Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.13 n.2 Mérida Apr./Jun. 2022 Epub June 20, 2022
https://doi.org/10.22319/rmcp.v13i2.5729
Articles
Diagnosis of the health quality of artisanal cheese dairies in Salinas, San Luis Potosí
a Universidad Autónoma de San Luis Potosí. Coordinación Académica Región Altiplano Oeste. Carretera Salinas-Santo Domingo #200, 78600, Salinas de Hidalgo, San Luis Potosí, México.
b Universidad Autónoma de San Luis Potosí. Instituto de Investigación de Zonas Desérticas. San Luis Potosí. México.
c Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. Jalisco, México.
The objective of this work was to evaluate the microbiological load of total coliforms (TCs), Staphylococcus aureus and Salmonella spp. in milk and fresh cheeses as indicators of process practices carried out in cheese dairies of different localities in the region of Salinas, San Luis Potosí. Fifteen establishments were sampled, milk and cheese samples were obtained, and they underwent a milk composition and microbiological analysis. Sixty-five percent of the production units do not pasteurize the milk, or they use natural rennet. The highest counts in milk were 42 x 109 and 40 x 109 CFU/ml for S. aureus and TCs, respectively. For cheese, the counts were 32 x 109 and 26 x 109 CFU/g for S. aureus and TCs, respectively. In addition, the presence of Salmonella was detected in milk and cheese. The lack of hygiene in the utensils and equipment in which the cheeses are made, as well as the use of natural rennet, can be a risk to the health of the consumer.
Key words Zoonosis; Bacterium; Public health; Cow’s milk; Goat’s milk
El objetivo de este trabajo fue evaluar la carga microbiológica de coliformes totales (CT), Staphylococcus aureus y Salmonella spp en leche y quesos frescos como indicadores de prácticas de proceso que se realizan en queserías de diferentes localidades de la región de Salinas, San Luis Potosí. Se muestrearon 15 establecimientos, se obtuvieron muestras de leche y queso y se les realizó un análisis de composición de la leche y microbiológico. El 65 % de las unidades de producción no pasteuriza la leche o utiliza cuajo natural. Los conteos más elevados en leche fueron de 42 x 109 y 40 x 109 UFC/ml para S. aureus y CT, respectivamente. Para queso, las cuentas de 32 x 109 y 26 x 109 UFC/g para S. aureus y CT, respectivamente. Además, se detectó presencia de Salmonella en leche y queso. Se concluye que la falta de higiene en los utensilios y equipos en los que se elaboran los quesos, así como el uso de cuajo natural, pueden ser un riesgo para la salud del consumidor.
Palabras clave Zoonosis; Bacteria; Salud pública; Leche de vaca; Leche de cabra
Introduction
Milk, due to its nutritional characteristics, is one of the foods of animal origin with the highest demand in the world. In Mexico in 2018, milk production was 12,008,239 thousand liters, of which about 73 % was destined to the production of dairy products and derivatives (8,784,055 thousand liters)1. The region of the Western Altiplano of the state of San Luis Potosí is characterized by being a milk-producing region, in greater proportion of cattle and in less of goats, in which most of the milk is destined to the production of artisanal fresh cheese. Traditional artisanal cheeses are important, not only for their nutritional and gustatory benefits, but also for their ability to generate and keep rural employment that involves some agents of the agro-industrial milk chain: farmers, cheesemakers and traders2. However, organization among producers is required to, for example, create an “artisanal denomination” with a level of regulation and protection that guarantees the preservation of dairy production systems based on traditional practices and their respective socioeconomic benefits3.
On the other hand, in these production units (PUs) of the Western Altiplano of San Luis Potosí, cheese is made from raw milk, with the use of non-standardized traditional methods, scarce technification and in inappropriate facilities (e.g., they use the kitchens of the producers’ homes). In this regard, most producers do not know about the concept of good manufacturing practices and the pasteurization process, which is a thermal process that allows controlling pathogenic bacteria currently found in milk, such as Mycobacterium tuberculosis, Listeria monocytogenes, as well as some species of Campylobacter, Salmonella, Escherichia coli and other fecal coliforms4. In addition, cross-contamination of milk after pasteurization must be controlled by applying strict cleaning and disinfection rules5. Also, in this type of PU, non-sterile natural rennet is sometimes used in the production of cheese, its production consists of drying extended fragments of abomasum of newborn calves in clotheslines under the sun for up to three days, later they are introduced into a plastic drum with whey, it is covered and left to ferment for at least five days for later use; this practice can be an important point of microbial contamination.
