Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.12 no.2 Mérida abr./jun. 2021 Epub 15-Nov-2021
https://doi.org/10.22319/rmcp.v12i2.5635
Technical note
Optimization of a DNA extraction protocol for hemolyzed and coagulated bovine blood for use in molecular detection of Anaplasma spp.
a Universidad San Francisco de Quito. Colegio de Ciencias Biológicas y Ambientales, Campus Cumbayá, Diego de Robles s/n, 170901. Quito, Ecuador.
b Pontificia Universidad Católica del Ecuador. Facultad de Ciencias Exactas y Naturales. Quito, Ecuador.
c Universidad San Francisco de Quito. Colegio de Ciencias de la Salud, Escuela de Medicina Veterinaria. Quito, Ecuador.
Anaplasma spp. bacteria cause anaplasmosis, a disease which negatively affects livestock production worldwide. Molecular detection by PCR requires efficient extraction of DNA from whole blood, which in turn depends on blood sample quality. Failures in sampling procedures and/or sample storage can lead to hemolysis and blood clotting, which can hamper diagnosis. An established DNA extraction protocol using Chelex® 100 resin was modified to optimize detection of Anaplasma spp. in hemolyzed and coagulated bovine blood samples, as well as reduce its cost. The optimized protocol extracted highly pure DNA effective in PCR analysis. Efficiency of the optimized protocol was compared with two commercial DNA extraction kits. When used in PCR detection of Anaplasma spp., the concordance values for all three were high (Cohen’s Kappa = 0.72). The optimized protocol is effective at extracting DNA from complex blood samples and is much less costly than commercial methods, a clear advantage when operating under limited budgets.
Key words Anaplasma; Anaplasmosis; Molecular detection; DNA extraction; Chelex® 100; Blood
La anaplasmosis es una enfermedad que puede afectar de manera significativa a la producción en el sector pecuario. Para la detección molecular por PCR de Anaplasma spp., se requiere de una extracción eficiente de ADN a partir de sangre completa, lo que a su vez depende de la calidad de la muestra de sangre. Fallas en los procedimientos de muestreo o conservación de las muestras pueden ocasionar hemolisis y coagulación de la sangre, lo que a su vez puede obstaculizar el diagnóstico. El objetivo de esta investigación fue optimizar un protocolo casero de bajo costo que utiliza resina de Chelex® 100 y permite detectar de manera eficiente a Anaplasma spp. en 30 muestras de sangre bovina hemolizada y coagulada. La eficiencia del protocolo optimizado se comparó con dos kits comerciales de extracción de ADN mostrando una alta concordancia (Kappa de Cohen = 0.72) en la detección molecular de Anaplasma spp. por PCR. Se recomienda esta metodología para superar las limitaciones de investigación que pueden surgir al extraer ADN a partir de muestras de sangre complejas.
Palabras clave Anaplasma; Anaplasmosis; Detección molecular; Extracción de ADN; Chelex® 100; Sangre
Anaplasmosis is a disease recognized for its worldwide impact on public health and livestock production1,2. Caused by bacteria of the genus Anaplasma, it results in a debilitating disease in cattle that can be fatal in some cases. The presence of anaplasmosis in a livestock production system can lead to economic losses from decreased milk production, delayed growth and low weight gain3,4. Due to a lack of epidemiological data in Latin American countries, it is difficult to quantify the true economic impact of bovine anaplasmosis in the region5. Managing anaplasmosis can be complex because of the existence of asymptomatic carriers. These act as reservoirs, contributing to the disease’s spread and raising infection rates in susceptible populations5.
Compared to conventional methods such as culture, serology and light microscopy, the polymerase chain reaction (PCR) method provides greater specificity and sensitivity in detecting Anaplasma spp.6,7. Furthermore, the probability of cross-detection of other hemoparasites is minimal when using PCR6. Extraction of DNA from clinical samples is a vital step prior to PCR analysis. This is why DNA extraction efficiency is essential to producing reliable and repeatable results. Detection of Anaplasma spp. by PCR ideally requires a whole blood sample since this genus can parasitize different blood cells7. However, the presence of various elements of the blood and/or failures in sampling, and/or sample transport or conservation can generate changes that make it difficult to extract genetic material or that inhibit the PCR reaction8,9. For example, alterations can occur in sample homogeneity, and hemolysis and clots can be observed, all of which complicate cell lysis and genetic material release during extraction. In addition, hemolyzed blood contains a higher concentration of hemoglobin and its derivatives, which can compromise PCR diagnostic efficacy. This is why hemolyzed and clotted blood is generally not used in molecular screening tests.
