Introduction
The economic profit of a dairy farm increases as reproductive efficiency improves and the ultrasound is a tool that helps to improve decision-making regarding reproductive management in dairy and beef herds1. Ultrasonography images may provide information regarding the physiological stage of the main components of the reproductive tract (ovary, follicle, corpus luteum (CL) and uterus) (Figures 1 to 9), which is valuable for accurate work decisions of animal reproduction practitioners and researchers. In addition, ultrasound allows to diagnose pathological conditions of the reproductive tract in real-time on field conditions in a highly reliable way.
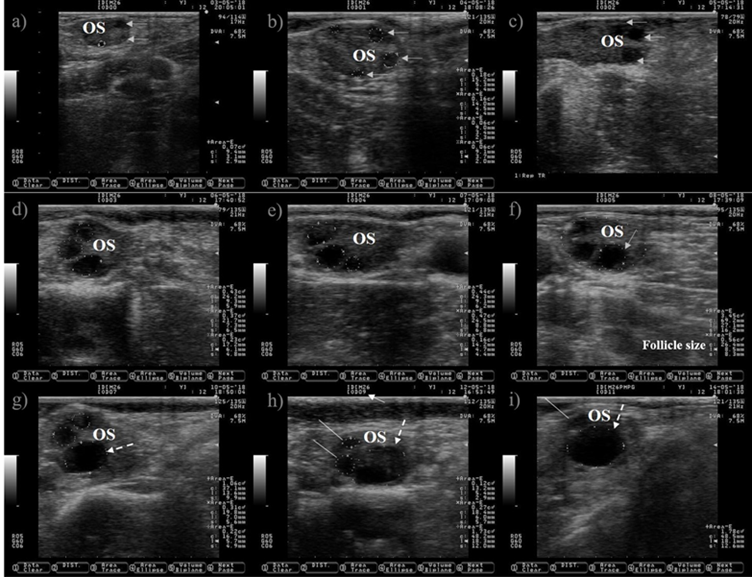
Ultrasound allows to visualize only the selection and dominance phase of the follicular wave, but not the recruitment phase because it takes place at very early stage of follicular development. The preovulatory follicle at day zero and the subsequent corpus luteum were located in the left ovary, but only pictures of the right ovary are shown because is where the follicle wave emerged. a) a small antral follicle is encircled with a dotted line at day zero (the anechogenic characteristics of the follicular fluid makes the follicle appears as dark circle), gray arrows are indicating the presence of other two antral follicles. b) the follicle wave has emerged, the four follicles are encircled with dotted lines at day one, three of them were previously observed at day zero (gray arrows). c) the gray arrows are pointing to the three follicles observed since day one, the increase in its size is easily observed at day two. The growth of the three follicles (dotted lines) observed since day zero is depicted in Pictures c) and d) (day three and four). e) the dominant follicle of the follicular wave is selected (selection of the dominant follicle occurs when the largest follicle reaches approximately 8.5 mm in diameter11), this marks the end of the selection and the commencement of the dominance phase. The growth of the dominant follicle (dashed arrows) and the atresia of the subordinate follicles (white lines) are depicted in Pictures g) to i). OS: ovary stroma. White arrow: day of the estrous cycle. Pictures were taken using a 7.5 MHz probe.
Figure 1 Pictures depicting follicle wave growth in dairy cattle from estrus (day 0) to day 11 of the estrous cycle
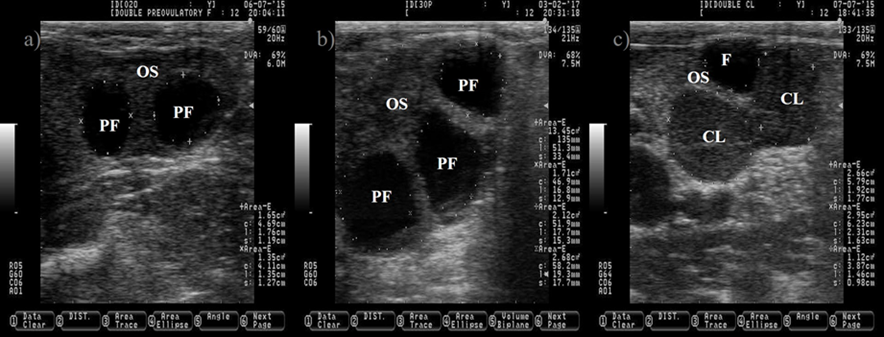
The follicular fluid is anechogenic, which make the PF appears as a dark circle. The ovulation of two PF will produce two corpora lutea (CL, c). The CL is usually darker (hypoechogenic) than the ovary stroma (OS). Pictures were taken using a 7.5 MHz probe at random stage of the estrous cycle.