Gastrointestinal diseases due to the consumption of contaminated foods can occur at any time of the year, but the risk increases in the hot season. The most frequent clinical conditions are fever, vomiting, abdominal pain and moderate or severe diarrhea6. For health institutions such as the Mexican Institute of Social Security (IMSS, for its acronym in Spanish), these diseases are: brucellosis, cholera, typhoid, gastroenteritis, shigellosis, salmonellosis and diarrhea, which represent a severe public health problem for San Luis Potosi state and the country7,8. Therefore, dairy products must comply with the sanitary provisions and specifications of NOM 2439. Which indicates that Salmonella spp. must be absent in 25 g of cheese or milliliter of milk, a maximum of 100 colony-forming units per gram or milliliter (CFU/g or ml) is allowed for total coliforms and a maximum of 1,000 CFU/g is allowed for Staphylococcus aureus. Therefore, the objective of the present work was to evaluate the microbiological load of total coliforms, S. aureus and Salmonella spp. in milk and cheeses as indicators of the process practices carried out in the cheese dairies of different localities of the region of Salinas, San Luis Potosí, and to be able to recommend good manufacturing practices that help improve the health conditions of production, without altering the characteristics of the dairy product.
Material and methods
Sampling site
The study was carried out from January to April 2018 in a part of the Western Altiplano region of the state, in some localities of the municipality of Salinas, San Luis Potosí and Villa González Ortega, Zacatecas, which are located between the following coordinates: 101°43” W and 22°38” N, with an altitude of 2,070 m asl, their climate is dry and their average temperature is 18 °C10.
Field visits were made to various localities of the region that produce cow and goat cheeses. Following the recommendations of the NOM 10911 project, samples of milk from the first milking of the day and ground fresh cheese were taken in 15 different PUs. Three aliquots of 10 ml of the accumulated milk of the day were taken from the tank or bottle (40 L) and placed in sterile bottles. Three samples of approximately 50 g of different cheeses were placed in individual hermetic bags of the Ziploc type. The milk and cheese samples were placed in a plastic cooler, previously sanitized and disinfected with alcohol to avoid cross-contamination, which contained ice for temperature control. Immediately afterwards, they were transferred to the laboratory for their compositional and microbiological analysis.
Two samples of commercial milk and two samples of commercial cheese were used as control treatment. In addition, a survey was applied at the sampled sites to obtain information on the conditions under which dairy products are produced and processed.
Milk composition
Milk samples were analyzed with a lactoscan equipment (Milkotester, Master Eco) with ultrasonic sensors to determine percentage content of fat, protein, lactose, non-fat solids, salts, freezing point, density and added water.
Microbiological analysis
The agars RVBA (red-violet-bile-lactose, BD-BIOXONTM), Salmonella-Shigella (BD-BIOXONTM), Baird Parker (BD-BIOXONTM) were prepared according to the sterility instructions indicated by each of the containers, for the growth of specific microorganisms such as total coliforms, Salmonella spp. and Staphylococcus aureus, respectively.
In the laboratory, milk and cheese samples were prepared using the method of dilutions in peptone water in accordance with NOM 11012. For solid samples, a sample of 1.0 g of cheese was taken, diluted and mixed homogeneously in 9 ml of lactose broth or 0.1 % peptone water to crush the solid, this constituted the first dilution of the sample (101). Subsequently, 10 tubes of 20 ml per each sample were prepared, 10 ml of the mixture was placed in the first tube and the tubes from two to ten contained 9.0 ml of 0.1 % peptone water, then 1.0 ml of the initial dilution of the first tube was taken and mixed homogeneously in the second tube, the procedure was repeated up to tube nine (109).
For liquid samples (milk), 10 previously sterilized tubes of 20 ml were prepared, a sample of 10 ml milk was taken and emptied into the first tube (100), the tubes from two to ten contained 9.0 ml of 0.1 % peptone water, then 1.0 ml of milk was taken from the first tube and mixed homogeneously in the second tube, the procedure was repeated up to tube nine (109). Once the dilutions were finished, the technique described by Miles and Misra, modified by Slack and Wheldon13, was used, in which 20 μl of each dilution were deposited on the surface of an agar plate for plate count, performing the drip from a height of 2.5 cm and depositing three drops per each dilution. The presence of Salmonella in cheese and milk, a sample of 25 ml of milk or 25 g of cheese was taken, diluted and mixed homogeneously in 225 ml of lactose broth or 0.1 % peptone water to crush it and it was incubated for 18 h at 36 °C, this constituted the first dilution of the sample (101). It was verified with NOM 11414 in Salmonella-Shigella agar and placed in an incubator (Yamato IN 804) for 24 h at 35 °C, translucent colonies, occasionally opaque, some colonies with black dots in the center. To determine the presence of S. aureus, the guidelines of NOM 11515 were followed, using Baird Parker agar (+Potassium tellurite) and incubating at 35 °C for 48 h, plates with between 15 and 150 black colonies are selected and the presence of total coliforms was analyzed with NOM 11316, using red-violet-bile-lactose agar (RVBA) and they were incubated at 35°C for 24 h, plates with between 15 and 150 dark red colonies are selected.