Several DNA extraction kits are currently available on the market. They are designed to extract low concentrations of high quality DNA10 from complex or poor quality samples such as clotted and hemolyzed blood11. However, high cost limits generally their use in research subject to limited budgets, a common situation in developing countries. One exception is the Chelex® 100 resin DNA extraction method, which is simple and inexpensive, and is therefore used with a wide variety of tissues12,13. Initially, the cells are lysed to release the DNA, be it with heat treatment or, if necessary, tissue maceration. The Chelex® 100 resin acts as a chelator by capturing magnesium ions, consequently preventing DNA degradation due to nuclease action12. The Chelex® 100 resin protocol of Singh et al14 is particularly efficient at extracting high quantities of highly pure DNA. Designed for analysis of dried blood on filter paper, use of this protocol has not yet been described for other types of samples. The present study objective was to optimize this DNA extraction protocol for hemolyzed and coagulated bovine blood samples, to make it applicable for molecular detection of pathogens in animals, specifically, Anaplasma spp. in cattle.
Analyses were run using 40 blood samples collected in September 2018 as part of the “Farm Plans” (Planes de Finca) project15. The project is promoted by the Autonomous Decentralized Government of the Province of Esmeraldas (Gobierno Autónomo Descentralizado de la Provincia de Esmeraldas - GADPE) and the Inter-American Institute for Cooperation in Agriculture (Instituto Interamericano de Cooperación para la Agricultura - IICA), with the participation of San Francisco University Quito (Universidad San Francisco de Quito). The samples were collected from crossbreed Bos taurus cattle with at least 50 ticks per animal. Blood samples (5 ml each) were taken from the coccygeal or jugular vein in tubes containing anticoagulant. The samples were refrigerated at 4 to 5 °C during transport to the laboratory of the Teaching Veterinary Hospital of San Francisco University Quito, where they were frozen until processing.
Upon thawing, the samples were confirmed to be hemolyzed and to contain small- to medium-sized clots. Extraction of DNA was done in a subgroup of 10 samples with these characteristics and previously confirmed to be positive for Anaplasma sp. The analysis was done following the protocol designed by Singh et al (see Table 1 for summary)14. Because this protocol was designed for dried blood samples on filter paper, an assay was performed using different volumes of blood (5 µl, 50 µl, and 100 µl). Steps were added to the protocol, specifically in the protein precipitation phase, to improve DNA purity using the sample volume defined in the protocol. Five steps were added: a) Add 75% alcohol at a 1:1 ratio with the volume of supernatant recovered in step 10; b) Let it rest overnight (approx. 16 h) at -20 °C; c) Centrifuge mixture at 13,000 rpm for 1.5 min; d) Recover supernatant; and e) Implement step 11 as shown in Table 1.
Table 1 DNA extraction protocol of Singh et al14
|
DNA Extraction 1. Heat to 100 °C, 300 μl 7% Chelex® 100 resin stock solution for 10 min. 2. Add 5 μl blood and heat mixture for 8 min at 100 °C. 3. Mix with a vortex for 15 sec and reheat for 7 min at 100 °C. 4. Centrifuge for 1.5 min at 12,000 rpm. 5. Recover supernatant and discard pellet. Protein precipitation 6. Add 7.5 M ammonium acetate stock solution to supernatant such that the final solution has a 2.5 M concentration. 7. Rest mixture for 5 min on ice. 8. Mix with a vortex for 10 sec. 9. Centrifuge at 13,000 rpm for 10 min at -4 °C. 10. Recover supernatant in new tube. DNA precipitation 11. Add 3 M ammonium acetate stock solution to supernatant such that the final solution has a 0.3 M concentration. 12. Add 200 μL absolute alcohol. 13. Mix with a vortex for 5 sec. 14. Rest for 4 h at -20 °C. 15. Centrifuge at 13,000 rpm for 10 min at -4 °C. Discard supernatant. 16. Wash pellet two times: first with 200 μL cold 75% alcohol; second with 200 μL cold 100% alcohol. Follow each with a centrifugation at 13,000 rpm for 10 min at -4 °C and discard the supernatant. 17. Allow pellet to air dry for 10 min. |
The quantity and purity of extracted DNA was compared between the reference protocol14 and that with modifications in the protein precipitation phase. The concentration and quality of sample DNA were measured by spectrophotometry in a NanoVue Plus Spectrophotometer (GE Healthcare, USA). The molecular analysis was done under the Framework Contract for Access to Genetic Resources No. MAE-DNB-CM-2018-0106.
