Introduction
Bovine respiratory disease (BRD) is one of the major health problems in calves and adult cattle, and has great economic impact on the cattle industry1-3. BRD in herds causes economic losses due to increased treatment costs, decreased production and culling4. BRD has a complex etiology, which involves bacterial and viral agents. Additionally, some predisposing factors such as management failures, environmental and host defense problems are influential on infection occurrence5.
The most frequent bacterial agents isolated from respiratory disease are Pasteurella multocida (P. multocida), Mannheimia haemolytica (M. haemolytica), Histophilus somni (H. somni) and Mycoplasma bovis (M. bovis)6,7. Pasteurella multocida is one of the primary bacterial pathogens and leads to clinical symptoms during BRD in neonatal calves and cattle. The bacterium, which is detected not only in infected but also healthy cattle, is isolated from lung, nasopharyngeal and nasal swabs and trans-tracheal washes. Therefore, diagnose of P. multocida becomes an issue, if clinical symptoms associated with pneumonia are detected in cattle8.
Similarly, M. haemolytica is normally presented in nasal pharyngeal mucosa in healthy cattle. However, the bacterium becomes a pathogen under inadequate conditions such as nutritional deficiency and overcrowded housing, and viral infections. Following to rapid proliferation of M. haemolytica within the infected lung, severe fibrinopurulent bronchopneumonia is presented. Additionally, it produces a potent leukotoxin that destroys the macrophages and neutrophils9,10. Because of these properties, this bacterium is accepted as the most harmful pathogen for lung.
H. somni is a bacterium which is normally presented not only in respiratory but also in reproductive tract. Similar with mentioned pathogens above, H. somni is also causing infections such as thrombotic meningoencephalitis (TME), pneumonia, septicemia, mastitis, arthritis, myocarditis and reproductive infections under inappropriate conditions with clinical symptoms5,11-13.
M. bovis is not only respiratory disease but also arthritis, mastitis, genital infections and abortus3. Moderate infections in cattle has the potential to cause an infection with severe clinical manifestations, as well as difficulty diagnosis; penicillin and its derivates are also an important problem in cattle breeding enterprises with the resistance mechanism of antibiotics14. At the same time, the rapid spread of bacteria in the cattle herd was a result of M. bovis makes it important15.
BRD is known as polymicrobial infection in cattle herds and mainly recorded in younger cows13. Thus, diagnosis of BRD is required to use different methods (conventional and molecular) to determine the bacteria that are effective in etiology. In particular, the use of different media, incubation conditions (temperature and O2 ratio), and differences between methods in conventional diagnostic methods require the use of faster methods. Molecular diagnosis of the pathogens based on Polymerase Chain Reaction (PCR) techniques can be used for identification and detailed evaluations. Molecular techniques, which are more sensitive than bacteriological methods, are preferred especially for direct identification of pathogens from tissue samples 8,9,15,16.
The aim of this study was to determine of P. multocida, M. haemolytica, H. somni and M. bovis of macroscopically healthy cattle lungs by PCR.
Material and methods
Sampling procedure
A total of 83 lung samples were collected from the different slaughterhouses. A piece of lung sample was taken from lungs without any lesions and placed in sterile tubes transported to the laboratory in cool chain.
Culture and DNA extraction
A piece of sample was taken and placed in eppendorf tube. Briefly, each sample was put into sterile petri dishes and then samples were break into parts using sterile bistouries and pens. Broth culture was only used for M. bovis. A piece of lung sample for M. bovis isolation inoculated in PPLO broth medium and incubated at 37 oC in %5 CO2 for 5 d. PPLO broth cultures were used for M. bovis DNA extraction, lyzed lung samples were used for other bacteria DNA extraction. DNA was extracted from the lung samples by using genomic DNA purification kit (Qiagen-DNeasy Blood and Tissue Kit-USA). Manufacturer instructions were followed.
Polymerase Chain Reaction
PCR procedures, which involved cycle conditions and reaction mixture, were performed according to previous reports17,18,19 (Table 1). The reaction mixture was prepared with a total volume of 50 μl contained 3 mM MgCl2, 200 μl dNTP, 0.5 μM each of primer and 1.25 units Taq DNA polymerase (Vivantis, MY) with minor revisions for pathogens. Extracted DNA (1 μL) was used as template. Amplification was carried by thermalcycler (The SuperCycler Trinity, Kyratech, AU). All samples of PCR amplification products (10 μL) were subjected to electrophoresis. DNA was visualized by UV fluorescence after staining with ethidium bromide.