Statistical analysis
The descriptive statistics for milk quality parameters resulting from the physicochemical analysis were obtained; the production units were classified by means of a cluster analysis with the hclust function that groups similar and dissimilar farms; to compare the resulting cluster groups, an analysis of variance was performed for a completely randomized design; when this indicated that there was a treatment effect (P<0.05), a Tukey mean test with a significance of P<0.05 was performed. Descriptive statistics of the data from the microbiological analysis CFU/g or ml of cheese or milk that contained Salmonella spp., total coliforms and S. aureus were obtained. The statistical software R Core Team17 was used to analyze the data.
Results
Characteristics of production units
Of the fifteen production units (PUs), only three have goats and the rest have cows as described in Table 1. The PUs with cows have on average 17.3 ± 14.2 heads, of which 7.5 ± 6 are milking cows, with a production of 7.08 ± 2.71 L/d. The three production units with goats have on average 45 ± 5 heads, of which 25 ± 5 are milking goats, with an average production of 1.0 ± 0.5 L/d. Milking is done manually twice a day, in the morning and in the afternoon.
Table 1 Localities sampled, number of cows and goats by farm, milking and daily milk production
| Production unit |
Locality | Total, animals | Milking animals |
Production (L/animal*d) |
Species |
|---|---|---|---|---|---|
| 1 | Punteros* | 35 | 25 | 4.0 | Cow |
| 2 | El Potro* | 8 | 8 | 11.0 | Cow |
| 3 | El Potro* | 11 | 7 | 8.0 | Cow |
| 4 | El Potro* | 13 | 10 | 10.0 | Cow |
| 5 | El Alegre* | 40 | 20 | 1.5 | Goat |
| 6 | El Alegre* | 50 | 30 | 0.7 | Goat |
| 7 | El Alegre* | 45 | 25 | 0.5 | Goat |
| 8 | Salinas* | 8 | 7 | 5.0 | Cow |
| 9 | Salinas* | 6 | 4 | 8.0 | Cow |
| 10 | El Refugio** | 50 | 7 | 6.0 | Cow |
| 11 | El Refugio ** | 2 | 1 | 4.0 | Cow |
| 12 | El Refugio ** | 30 | 3 | 5.0 | Cow |
| 13 | El Refugio ** | 9 | 5 | 4.0 | Cow |
| 14 | Zumpango** | 20 | 8 | 10.0 | Cow |
| 15 | Zumpango** | 15 | 5 | 10.0 | Cow |
*Salinas de Hidalgo; ** Villa González Ortega, Zacatecas.
Milk composition
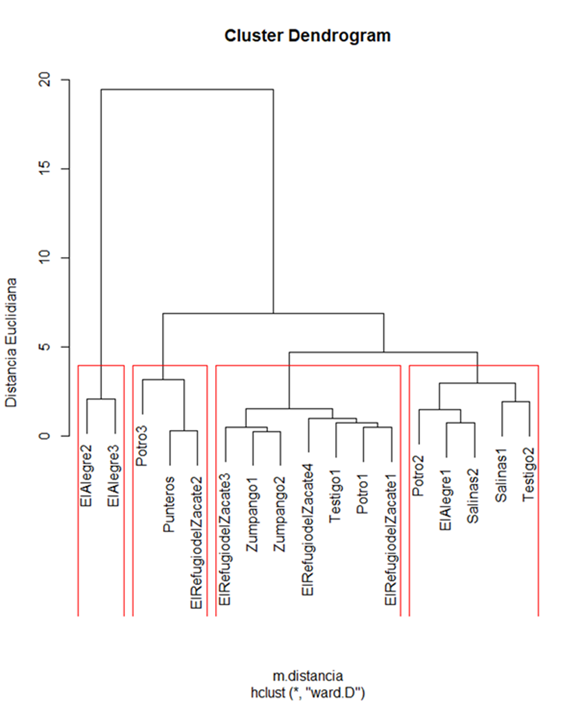
Four groups were identified as a result of the cluster analysis (Figure 1), which are detailed in Table 2, resulting in four groups, separating in group I where the PUs that have goats, mainly of the Saanen breed, are, this grouping is based on the similar content of nutrients in milk. In percentage of fat, cluster I showed a statistical difference (P<0.05) in contrast to the other groups (cow’s milk samples), which show some similarity between them. The percentage of protein, lactose, non-fat solids and salts showed no difference (P>0.05) between groups. In the variable total solids, there were significant differences between clusters (P<0.05), the highest value was obtained in cluster I (14.5 %) because it was goat’s milk, and the lowest value was shown by cluster IV (10.8 %). The variable density showed statistical differences, the groups of cow’s milk samples (cluster II, III and IV) obtained the highest value (1,024 kg/m3) and were statistically the same, in contrast, cluster I had an average value of 1,019 kg/m3. Finally, the cryoscopic point in cluster I decreased as a result of a higher content of lactose and salts in milk.