To demonstrate the efficiency of the modifications made to the established protocol14, DNA was extracted from 30 hemolyzed and coagulated blood samples for which Anaplasma spp. positivity was unknown. Extraction of DNA was done using the modified protocol as described above, hereafter referred to as the modified Chelex protocol (MCP). From the same samples, DNA was extracted using the commercial kits DNeasy Blood & Tissue Kits Print Qiagen® and AccuPrep® Genomic DNA Extraction Kit Bioneer®, following the manufacturers’ recommendations for DNA extraction from whole blood. Confirmation of MCP efficacy in molecular detection of Anaplasma spp. was done by applying a PCR reaction specific to the Anaplasma bacterial genus16. The amplicons obtained from 13 positive samples (n = 40) were sequenced at Functional Biosciences, Inc. (Wisconsin, USA) to confirm the presence of Anaplasma spp. DNA. The constitutive gene that codes for the protein β-Actin, always present in bovine blood samples17, was used to rule out the possibility of false negatives due to inhibition of the PCR reaction. The details of the primers and the product size of both PCR reactions are shown in Table 2.
Table 2 Primer sequences
| Gene | Primer | Sequence: 5' - 3' | Size | Ref. |
|---|---|---|---|---|
| 16S rDNA | AnaplsppF | AGAAGAAGTCCCGGCAAACT | 800 bp | (18) |
| AnaplR3 | GAGACGACTTTTACGGATTAGCTC | |||
| β-actin | XAHR 17 | CGGAACCGCTCATTGCC | 289 bp | (19) |
| XAHR 20 | ACCCACACTGTGCCCATCTA |
The results were analyzed with a Shapiro-Wilks normality test and non-parametric tests. Differences in the amount of DNA extracted between blood volumes (5 µl, 50 µl and 100 µl) were identified with the Friedman test and a Wilcoxon post-hoc analysis; the P values were adjusted with the Bonferroni correction19. Comparison of DNA purity from the protein precipitation assays was done with the Wilcoxon non-parametric test. The Cohen’s Kappa test and McNemar test were applied to identify concordance between the PCR results from the three extraction methods20,21. All statistical tests were run at a 95% significance level and using the R v.3.3.0 software18.
The reference protocol14 was used in 10 hemolyzed and coagulated blood samples. To define optimum sample volume, three different volumes (5 µl, 50 µl and 100 µl) of liquid blood from each sample were tested. The DNA concentrations differed between the 5 µl volume and 50 µL and 100 µL volumes (Friedman test: χ2(2) = 16.800, P= 0.017) (Figure 1), but not between the 50 µL and 100 µL volumes (Wilcoxon: Z= -2,293, P= 0.063). With the intent of using the lowest possible volume, the 50 µl volume was used in the following steps.