Table 1 PCR conditions and oligonucleotids sequences
| Pathogen | Cycle condition (°C/min) | Oligonucleotids | Base pair (bp) | Reference |
|---|---|---|---|---|
|
P. multocida |
94/1 69/1 30 cyc 72/1 |
F:GGCTGGGAAGCCAAATCAAAG R:CGAGGGACTACAATTACTGTAA | 1432 | Miflin and Blackall, 2001 |
|
M. haemolytica |
94/1 55/1 30 cyc 72/1 |
F:TGTGGATGCGTTTGAAGAAGG R:ACTTGCTTTGAGGTGATCCG | 1145 | Akan et al, 2006 |
| H. somni | 94/1 55/1 35 cyc 72/1 |
F:GAAGGCGATTAGTTTAAGAG R:TTCGGGCACCAAGTRTTCA | 400 | Angen et al, 1998 |
| M. bovis | 94/1 54/1 30 cyc 72/1 |
F: TATTGGATCAACTGCTGGAT R: AGATGCTCCACTTATCTTAG | 447 | Foddai et al, 2005 |
Results
In molecular evaluation, positive results were achieved for P. multocida, M. haemolytica, H. somni and M. bovis in 4 (4.8 %), 4 (4.8 %), 6 (7.3 %) and 3 (3.6 %) of the samples, respectively (Figure 1-3). Mix infections were detected in five samples. Of the samples, two were positive for both P. multocida and M. haemolytica, two were positive for both M. haemolytica and H. somni and one was positive for both P. multocida and H. somni. However, a positive sample, which carried all of the pathogens, was not detected.
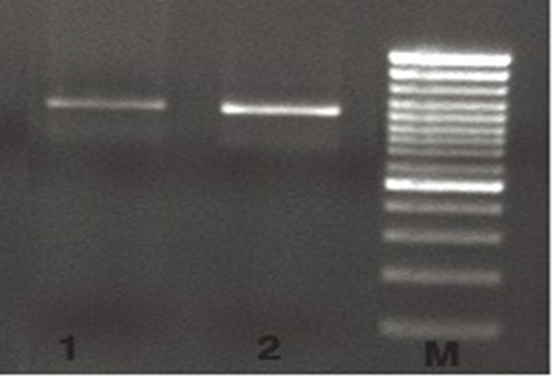
M= Marker (100 bp DNA Ladder Plus), 1-2= M. haemolytica
Figure 2 Molecular evaluation of M. haemolytica
Discussion
Bovine respiratory diseases, which cause economic losses due to production decrease and culling, have major importance in cattle herds. Although, cattle of all ages and sex are susceptible to the disease, it is more harmful for calves6,20,21. Bacteria that cause respiratory infections can be transmitted to sensitive animals from healed or immunologically strong animals (no clinical signs)2,22. In addition to pathogens, some predisposing factors such as overcrowded and bad-ventilated barns, inadequate feeding and other infectious diseases increased the infection risk. In these herds, transmission usually occurs horizontally7,9,23. Previous studies were usually focused on the detection of P. multocida, M. haemolytica, H. somni and M. bovis in the tonsils, nasopharynx and upper respiratory tract in carrier animals2,24,25. Various results about the presence of pathogens in cows were achieved in the reports. Positivity of P. multocida, M. haemolytica, H. somni and M. bovis in cattle varied between 0.4-57.4 %, 1.6-35 %, 2-45 % and 14-59 % in previous reports (Figure 4)9,19,26-30.
In the present study, the number of positive animals identified for P. multocida was found lower than Onat et al28 but was found higher than others24,27. Positivity of M. haemolytica was found lower than some other works2,27,29, but was found higher than Hajikolaei et al20. In terms of positivity H. somni and M. bovis was found different from other studies. In studies, in both bacteria was evaluated different diagnostic method in pneumonic cows15,30,31. Additionally, the variation among the results may be associated with difference in diagnostic methods, vaccination, and bacterial properties2,23,29. For instance, conventional culture methods can be inadequate in detection of the pathogens in healthy cows due to lower bacterial count in the samples. Likewise, vaccination can reduce bacteria carriage and lesions16,32,33. In addition, detection of some respiratory system pathogens as H. somni and M. bovis in culture media is difficult although the samples are collected from infected cows9,19. Thus, PCR tests, which involve specific primers for 16S rRNA, are suggested for identification of this bacterium during mix respiratory infections5,13. Therefore, genetic material basis molecular technics, which allow the detection of the pathogens even though lower bacterial count, would be preferable rather than culture methods in determining of carrier cows 16,34
The presence of these bacteria in the absence of clinical symptoms in animals or macroscopic lesions in the necropsy supports the opportunistic character of these bacteria. However, in these cases, histopathological examinations should be done and the animal's health status should be questioned. Additionally, because the occurrence of the disease has association with carriage22,24,27,35, detection of reservoir animals is important for reduction of the risk in herds. So that, detection of carrier cows, which have potential risk for contamination, is an approach for control22.
Conclusions and implications
In conclusion, P. multocida, M. haemolytica, H. somni and M. bovis, which cause economic losses and death in animals, are also important opportunistic pathogens. Therefore, the immune system should be developed by vaccination in animals. Moreover, housing conditions and management, awareness of the staff (owner) should be improved to establish an effective and sustainable control program for respiratory system diseases.











 texto em
texto em 