Table 2 Nutritional composition of milk samples
| Variable | Cluster I | Cluster II | Cluster III | Cluster IV |
P- value |
|---|---|---|---|---|---|
| Fat, % | 6.3 ± 0.71a | 4.3 ± 0.53b | 3.3 ± 0.25bc | 2.7 ± 0.65c | *** |
| Protein, % | 3.2 ± 0.07 | 3.1 ± 0.12 | 3.1 ± 0.21 | 2.9 ± 0.22 | N.S. |
| Lactose, % | 5.0 ± 0.07 | 4.7 ± 0.23 | 4.5 ± 0.19 | 4.5 ± 0.30 | N.S. |
| Total solids, % | 14.5 ± 0.57a | 13.0 ± 0.15b | 11.7 ± 0.26c | 10.8 ± 0.48d | *** |
| Non-fat solids, % | 9.0 ± 0.13 | 8.7 ± 0.4 | 8.5 ± 0.12 | 8.4 ± 0.51 | N.S. |
| Density, kg/m3 | 1018.8 ± 1.1b | 1024.8 ± 1.2a | 1024.3 ± 0.2a | 1023.5 ± 0.8a | *** |
| Salts, % | 0.7 ± 0.0 | 0.67 ± 0.06 | 0.69 ± 0.04 | 0.64 ± 0.05 | N.S. |
| Cryoscopic point, °C | -0.62 ± 0.01b | -0.55 ± 0.03a | -0.53 ± 0.02a | -0.53 ± 0.04a | ** |
abc Mean values with different literals are statistically different (P<0.05) by row.
N.S. = not significant; ** = P<0.001; *** = P<0.0001.
Microbiological analysis
Milk
The microbiological quality specifications of dairy products established by NOM 2437 are the same for cow’s, sheep’s and goat’s milk products. The results of the microbiological analysis of the milk samples can be seen in Table 3. It is evident that those producers who carry out a thermal process to avoid the decomposition of milk, which consists of boiling the milk, letting it cool at room temperature and then refrigerating it (pasteurization without control), show the lowest microbial loads, compared to those who do not perform a thermal process.
Table 3 Microbiological analysis (CFU/ml) of milk samples in different cheese dairies in Salinas, S.L.P. and Villa González, Zacatecas
| Production unit | S. aureus | TCs |
Salmonella spp. |
They pasteurize milk |
|---|---|---|---|---|
| 1 | 10x101±1x10 | 12x101±1x10 | Absent | No |
| 2 | Absent | Absent | Absent | Yes |
| 3 | 77x102±1x102 | 29x101±3x10 | Present | Yes |
| 4 | 43x102±5x101 | 13x103±2x102 | Absent | Yes |
| 5 | 36x104±5x103 | Absent | Present | Yes |
| 6 | 40x104±1x103 | 21x105±7x104 | Present | No |
| 7 | 36x101±1x10 | 24x105±4x104 | Present | No |
| 8 | 32x108±4x107 | Absent | Present | No |
| 9 | 43x107±5x106 | 12x108±2x107 | Present | No |
| 10 | 40x101±1x10 | 80x102±1x102 | Present | No |
| 11 | 67x102±5x101 | 37x106±1x106 | Present | No |
| 12 | 12x103±2x102 | 40x109±1x108 | Present | No |
| 13 | 72x107±1x107 | 24 x105±6x104 | Present | No |
| 14 | 42x109±4x108 | 57 x108±5x107 | Present | No |
| 15 | 13x103±2x102 | 25 x103±2x102 | Present | No |
| T1 | Absent | Absent | Absent | Yes |
| T2 | Absent | Absent | Absent | Yes |
TCs = Total coliforms; T1, T2: Commercial samples considered as controls.
Values express means and standard deviation CFU/g.
Regarding the analysis and identification of S. aureus, it was observed that most of the samples have values higher than those allowed; the highest data recorded was in PU 14 with an average value of 42 x 109 CFU/ml, PUs #1, #7 and #10 showed the lowest values, 10 x 101, 36 x 101 and 40 x 101 CFU/ml, respectively. This bacterium is absent in the two control samples and in PU #2. The highest load of total coliform (TCs) was observed in PU #12, with a value of 40 x 109 CFU/ml and the lowest load in PUs #1 and #3, with values of 12 x 101 and 29 x 101 CFU/ml, respectively. In contrast, this microorganism was absent in the control samples and PUs #2, #5 and #8.
The results of the analysis of Salmonella spp. show that this bacterium was present in most of the samples, mainly in those where there is no pasteurization of milk. On the other hand, there was an absence of this microorganism in PUs #1, #2 and #4 and the control samples.
Dairy products (cheese)
The results of the microbiological analysis of the cheese samples are shown in Table 4. Initially, it is important to mention that the presence of S. aureus was detected in most of the samples collected in the PUs visited. The PUs where the highest load of this microorganism was recorded were #13 and #15, where the microbial load corresponded to 32 x 109 and 63 x 109 CFU/g in cheese made with cow’s milk and artificial rennet. In contrast, the lowest microbial load, with values of 23 x 101 and 32 x101 CFU/g, was obtained in PUs #1 and #10. In addition, the control samples and PU # 5 had absence of this bacterium.