Figure 2 Pictures depicting two (a) and three preovulatory follicles (PF, b) at estrus
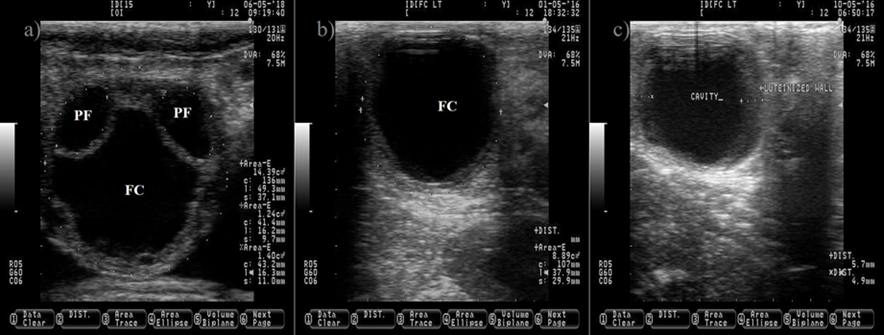
The anechogenic follicular fluid in the PF and FC makes them appear as a dark circle. Pictures b and c depict a FC after induced luteinization by GnRH injection, notice the increment on the thickness of the follicular wall (c). Pictures were taken using a 7.5 MHz probe.
Figure 3 Ovarian cyst in dairy cattle. a) picture depicting two normal pre-ovulatory (PF) follicles and one large follicular cyst (FC)

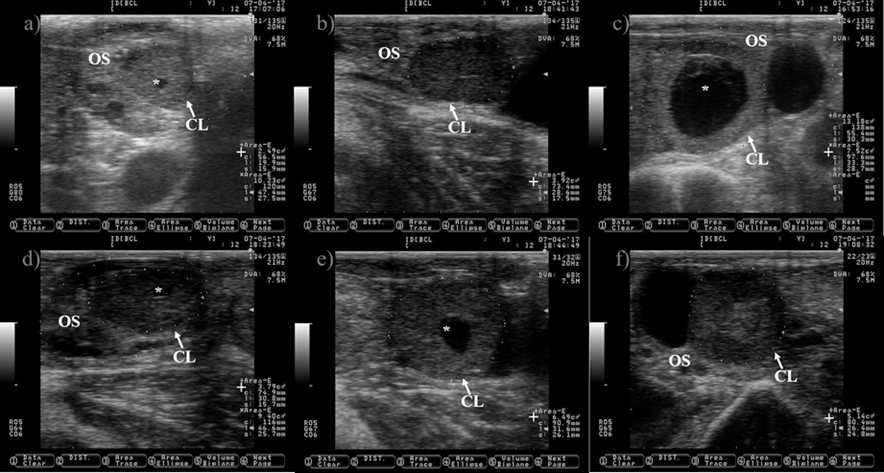
Pictures were taken at approximately every 24 h from day before to 7 d after ovulation. a) preovulatory follicle (PF). b) early CL after ovulation of the PF, notice the presences of luteal cavity (*), the CL and the ovary stroma (OS) are almost isoechogenic until 2 d after ovulation (b-d), but the CL becomes darker (hypoechogenic) than the OS as it ages (c-i). e) the CL is well differentiated from the OS at 3 d after ovulation. f-i) pictures depict a growing CL, the luteal cavity is clearly identified at this stage (4-7 d after ovulation). i) the CL has almost triple its area (+) since ovulation day (b). Pictures were taken using a 7.5 MHz probe.