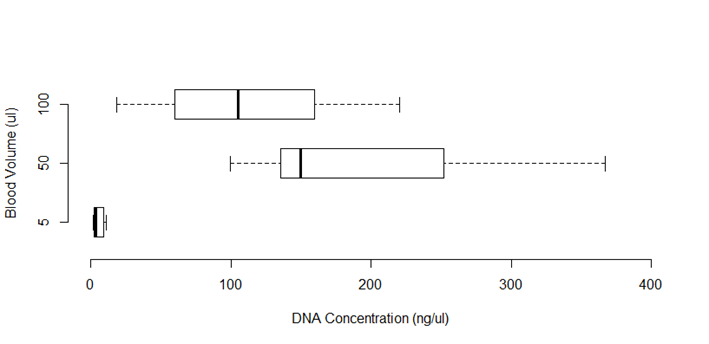
Figure 1 DNA concentration (ng/µl) obtained from different volumes of blood (n=10), using extraction method of Singh et al14 (χ2(2) = 16,800; P=0.017*)
When using the reference protocol14, the purity of the DNA extracted from the coagulated and hemolyzed blood samples yielded a median A260/280 ratio of 0.820 (n = 10). In the MCP, modification of the protein precipitation phase increased the median A260/280 ratio to 1.970 (n= 10). Compared to the reference protocol14, the MCP allows significant increases in the 260/280 (Wilcoxon: Z= -2.803, P= 0.005) and 260/230 (Wilcoxon: Z= -2.666, P= 0.002) ratios (Figure 2). Worth mentioning is that the DNA concentration in these samples decreased significantly (Wilcoxon: Z= -2.803, P= 0.005) compared to that obtained with the reference protocol14. However, this did not interfere with molecular detection of Anaplasma spp. in any of the samples. An added advantage is that the nonspecific bands observed when amplifying samples extracted with the reference protocol14 did not appear when using the MCP.
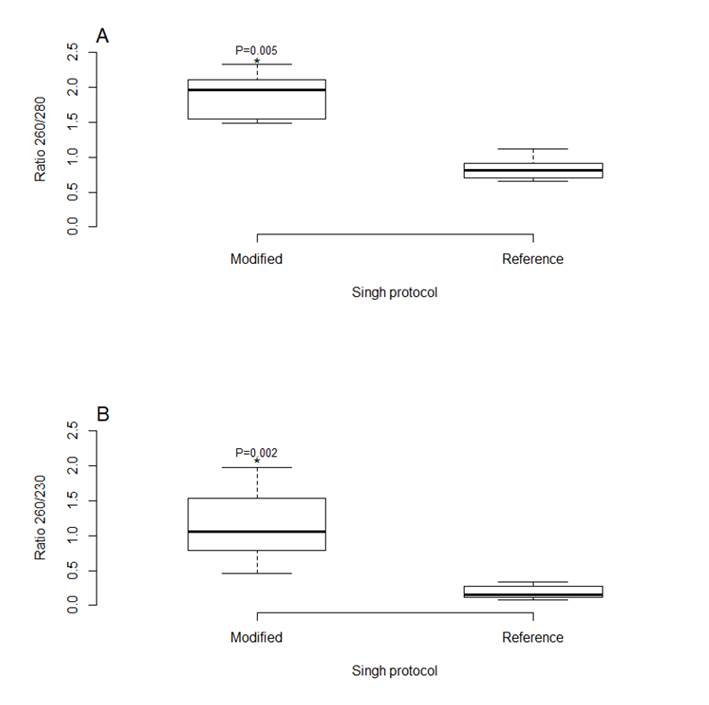
Figure 2: Comparison between the Singh et al14 extraction protocol and the modified Chelex protocol (MCP) in terms of A) DNA purity and B) DNA quantity (n=10)
The DNA extracted from 30 samples of coagulated and hemolyzed blood with two commercial kits and the MCP exhibited no inhibition of the PCR reaction. The same was true in terms of analysis of the presence of Anaplasma spp. (Table 3), with high Cohen’s Kappa concordance index values (0.72) between the MCP and the commercial kits. The McNemar exact test identified no significant differences between the kits and the MCP in the proportion of positive samples detected by PCR (McNemar’s Chi-squared 0.500; P= 0.5).
Table 3 Anaplasma spp. positivity results in blood samples processed with different methods (n=30)
| Qiagen Kit | Bioneer Kit | ||||
|---|---|---|---|---|---|
| MCP | Positives | Negatives | Positives | Negatives | |
| Positives | 25 | 0 | 25 | 0 | |
| Negatives | 2 | 3 | 2 | 3 | |
MCP= modified Chelex protocol.
Bovine anaplasmosis’ negative impacts in the livestock sector1,2 can include anemia, weakness, reduced growth and milk production, abortions and even mortality in infected cattle22. The true prevalence of anaplasmosis in cattle in Latin America and the Caribbean is unknown. This is partially due to the fact that 80 % of livestock producers in these regions are small family farmers living in rural and marginal areas23, and their meagre financial resources limit efforts towards vaccination and infectious disease control24. Limited resources in developing countries is also a problem when doing research or implementing disease prevention campaigns since laboratory supplies can cost two to ten times more than in developed countries25. This highlights the need to develop techniques that accurately detect Anaplasma spp. and are affordable for both the agencies in charge of controlling anaplasmosis and for small and large farmers.