The analysis of TCs, PUs #6 and #15 had the highest values, 13 x 109 and 26 x 109 CFU/g, respectively, where the highlight of these results is that the first PU uses artificial rennet and the second natural rennet. In contrast, PU #10 had the lowest data on TC load, 74 x 101 CFU/g. In addition, in the samples of control cheese and PU # 5, they presented absence of TCs.
Table 4 Microbiological analysis of cheese samples and type of rennet used in the production units
| Production unit |
S. aureus | TCs | Salmonella spp. | Type of rennet |
|---|---|---|---|---|
| 1 | 23x101±6x10 | 32x103±1x102 | Present | Artificial |
| 2 | 83x105±2x104 | 70x103±9x102 | Present | Natural |
| 3 | 18x103±1x102 | 16x105±6x104 | Present | Natural |
| 4 | 21x102±1x10 | 24x108±1x108 | Present | Natural |
| 5 | Absent | Absent | Present | Artificial |
| 6 | 41x103±1x102 | 13x109±1x108 | Present | Artificial |
| 7 | 60x104±2x104 | 28x103±2x102 | Present | Natural |
| 8 | 87x103±6x102 | 38x103±3x103 | Present | Artificial |
| 9 | 31x104±1x103 | 17x104±1x103 | Present | Natural |
| 10 | 32x101±8x10 | 74x101±5x10 | Present | Natural |
| 11 | 10x102±1x101 | 32x105±2x104 | Present | Natural |
| 12 | 12x102±1x101 | 49x105±1x104 | Present | Natural |
| 13 | 32x109±2x108 | 48x106±2x105 | Present | Natural |
| 14 | 19x105±3x104 | 11x102±1x101 | Present | Natural |
| 15 | 63x109±1x108 | 26x109±3x108 | Present | Natural |
| T1 | Absent | Absent | Absent | Artificial |
| T2 | Absent | Absent | Absent | Artificial |
TCs = total coliforms; T1, T2= commercial samples considered as controls.
Values express means and standard deviation CFU/g.
In the determination of Salmonella spp., presence was observed in all samples of the PUs analyzed, as shown in Table 4. Finally, only commercial cheese samples showed an absence of Salmonella spp.
Discussion
The results of the physicochemical analysis in cluster I (goat’s milk samples), the percentage values are very similar to those reported for Saanen goats in Mexico18. In addition, Salinas-González et al19 point out that these values vary with time, being higher in September. Studies on the factors that affect the stability of the freezing point of milk indicate that the decrease in the cryoscopic point is related to a higher content of lactose and salts (calcium, phosphorus, magnesium), as well as to the passage of time (days) after birth20,21.
Regarding the physicochemical quality of milk from clusters II, III and IV (milk samples), the three groups are within the reference data of NOM 15522. Álvarez-Fuentes et al23 mention that small-scale dairy farms located in the south of Mexico City face the challenge of producing milk in quantity and quality; in this regard, the authors point out that the quality of milk is different according to the time of year (dry, rainy and winter periods), the results of milk quality (fat, protein, lactose and total solids) that they recorded in the dry season are very similar to those obtained in this work.
In this study, milk and cheese samples were taken from local cheese dairies to analyze pathogenic microbial loads. In addition, a request and comparison of records of the diseases caused by the consumption of dairy products of 2018 of the Basic Community Hospital of Salinas was made (personal communication, Dr. Sugey Bastidas Gastelum, director), and the response was that the hospital only follows up on cases of brucellosis (4 cases in that year) because it is considered as primordial by Epidemiological Surveillance, other diseases possibly caused by Escherichia coli, Staphylococcus aureus and Salmonella in dairy products and other foods are not analyzed because they do not have their own laboratory for sample processing.
For fluid milk, NOM 2439 indicates that the maximum allowable loads for total coliforms and S. aureus must be ≤10 CFU/ml by direct seeding, while Salmonella must be absent. The low counts of microorganisms in PUs 1, 5 and 10 may be due to the fact that these units were the only ones that have exclusive facilities for the handling of milk and cheese making process, and maintain hygiene measures (use of cap, masks, boots and cleaning of equipment, floor and utensils); despite these practices, the facilities do not have hermetic sealing doors that protect from the entry of dust. Table 3 shows that the regulations are not complied with in several PUs, this may be due to the fact that the milk is not pasteurized due to the lack of equipment, knowledge of pasteurization times and temperatures and the influence on the state of health of the animals, cleaning during milking, milking utensils and place of storage of milk. In a study conducted by Fuentes-Coto et al24, the microbial load of organic milk was analyzed, the presence of mesophilic and coliform bacteria was detected in milk and dairy products, where the amounts of CFU/ml were above the allowed limit.