Figure 4 Growth of the corpus luteum (CL) after ovulation in Holstein dairy cattle
a, c, d and e) CL with luteal cavity (*) of different sizes. b and f) CL without luteal cavity.
OS= ovary stroma; += area of the corpus luteum. Pictures were taken using a 7.5 MHz probe.
Figure 5 Pictures depicting different corpus luteum (CL) shapes at nine days after estrus in Holstein dairy cattle
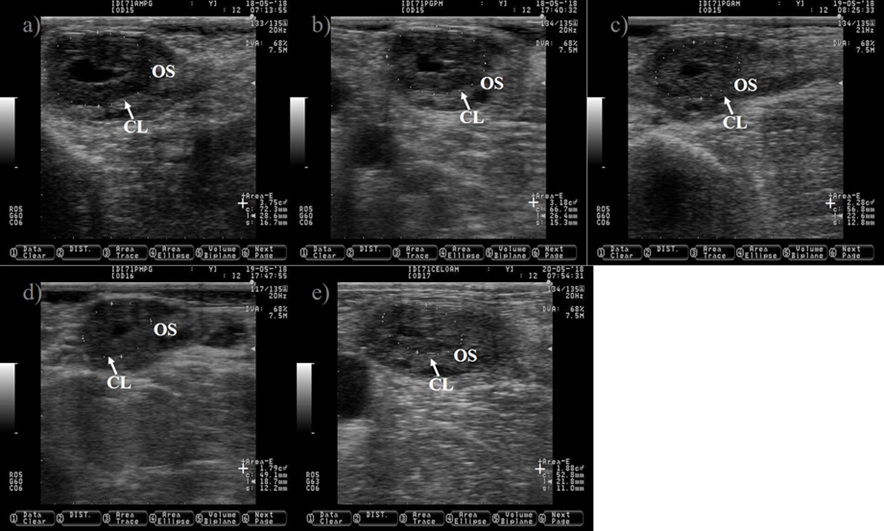
Pictures were taken at approximately 12 h interval after prostaglandin injection until estrus detection using a 7.5 MHz probe. a) CL just before prostaglandin injection. b) CL 10 h after prostaglandin injection, notice the reduction in its size (+). c) CL showing 39 % reduction in its size 25 h after prostaglandin injection. d) CL showing 52 % reduction in its size 34 h after prostaglandin injection. e) picture depicting the regressed CL 48 h after prostaglandin injection, notice that the CL and the ovary stroma (OS) are almost isoechogenic. The picture was captured a few hours after the cow was detected in estrus, the preovulatory follicle was located in the opposite ovary and it is not shown.
Figure 6 Pictures depicting corpus luteum (CL) regression after prostaglandin injection in Holstein dairy cattle

An ovary and regressed corpus luteum (CL) are encircled with + and x. The pre-ovulatory follicle (PF) is the black circle in the top center of the ovary. OS= ovary stroma. Pictures were taken using a 7.5 MHz probe.
Figure 7 Remains of the corpus luteum at estrus in Holstein dairy cattle
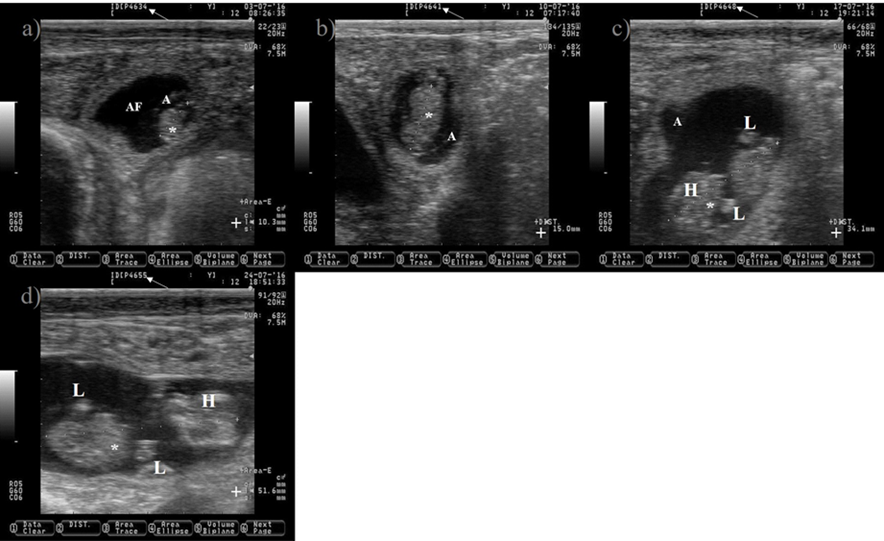
White arrow is pointing at days of gestation. a) 34-d old embryo. b) 41-d old embryo. c) 48-d old embryo. e) 55-d old embryo.