Molecular detection of pathogens by PCR from clinical samples has become a widely used methodology for infectious disease diagnosis and monitoring26. This technique also allows subsequent characterization of the pathogen by sequencing its genetic material, which produces data useful in understanding disease epidemiology. A vital step prior to molecular detection is extraction of genetic material from samples. This procedure can be affected by sample collection, transport and storage practices. Each of these steps must ensure that pathogen genetic material remains intact until extraction procedure implementation. However, pre-extraction conditions are not always ideal. For example, sample collection from animals in difficult-to-access rural areas makes optimal sample collection and conservation a challenge. Under these conditions samples commonly exhibit hemolysis and clots, which make subsequent analysis difficult because they can inhibit the pathogen molecular detection reaction.
The present results confirmed that the modifications made to the DNA extraction protocol of Singh et al14 allow DNA extraction from hemolyzed and coagulated blood which can then be used in molecular detection of Anaplasma spp. When the MCP was used to extract DNA from 50 µL of sample, the purity values in both the 260/280 and 260/230 ratios were higher than when using the reference protocol14. The MCP did produce less DNA than the tested commercial kits, but with no differences in terms of Anaplasma spp. positivity. The Kappa concordance index value (0.72) indicates high concordance among the results from all three extraction methods20. Therefore, blood volume standardization and modification of the reference protocol14 produced equivalent results in the MCP and the commercial kits. Future research could determine if the MCP is also effective at detecting other pathogens found in the blood of cattle and other animals.
The MCP also allowed DNA extraction from Anaplasma spp. from bovine blood samples at considerably less cost than the tested commercial kits. This relative cost effectiveness is based on the cost of reagents from authorized suppliers in Ecuador. Extraction of DNA from 250 blood samples using the MCP would cost approximately US$ 60, considering only reagents. In contrast, the cost of processing the same number of samples with the tested commercial kits would be US$ 600 with the Bioneer® kit and US$ 2,000 with the Qiagen® kit; again this includes only reagents and not the equipment needed to run the extraction (the Bioneer® kit requires its own specialized equipment).
One of the principal reasons for the high cost of reagents in countries such as Ecuador, Colombia and Mexico, among others, is that they are not manufactured in Latin America. Import fees and the commissions charged by local suppliers therefore greatly increase the cost of scientific analyses. A chronic lack of financial resources is perhaps the greatest limiting factor when attempting to carry out research in developing countries. Functioning under these circumstances requires creative development of reliable low-cost techniques for infectious disease research and monitoring. This is an important challenge to overcome to better understand disease epidemiology, and to design and implement effective control/monitoring plans that benefit animal production and protect exposed human populations.
The low-cost modified Chelex® resin protocol developed here for DNA extraction from hemolyzed and clotted blood produces DNA of high quality for molecular detection of Anaplasma spp. by PCR. Results are equivalent to those obtained with commercial kits. The proposed protocol is ideal for monitoring Anaplasma spp. in cattle under limited research and/or disease control budgets. Creation of alternative low-cost protocols for pathogen detection and molecular analysis makes research more accessible, even under the conditions prevailing in developing countries. This will increase the ability of livestock sector and public health agencies to proceed efficiently and effectively. Considering the cost effectiveness of the modified Chelex protocol (MCP), it is well worth testing its usefulness in detecting other pathogens and its extraction efficiency with other sample types.
Acknowledgements
The research reported was supported by the Gobierno Autónomo Descentralizado de la Provincia de Esmeraldas (GADPE) and the Instituto Interamericano de Cooperación para la Agricultura (IICA). It was financed with research funds from the Colegio de Ciencias Biológicas- USFQ (2019-2020) granted to Verónica Barragán as well as research funds from the Escuela de Medicina Veterinaria- USFQ (2017-2018) granted to Lenin Vinueza. Publication of this article was financed by the Fondo para Publicación de Artículos Académicos of the Universidad San Francisco de Quito USFQ. Thanks are due to Fernanda Loaiza for technical suggestions at the beginning of the research.
