembre de 1994. [ Links ]
7. Norma Oficial Mexicana NOM-110-SSA1-1994. Bienes y servicios. Para la preparación y dilución de muestras de alimentos para su análisis microbiológico. México: Diario Oficial de la Federación, 28 de abril de 1994. [ Links ]
8. Norma Oficial Mexicana NOM-111-SSA1-1994. Bienes y servicios. Método para la cuenta de mohos y levaduras en alimentos. México: Diario Oficial de la Federación, de abril de 1994. [ Links ]
9. Norma Oficial Mexicana NOM-092-SSA1-1994. Bienes y servicios. Método para la cuenta de bacterias aerobias en placa. México: Diario Oficial de la Federación, 23 de marzo de 1994. [ Links ]
10. Norma Oficial Mexicana. NOM-113-SSA1-1994 Bienes y servicios. Método para la cuenta de microorganismos coliformes totales en placa. México: Diario Oficial de la Federación, 28 de abril de 1994. [ Links ]
11. Norma Oficial Mexicana NOM-112-SSA1-1994. Bienes y servicios. Determinación de bacterias coliformes fecales. Técnica del número más probable (NMP). México: Diario Oficial de la Federación, 10 de mayo de 1995. [ Links ]
12. Norma Oficial Mexicana NOM-115-SSA1-1994. Bienes y servicios. Método para la determinación de Staphylococcus aureus en alimentos. México: Diario Oficial de la Federación, 20 de febrero de 1995. [ Links ]
13. Norma Oficial Mexicana NOM-114-SSA1-1994. Bienes y servicios. Método para la determinación de Salmonella en alimentos. México: Diario Oficial de la Federación, 28 de abril de 1994. [ Links ]
14. Norma Oficial Mexicana NOM-143-SSA1-1995. Bienes y servicios. Método de prueba microbiológico para alimentos. Determinación de Listeria monocytogenes. México: Diario Oficial de la Federación, 19 de noviembre de 1997. [ Links ]
15. Minitab V.14. Statistical software. User´s guideII: Data analysis and quality tools, graphics, and Macros 2003; USA. [ Links ]
16. Alejo-Martínez K, Ortiz-Hernández M, Recino-Metelín BR, González-Cortés N, Jiménez-Vera R. Tiempo de maduración y perfil microbiológico del queso de poro artesanal. Rev Iberoamericana Cienc 2015;2 (5):15-24. [ Links ]
17. Vázquez-Fontes C, Sánchez-Vera E, Castelán-Ortega O, Espinoza-Ortega A. Microbiological quality of artisan-made Mexican Botanero cheese in the Central Highlands. J Food Saf 2010;30:40-50. [ Links ]
18. Volken De SCF, Dalla RT, Zachia AA. Changes in the microbiological and physicochemical characteristics of Serrano cheese during manufacture and ripening. Braz J Microbiol 2003;34:260-266. [ Links ]
19. De Dea LJ, Bernini V, De Lorentiis A, Pecorari A, Neviani E, Gatti M. Parmigiano Reggiano cheese: evolution of cultivable and total lactic microflora and peptidase activities during manufacture and ripening. Dairy Sci Technol 2008;88:511-523. [ Links ]
20. Marino M, Maifreni M, Rondinini G. Microbiological characterization of artisanal Montaisa cheese: analysis of its indigenous lactic acid bacteria. FEMS Microbiol Letón 2003;229:133-140. [ Links ]
21. Solís MAD, Martínez LR, Solorio SJ, Estrada FJG, Avilés NF, Gutiérrez IAT, Castelán OOA. Características del queso Tepeque de la tierra caliente de Michoacán: Un queso producido en un sistema silvopastoril intensivo. Trop Subtrop Agroecosystems 2013;16:201-214. [ Links ]
22. Milani E, Shahidi F, Mortazavi SA, Reza-Vakili SA, Ghoddusi HB. Microbiological, biochemical and rheological changes throughout ripening of Kurdish cheese. J Food Saf 2014;34:168-175. [ Links ]
23. Norma Oficial Mexicana NOM-243-SSA1-2010. Productos y servicios. Leche, fórmula láctea, producto lácteo combinado y derivados lácteos. Disposiciones y especificaciones sanitarias. Métodos de prueba. México: Diario Oficial de la Federación, 27 de septiembre de 2010. [ Links ]
24. Romero-Castillo PA, Leyva-Ruelas G, Cruz-Castillo JG, Santos-Moreno A. Evaluación de la calidad sanitaria de queso crema tropical mexicano de la región de Tonalá, Chiapas. Rev Mex Ing Quim 2009;8:111-119. [ Links ]
25. Beresford TP, Fitzsimons NA, Brennan NL, Cogan TM. Recent advances in cheese microbiology. Int Dairy J 2001;11:259-274. [ Links ]
26. Hernández MC, Hernández MA, Aguirre ME, Villegas de Gante AZ. Physicochemical, microbiological, textural and sensory characterization of Mexican Añejo cheese. Int J Dairy Technol 2010;63(4):552-560. [ Links ]
27. Amran AM, Abbas AA. Microbiological changes and determination of some chemical characteristics for local Yemeni cheese. Jordan J Biol Sci 2011;4 (2):93-100. [ Links ]
28. Castro-Castillo G, Martínez-Castañeda FE, Martínez-Campos AR, Espinoza-Ortega A. Caracterización de la microbiota nativa del queso Oaxaca tradicional en tres fases de elaboración. Rev Soc Ven Microbiol 2013;33(2):105-109. [ Links ]
29. González-Montiel L, Franco-Fernández MJ. Perfil microbiológico del queso de aro consumido en la Cañada Oaxaqueña. Brazilian J Food Technol 2015;18(3):250-257. [ Links ]
30. Sengul M, Ertugay MF. Microbiological and chemical properties of cheese Helva produced in Turkey. Int J Food Prop 2006;9:185-193. [ Links ]
31. Vasek OM, Mazza SM, Giori GS. Physicochemical and microbiological evaluation of corrientes artisanal cheese during ripening. Food Sci Technol 2013;33(1):151-160. [ Links ]
32. Martins JM, Galinari E, Pimentel-Filho NJ, Ribeiro JJI, Furtado MM, Ferreira CLLF. Determining the minimum ripening time of artisanal Minas cheese, a traditional Brazilian cheese. Braz J Microbiol 2015;46(1):219-230. [ Links ]
33. Meyer-Broseta S, Diot A, Bastian S, Rivière J, Cerf O. Estimation of low bacteria concentration: Listeria monocytogenes in raw milk. Int J Food Microbiol 2003;80:1-15. [ Links ]
34. Arguello P, Lucero O, Castillo G, Escobar S, Albuja A, Gallegos J, Carrascal A. Calidad microbiológica de los quesos artesanales elaborados en zonas rurales de Riobamba (Ecuador). Perspectiva 2015;16 (18):65-74. [ Links ]
Received: March 08, 2021; Accepted: April 07, 2022











 texto en
texto en 