*= embryo; A= amnion; AF= allantoic fluid; += size of the embryo; H= head; L= limbs. Pictures were taken using a 7.5 MHz probe. Pictures correspond to the same embryo.
Figure 8 Pictures depicting embryo development in Holstein dairy cattle from 34 to 55 d of gestation

White arrows indicating the location of pus (the pus is observed as bright and white spots of different shapes and sizes). White lines are delimiting the uterine horns. Pictures were taken using a 7.5 MHz probe.
Figure 9 Pictures depicting the presence of pus (subclinical endometritis) in the uterus of Holstein dairy cattle at random stages of lactation
There are outstanding reviews discussing technical and practical aspects about ultrasound applications in dairy cattle reproductive management2-7. A search including only “Mexico” and using the key words “cattle, follicle and corpus luteum size” in SCOPUS reveals that during the period from 2015 to 2019 only nine scientific articles were wrote by Mexican researchers, which is lower than the 95 scientific articles found when the search was restricted to the Unites States of America, during the same period. The reasons for this might include a lower number of researchers in Mexico than in the Unites States of America, the restricted access to guiding information to empower new ultrasound practitioners and the economic investment needed to obtain an ultrasound6. The professional guidance is always recommended when adopting new technologies, but when factors such as time and geographical distance limit the access to professional expertise, then the self-training is the best option. However, the first encounters with the vast amount of scientific literature can be overwhelming to new ultrasound practitioners, because not only the revision of literature related to ultrasonography is mandatory, but also the reproductive physiology and management of dairy cattle must be attended. Despite the available papers reviewing the ultrasound applications to dairy cattle reproductive management, there is still space to enrich the existent literature by merging field experiences performing ultrasonography with cattle reproductive physiology and management.
Therefore, the objective is to merge a physiological description with reproductive management and technical aspects of original ultrasound pictures of the most relevant reproductive events in dairy cattle to promote ultrasound use during dairy cattle reproductive management by practitioners and researchers.
Follicular events
The ovarian follicles grow in cyclic and organized events known as follicular waves (Figure 1). A follicular wave comprises three fixed (recruitment, selection and dominance) and two conditional (atresia or ovulation) stages8. During the fixed stages a dominant follicle is selected from a cohort of growing follicles, while the unselected (subordinate) follicles suffer atresia. A growing follicle must have access to IGF-I, express LH receptors and synthesize estradiol under low FSH concentrations conditions in order to reach the stage of dominance9. After dominance, the selected follicle will undergo atresia or ovulation depending on the hormonal environment. Ovulation will occur if the follicle reaches the dominance stage under declining blood progesterone concentrations, otherwise it will suffer atresia and a new wave will emerge. Follicle wave emerging is characterized by the appearance of several follicles between 3-4 mm10. Two or three waves are commonly observed during the estrous cycle in cattle11.
The preovulatory follicle is the dominant follicle that naturally emerges from the last follicle wave of the estrous cycle in cyclic non-pregnant cows and, under normal circumstances, is destined to ovulate. The estradiol synthesized by the preovulatoy follicle is responsible for the characteristic signs of estrus in cattle and induces the GnRH/LH surge, which triggers ovulation. Researchers have tried to establish a relationship between preovulatory follicle size and pregnancy success after artificial insemination. In this regard, follicles reach ovulatory capacity in response to GnRH at 10 mm size12. However, GnRH induced ovulation of small (≤ 11 mm) or large (> 20 mm) follicles negatively affects fertility and increases the probability of pregnancy loss13,14. Explanatory reasons for the aforementioned include a reduced oocyte competence and an impaired luteal function when small or large follicles are induced to ovulate14,15. Contrary, preovulatory follicle size does not compromise pregnancy rate when ovulation occurred spontaneously13. Therefore, the measuring of follicle to ensure an adequate size is advisable during protocols that involve ovulation induction. It is common to observe a wide variation within and between new ultrasound practitioners when measuring follicle and CL size. To avoid this variation, it is recommended to always explore the entire ovary and then capture the largest view of the desired ovarian structure. In addition, physical pressure on the preovulatory follicle should be minimized because of the risk of shape deformation and rupture, which might occur when the ovary is mishandled.