On the other hand, Álvarez-Fuentes et al23 mention that the presence of mesophilic bacteria and the somatic cell count are related to the type of cleaning performed on the udders (traditional, partial and complete); however, regarding the microbial load, even though the work describes low counts of CFU/ml, the species of microorganisms are not specified.
Although the microbiological analysis in the milk reveals that the microbial loads are low in some farms, in some cases, the samples of fresh cheese had microbial loads higher than allowed by NOM 2439, it establishes a maximum of 100 CFU/g for TCs, a maximum allowed of 1,000 CFU/g for S. aureus, and Salmonella spp. must be absent in 25 g of sample.
One of the factors of the high microbial loads detected in this study is probably due to the fact that, in PU # 15, natural rennet fermented in non-sterile conditions is used as a coagulating agent. In the interviews that were carried out with the producers, they comment that the use of natural rennet is mainly due to the fact that it impregnates flavors and aromas desirable by consumers of fresh cheese. The results of Table 4 are similar to those reported in other cheese-producing areas in Mexico, some authors25 report counts of 9.27 log10 CFU/g for TCs and Salmonella present in fresh cheese. The aro cheese that is marketed in the municipality of Teotitlán de Flores Magón, Oaxaca, Mexico, had counts of 6.94 for TCs, 6.74 for E. coli, 5.76 log10 CFU/g for S. aureus and Salmonella was present, consequently, no sample analyzed complies with the regulations26. Also, artisanal botanero cheese from the northwest of the state of Mexico has serious deficiencies in its microbiological quality, since the counts exceeded the permitted limits of pathogens27. In the findings of this work, only the results of the control samples comply with the regulations. On the other hand, PU #5 partially complies with the counts of TCs and S. aureus. However, there is the presence of Salmonella colonies, which is associated with the use of unpasteurized milk25. In other geographical regions, such as Cajamarca, Peru, industrial fresh cheese from six companies was analyzed, under the guidelines described in the Sanitary Standard of microbiological criteria of sanitary quality and safety for foods and beverages for human consumption in that country. In the results, it is highlighted that all companies comply with what is established by the standard for Salmonella spp., since all the samples showed absence, with respect to other pathogens, only one company presents better microbiological conditions for the production of fresh cheese28. The same happens in Egypt and Middle Eastern countries with soft cheeses (Domiati), where they also have loads of aerobic bacteria S. aureus, TCs, E. coli and yeasts29. In the north of Iran, Kurdish cheese, named after the region where it is produced (Kurdistan, Iran), is a cheese prepared with cow’s or sheep’s raw milk, the loads of pathogenic microorganisms in the first days are very similar to the loads found in this study; the presence of Salmonella, E. coli of 5.27 log10 CFU/g and 8.22 log10 CFU/g for TCs was found. In addition, they point out that the maturation of cheese (60 d), the loads decrease significantly due to the presence of lactic acid bacteria30.
The presence of pathogenic microorganisms in dairy farms is due to several factors, for example, Salmonella is present in other domestic animal species such as pigs and poultry, which can become infected because they are within the production unit, in the environment due to the management of manure and its importance lies in the fact that it can survive for prolonged periods of time in the environment and cause salmonellosis in humans31,32. On the other hand, Staphylococcus aureus is a very common pathogen that causes mastitis in dairy cows, in this regard, if milk is not pasteurized, this bacterium can be found even in milk storage tanks33. In the case of coliforms, Van Kessel et al34 point out that these enteropathogenic microorganisms are very persistent in dairy farms; their origin is the intestine of animals, as well as their feces and water contaminated with manure, with a high risk of contamination of milk and storage tanks. The authors described agree in pointing out that the poor sanitary quality of dairy products throughout the milk and cheese production chain is a health problem, since these can be a vehicle for the transmission of food diseases, due to their high content of: E. coli, Listeria monocytogenes, S. aureus, Salmonella and possibly Brucella spp.26,35,36. Therefore, they recommend improving the quality at the farm level with hygienic milking measures, establishment of a cold chain, adequate transport and good sanitary measures of milk, such as: hygiene of the facilities where the cheeses are made, use of appropriate clothing, use of drinking water, washing of utensils, tables and hand disinfection25.
Conclusions and implications
According to the results of the analysis of milk and cheese samples, most production units do not meet quality standards in dairy products, because they do not have good hygiene practices throughout the entire production process, so it is necessary to implement actions that make producers aware of taking better health measures to reduce possible sources of contamination and that it does not represent a risk to the health of consumers, causing possible gastrointestinal diseases, mainly. It is necessary to conduct more studies on the process of making fresh cheese, such as taking samples of utensils, analysis of natural rennet and carrying out some process of sterilization of natural rennet by physical means. As well as to hold workshops of good practices in the production of dairy products for producers.
Acknowledgements
The authors thank the producers for providing us with the samples and the Research Support Fund C18-FAI-05-57.57 UASLP.