The cow normally ovulates one follicle between 28 to 31 h after the onset of estrus16,17, but occasionally more than one follicle ovulates (Figure 2), increasing the incidence of twin pregnancies. Pregnancies carrying more than one product are undesired because it impairs reproductive performance and reduce the productive life span of the dam18. Unfortunately, the incidence of twin pregnancies has increased over the years. The improvement in nutrition, management practices and the genetic progress to increase milk production predispose to the augmented incidence of twin pregnancies19. In addition, cows carrying the Trio allele are more likely to carry twins20. Fortunately, the incidence of twin pregnancy can be prevented by ablation of multiple follicles before artificial insemination, leaving only one preovulatory follicle21.
Ovulation is expected to occur after estrus. However, 3.4 and 12.4 % of cows did not ovulate after estrus during the cool and warm season in a study carried out in Spain22. Ovulation failure (anovulation) completely blocks the opportunity of pregnancy. Therefore, disappearance of the preovulatory follicle (ovulation) should be confirmed by ultrasound after estrus (Figure 4).
The causes of anovulatory condition include a low LH pulse frequency after estrus23 and the formation of follicle cyst (Figure 3). A follicle cyst is a large follicle that fails to ovulate and persist for an abnormal period of time in the absence of a CL, causing a recurrent estrus behavior. A sub-luteal progesterone concentration24 that allows a rise of LH pulse frequency to sustain follicle growth25, but not a preovulatory LH surge to induce ovulation26, favors follicle cyst formation. In addition, the induction of a preovulatory LH surge without subsequent progesterone exposure is also effective to induce follicle cyst formation27.
The follicle cyst appears as a large ovarian structure (> 25 mm in diameter) with a thin wall (< 3 mm), a non-echogenic antrum, a large estradiol: progesterone ratio and it can rupture if mishandled during palpation (Figure 3). Another type of cyst is the follicle-luteal, also known as luteinized follicle or luteinized cyst. The luteinized follicle cyst has a thick wall (> 3 mm), a reduced cavity, a small estradiol: progesterone ratio and it will not rupture during palpation (Figure 3)28,29. A luteinized follicle cyst might appear after treatment of follicle cyst with GnRH injections. Ovulation and release of the oocyte from the preovulatory follicle are paramount events that open the possibility for pregnancy. After these events, the next expected ovarian structure to develop is the CL.
Corpus luteum development and regression
The CL is a transient endocrine ovary gland that regulates the length of the estrous cycle and produces progesterone to create a suitable uterine environment for pregnancy. The CL originates from the transformation of granulosa and theca follicular cells into large and small luteal cells triggered by the preovulatory LH peak30. The tracking of the growth of the CL during the estrous cycle might begin at 12 to 24 h after ovulation (Figure 4)31. The CL reaches its maximum size between days nine and 10 of the estrous cycle32,33. It appears as a semi-circle solid shape structure or with a central cavity (Figure 5). The cavity is filled with a serous transudate or blood34. The incidence of CL with cavities might reach up to 79%31. The ovulation of larger follicle predisposes to cavities formation35, without compromising progesterone production or pregnancy rate in Holstein cattle35,36.
The measurement of the CL after insemination is relevant because blood progesterone concentration at mid-luteal phase depends on its size37 and a positive association between progesterone concentrations and area of the growing CL has been reported38. In addition, dairy cattle with good genetic merit for fertility traits have greater blood progesterone concentrations and bigger CL than those with poor genetic merit39,40. The CL grows faster in pregnant than in non-pregnant cows from day six to nine of the estrous cycle41, a reason to explain the difference in CL growth rate is unknown. However, a faster growth rate might be associated with a healthier corpus luteum.