REFERENCES
1. SADER-SIAP. Boletín de leche, julio-septiembre 2019. Secretaría de Agricultura y Desarrollo Rural, Servicio de Información Agroalimentaria y Pesquera: Secretaría de Agricultura y Desarrollo Rural, Servicio de Información Agroalimentaria y Pesquera: https://www.inforural.com.mx/wp-content/uploads/2020/02/Bolet%C3%ADn-de-Leche-enero-septiembre-2019-env%C3%ADo_.pdf . Consultado 6 Nov, 2020. [ Links ]
2. Villegas de Gante A, Cervantes-Escoto F. La genuinidad y tipicidad en la revalorización de los quesos artesanales mexicanos. Estudios Sociales 2011;19(38):146-164. https://www.redalyc.org/articulo.oa?id=41719205006 . Consultado 10 Abr, 2021. [ Links ]
3. Cervantes-Escoto F, Islas-Moreno A, Camacho-Vera JH. Innovando la quesería tradicional mexicana sin perder artesanalidad y genuinidad. Estudios Sociales . Revista de Alimentación Contemporánea y Desarrollo Regional 2019;29(54):18. http://www.scielo.org.mx/pdf/esracdr/v29n54/2395-9169-esracdr-29-54-e19794.pdf . Consultado 10 Abr, 2021. [ Links ]
4. Madigan TM, Martinko JM, Dunlap PV, Clark DP. Brock Biología de los microorganismos. Madrid España: Pearson Educación; 2009. [ Links ]
5. Smigic N, Djekic I, Tomasevic I, Miocinovic E, Gvozdenovic R. Implication of food safety measures on microbiological quality of raw and pasteurized milk. Food Control 2011;25:728-731. [ Links ]
6. Caballero TA, Carrera VA, Legomin FE. Evaluación de la vigilancia microbiológica de alimentos que se venden en las calles. Rev Cubana Aliment Nutr 1998;12:7-10. [ Links ]
7. Instituto Mexicano del Seguro Social. Enfermedades gastrointestinales. http://www.imss.gob.mx/salud-en-linea/enfermedades-gastrointestinales . Consultado 15 May, 2020. [ Links ]
8. Secretaría de Salud. Enfermedades transmitidas por alimentos. Dirección General Adjunta de Epidemiología. https://www.gob.mx/cms/uploads/attachment/file/334668/7._Vigilanca_Epidemiol_gica-Ma._Eugenia-DGE-SSA.pdf . Consultado 20 Nov, 2020. [ Links ]
9. Norma Oficial Mexicana NOM 243-SSA1-2010. Productos y servicios. Leche, fórmula láctea, producto lácteo combinado y derivados lácteos. Disposiciones y especificaciones sanitarias. Métodos de prueba. México: Diario Oficial de la Federación, 27 de septiembre del 2010. [ Links ]
10. Instituto Nacional de Estadística y Geografía. Prontuario de información geográfica municipal de los Estados Unidos Mexicanos, Salinas, San Luis Potosí. http://www.beta.inegi.org.mx/app/areasgeograficas/?ag=24# . Consultado 20 Sep, 2019. [ Links ]
11. Norma Oficial Mexicana NOM-109-SSA1-1994. Bienes y servicios. Procedimientos para la toma, manejo y transporte de muestras de alimentos para su análisis microbiológico. México: Diario Oficial de la Federación, 4 de noviembre de 1994. [ Links ]
12. Norma Oficial Mexicana NOM-110-SSA1-1994. Bienes y servicios. Para la preparación y dilución de muestras de alimentos para su análisis microbiológico. México: Diario Oficial de la Federación, 28 de abril de 1994. [ Links ]
13. Slack MP, Wheldon DB. A simple and safe volumetric alternative to the method of Miles, Misra and Irwin for counting viable bacteria. J Med Microbiol 1978;11(4): 541-545. [ Links ]
14. Norma Oficial Mexicana NOM-114-SSA1-1994. Bienes y servicios. Método para la determinación de Salmonella en alimentos. México: Diario Oficial de la Federación, 28 de abril de 1994. [ Links ]
15. Norma Oficial Mexicana NOM-115-SSA1-1994. Bienes y servicios. Método para la determinación de Staphylococcus aureus en alimentos. México: Diario Oficial de la Federación, 25 de septiembre de 1995. [ Links ]
16. Norma Oficial Mexicana NOM-113-SSA1-1994. Bienes y servicios. Método para la cuenta de microorganismos coliformes totales en placa. México: Diario Oficial de la Federación, 10 de mayo de 1995. [ Links ]
17. R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. 2020. URL https://www.R-project.org/. [ Links ]
18. Torres-Vázquez JA, Valencia-Posadas M, Castillo-Juárez H, Montaldo HH. Genetic and phenotypic parameters of milk yield, milk composition and age at first kidding in Saanen goats from Mexico. Livest Sci 2009;126:147-153. [ Links ]
19. Salinas-González H, Maldonado JA, Torres-Hernández G, Triana-Gutiérrez M, Isidro-Requejo LM, Meda-Alducin P. Compositional quality of local goat milk in the Comarca Lagunera of Mexico. Rev Chapingo Ser Zonas Áridas 2015;14(2):175-184. [ Links ]
20. Henno M, Ots M, Jõudu I, Kaart T, Kärt O. Factors affecting the freezing point stability of milk from individual cows. Int Dairy J 2008;18:210-215. [ Links ]
21. Costa A, Visentin G, de Marchi M, Cassandro M, Penasa M. Genetic relationships of lactose and freezing point with minerals and coagulation traits predicted from milk mid-infrared spectra in Holstein cows. J Diary Sci 2019;102:7217-7225. [ Links ]
22. Norma Oficial Mexicana NOM-155-SCFI-2012. Leche-Denominaciones, especificaciones fisicoquímicas, información comercial y métodos de prueba: Diario Oficial de la Federación, 15 de marzo de 2012. [ Links ]
23. Álvarez-Fuentes G, Herrera-Haro JG, Alonso-Bastida G, Barreras-Serrano A. Calidad de la leche cruda en unidades de producción familiar del sur de Ciudad de México. Arch Med Vet 2012;44:237-242. [ Links ]
24. Fuentes-Coto G, Ruiz-Romero RA, Sánchez-Gómez JI, Ávila-Ramírez DN, Escutia-Sánchez J. Análisis microbiológico de leche de origen orgánico: atributos deseables para su transformación. Agric Soc Desarro 2013;10:419-432. [ Links ]
25. Sánchez-Valdés JJ, Colín-Navarro V, López-González F, Avilés-Nova F, Castelán-Ortega OA, Estrada-Flores JG. Diagnóstico de la calidad sanitaria en las queserías artesanales del municipio de Zacazonapan, Estado de México. Salud Publica Mex 2016;58:461-467. [ Links ]
26. González-Montiel L, Franco-Fernández MJ. Perfil microbiológico del queso de aro consumido en la Cañada Oaxaqueña. Brazilian J Food Technol 2015;18(3):250-257. [ Links ]
27. Vázquez-Fontes C, Sánchez-Vera E, Castelán-Ortega O. Espinoza-Ortega A. Microbiological quality of artisan-made Mexican botanero cheese in the central highlands. J Food Saf 2010;30:40-50. [ Links ]
28. Vázquez V, Salhuana JG, Jiménez L A, Abanto LM. Evaluation of the bacteriological quality of fresh cheeses from Cajamarca. Ecología Aplicada 2018;17(1):45-51. [ Links ]
29. El-Kholy AM, El-Shinawy SH, Meshref AMS, Korany AM. Microbiological quality of Domiati cheese and the influence of probiotics on the behavior of Staphylococcus aureus and Escherichia coli o157:h7 in domiati cheese. J Food Saf 2014;34(4):396-406. [ Links ]
30. Milani E, Shahidi F, Mortazavi SA, Vakili SAR, Ghoddusi H. Changes throughout ripening of Kurdish cheese. J Food Saf 2014;34:168-175. [ Links ]
31. Organización Mundial de la Salud. Salmonella (no tifoidea). https://www.who.int/es/news-room/fact-sheets/detail/salmonella-(non-typhoidal) . Consultado 12 Abr, 2021. [ Links ]
32. Rodriguez-Rivera LD, Wright EM, Siler JD, Elton M, Cummings KJ, Warnick LD, Wiedmann M. Subtype analysis of Salmonella isolated from subclinically infected dairy cattle and dairy farm environments reveals the presence of both human- and bovine-associated subtypes. Vet Microbiol 2014;170:307-316. [ Links ]
33. Zhao X, Yuan X, Hu M, Zhang Y, Li L, Zhang Q, Yuan X, Wang W, Liu Y. Prevalence and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus isolated from bulk tank milk in Shandong dairy farms. Food Control 2021;125:107836. [ Links ]
34. Van Kessel JS, Karns JS, Gorski L, McCluskey BJ, Perdue ML. Prevalence of Salmonellae, Listeria monocytogenes, and fecal coliforms in bulk tank milk on US dairies. J Dairy Sci 2004;87(9):2822-2830. [ Links ]
35. Verraes C, Vlaemynck G, Van Weyenberg S, De Zutter L, Daube G, Sindic M, Uyttendaele M, Herman L. A review of the microbiological hazards of dairy products made from raw milk. Int Dairy J 2015;50:32-44. [ Links ]
36. Bobbo T, Ruegg PL, Stocco G, Fiore E, Gianesella M, Morgante M, Pasotto D, Bittante G, Cecchinato A. Associations between pathogen-specific cases of subclinical mastitis and milk yield, quality, protein composition, and cheese-making traits in dairy cows. J Dairy Sci 2015;100:4868-4883. [ Links ]
Received: July 10, 2020; Accepted: September 24, 2021











 text in
text in