The analysis of CL ultrasound images has been performed to predict the stage of the estrous cycle and its functional status (growing or regressing). During the diestrus, the CL is darker, and its echotexture is more homogenous than during the metestrus and proestrus34. However, the functional assessment of the CL is difficult to determine by ultrasound images33,42. Corpora lutea at diestrus stage are easily identified, but to differentiate between those in metestrus (growing CL) and proestrus (regressing CL) is hard because of its morphological similitudes (Figures 4 and 7). In addition, when the area of the CL is between 1.3 and 3.2 cm2, it also difficult to determine if functional CL exists43. Therefore, to establish the functional status of the CL, it is necessary to perform multiple progesterone concentration analysis, to review the animal reproductive record or to carry on more than one CL measurement by ultrasound at least 2 d apart. In addition, the assessment of luteal blood flow by doppler ultrasound can be used to stablish corpus luteum functionality44.
The CL undergoes regression if pregnancy is not established. Regression of the CL is under the uterine prostaglandin (F2α) command. A loss of progesterone synthesis capacity and reduction in the size of the CL is observed during the stage of regression (Figure 6). The CL begins to shrink after d 14 of the estrous cycle33 or 3.2 d before the onset of estrus in non-pregnant cows32, but becomes sensitive to the luteolytic effects of prostaglandins at d 5 of the estrous cycle45. Prostaglandin reduces the size of the CL (23 to 47 %) within one to four days46 and decreases blood concentration of progesterone within 4 h post-injection by ceasing steroidogenic enzymes activity47 and by inducing luteal cell death48. In addition, the CL is colonized by immune cells49 that remove dead cells from the ovarian stroma during regression48, contributing to CL reduction in size at this stage. After regression of the CL, a preovulatory follicle and estrus will appear, remaining only trace of the former CL on the ovary (Figure 7). However, if pregnancy is established, uterine prostaglandin (F2α) synthesis is blocked. Therefore, regression does not occur and the CL extends its life span until the end of the next reproductive event (pregnancy).
Pregnancy diagnoses
A land-mark in the pathway to pregnancy is the production of interferon tau by the bovine conceptus, which prevents CL regression induced by prostaglandin F2α and promotes pregnancy recognition50. The presence of the bovine embryonic vesicle, CL and uterine fluid are indications of pregnancy (51, but the gold standard to diagnose a pregnancy is by the observation of an embryo with a heartbeat, which is rapidly detectable at 30 d after artificial insemination (Figure 8)52.
The pregnancy diagnoses should be performed with care to avoid physical damage to the embryo. The entire uterus and uterine horns should be revised to give an accurate diagnosis. A common mistake is to diagnose a cow as pregnant only by the visualization of uterine fluid, which is also observed in cows during the estrous stage.
The primary goal of early pregnancy diagnosis (28-30 d after artificial insemination) is to differentiate between pregnant and nonpregnant cows as soon as possible after artificial insemination. After diagnosis, the nonpregnant cows are prepared to rebreed, while the risk of pregnancy loss during the first 60 d of gestation obligates to scheduled pregnant cows to confirmatory diagnosis (40-60 d after artificial insemination) by ultrasound or hand palpation53,54. Causes of pregnancy loss during this period of time include the selection to increase milk production, impaired uterine environment, poor oocyte and embryo quality55.
The pregnancy diagnosis is followed by two optional events, which are fetus age estimation and sexing. Modern dairy farms keep accurate records of artificial insemination dates, avoiding the need for fetal age estimation. However, the measurement of the crown-rump, head length, trunk diameter and placentoma size might be carried out to estimate the age of the fetus56,57. The gender of the fetus is determined between 55 to 111 d of gestation58. However, fetal sexing is not a common practice in dairy farms, probably because ultrasound services are mostly required for early pregnancy diagnosis. In addition, artificial insemination of heifers with sexed semen ensures the born of calves with the desired sex in approximately 90 % of the inseminated animals.
Postpartum reproductive events
The next reproductive events after pregnancy diagnosis are calving, shedding of fetal membranes, uterine involution, the establishment of cyclical ovarian activity and diagnosis of uterine health before the first insemination post-partum. Even though all mentioned events must be supervised, the establishment of cyclical ovarian activity and diagnosis of uterine health before the first insemination might be the most relevant for ultrasound practitioners.
The achievement of a rapid presentation of ovulation and cyclical estrus after calving are the primary goals for good reproductive management. Ultrasound examination of the reproductive tract, at least every 2 wk after 20 d of lactation, is advisable. The presence of a corpus luteum is the best indicator that ovulation had successfully occurred. After ovulation and CL formation, the observation or data revision from computer software to detect signs of estrus is mandatory. Once cyclical estrus activity has been achieved, the eradication of subclinical uterine infections is necessary to establish a healthy uterine environment capable to host a pregnancy.
The clinical uterine infections (metritis and endometritis) are easily detected by observation, smell and palpation of classical signs such as enlarged uterus, vaginal discharge, dullness, and fetid smell. However, signs of subclinical uterine infection (subclinical endometritis) are hidden from simple observation, smell or palpation. A subclinical endometritis is an inflammation of the endometrium, without purulent vaginal discharge and external signs of illness, and it is diagnosed by an increased number of neutrophils in the endometrium using cytology (cytobrush)59.
Subclinical endometritis does not compromise the life of a cow, but fertility might be decreased. A reduced fertilization rate and embryo quality has been reported in superovulated cow with subclinical endometritis60, which partly explained the low pregnancy rate found in cows suffering the same malady61. Contrary, others have failed to establish a relationship between subclinical endometritis and poor reproductive performance in cattle62,63. Origin of the controversy among studies is not well understood because all of them used the same instrumental (cytobrush) to discern between healthy cows and those with subclinical endometritis. The nature of this controversy might be related to the number and intrauterine locations where the samples for cytology were obtained. It is recommended to take at least two samples in two different intrauterine locations, preferably in the horns, to accurate diagnose subclinical endometritis64. The mentioned studies61-63 only took one sample and only one of them did it in the uterine horns61. The cytobrush is one of the most common methods to diagnose subclinical endometritis. However, the uterine lavage, leukocyte esterase test trips and uterine biopsy have been used to successfully diagnose subclinical endometritis in cattle65,66. The main disadvantage of these methods is that they are time consuming and invasive, requiring a sample of endometrial fluid/cells taken from uterine horns. Ultrasound is an alternative, non-invasive and reliable tool to diagnose subclinical endometritis, by identification of uterine fluid at 20-47 d in milk66,67. However, the presences of pus in the uterus might be also considered as indication of subclinical endometritis. The pus is composed by neutrophils and it is a sign of inflammation68, and it is commonly observed along the uterine horns (Figure 9), normally in such small quantities that it is not observed as vaginal discharge, which comply with the definition of subclinical endometritis59.
Further considerations and areas of opportunity
The reason that justified the use of ultrasound must be known before to actually perform any ultrasonographic exploration in the cow. In addition, the safety of the practitioner, the animal and the equipment must be guaranteed at all times during the procedure. It is also advisable to gather as much information as possible about the clinical, nutritional, management, productive and reproductive history of the cows before carrying any physical exploration.
The latter will help to make an accurate diagnosis and to provide the most suitable treatment.
The selection of experimental units during reproductive research should be done at least 21 d before the study commence. Their reproductive tract must be revised by ultrasonography to ensure that all animals are in the desired condition (healthy: cyclic state, without ovarian cyst and free of uterine infection; unhealthy: with uterine infections or ovarian cysts). However, if the number of experimental units is limited to perform the research, then there will be at least 21 d to treat cows that require reproductive assistance. The oocyte competence and embryo quality are factors that significantly affect the chances of achieving pregnancy status in a cow after artificial insemination. It will be advantageous to measure specific and predictive characteristics related to those factors on field condition by ultrasonography.
Conclusions
The application of ultrasound is mandatory to better understand some of the most relevant reproductive events and to support decision-making during dairy cattle reproductive management. It is important that students and researchers embrace ultrasound as a routine tool for research and field work.











 texto en
texto en 