REFERENCES
1. Reyna-bello A. Anaplasma marginale: Logros y retos. En: González C, Madrid N, Soto E, editores. Logros y desafíos de la ganadería doble propósito. Maracaibo, Venezuela: Ediciones Astro Data SA; 2014:388-395. [ Links ]
2. OIE. Organización mundial de sanidad animal. Terrestrial manual-Bovine Anaplasmosis: 2018. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.01_BOVINE_ANAPLASMOSIS.pdf. [ Links ]
3. Goodger WJ, Carpenter T, Riemann H. Estimation of economic loss associated with anaplasmosis in California beef cattle. J Am Vet Med Assoc 1979;174(12):1333-1336. [ Links ]
4. Jonsson NN, Bock RE, Jorgensen WK. Productivity and health effects of anaplasmosis and babesiosis on Bos indicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Vet Parasitol 2008;155(2):1-9. [ Links ]
5. Rodríguez RI, Grisi L, Pérez de León A, Silva H, Torres JF, Fragoso H, et al. Potential economic impact assessment for cattle parasites in Mexico. Review. Rev Mex Cienc Pecu 2017;8(1):61-74. [ Links ]
6. Aubry P, Geale DW. A review of bovine anaplasmosis. Transbound Emerg Dis 2011;58(1):1-30. [ Links ]
7. Silaghi C, Santos AS, Gomes J, Christova I, Matei IA, Walder G, et al. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector-Borne Zoonotic Dis 2017;17(1):12-22. [ Links ]
8. Al-Soud WA, Rådström P. Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol 2001;39(2):485-493. [ Links ]
9. Sidstedt M, Hedman J, Romsos EL, Waitara L, Wadsö L, Steffen CR, et al. Inhibition mechanisms of hemoglobin, immunoglobulin G, and whole blood in digital and real-time PCR. Anal Bioanal Chem 2018;410(10):2569-2583. [ Links ]
10. Ghaheri M, Kahkrizi D, Yari K, Babaie A, Suthar RS, Kazemi E. A comparative evaluation of four DNA extraction protocols from whole blood sample. Cell Mol Biol 2016;62(3):119-123. [ Links ]
11. Smith K, Diggle MA, Clarke SC. Comparison of commercial DNA extraction kits for extraction of bacterial genomic DNA from whole-blood samples. J Clin Microbiol 2003;41(6):2440-2443. [ Links ]
12. Polski JM, Kimzey S, Percival RW, Grosso LE. Rapid and effective processing of blood specimens for diagnostic PCR using filter paper and Chelex-100. J Clin Pathol Mol Pathol 1998;51(4):215-217. [ Links ]
13. Walsh PS, Metzger DA, Higushi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 2013;54:134-139. [ Links ]
14. Singh UA, Kumari M, Iyengar S. Method for improving the quality of genomic DNA obtained from minute quantities of tissue and blood samples using Chelex 100 resin. Biol Proced Online 2018;20:12. [ Links ]
15. IICA. Instituto Interamericano de Cooperación para la Agricultura. Planes de Fincas Ganaderas en Esmeralda, Ecuador: 2017: https://repositorio.iica.int/handle/11324/8410. [ Links ]
16. Zobba R, Anfossi AG, Parpaglia MLP, Dore GM, Chessa B, Spezzigu A, et al. Molecular investigation and phylogeny of Anaplasma spp. in mediterranean ruminants reveal the presence of neutrophil-tropic strains closely related to A. platys. Appl Environ Microbiol 2014;80(1):271-280. [ Links ]
17. Du Breuil RM, Patel JM, Mendelow BV. Quantitation of β-actin-specific mRNA transcripts using xeno-competitive PCR. Genome Res 1993;3(1):57-59. [ Links ]
18. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. URL http://www.R-project.org/. [ Links ]
19. McLaughlin MJ, Sainani KL. Bonferroni, holm, and hochberg corrections: Fun names, serious changes to P values. PM R 2014;6(6):544-546. [ Links ]
20. McHugh ML. Interrater reliability: The kappa statistic. Biochem Medica 2012;22(3):276-282. [ Links ]
21. Sánchez-Otero J. Introducción a la estadística no paramétrica y al análisis multivariado. Quito, Ecuador: Giro Creativo; 2016:12-13. [ Links ]
22. Suarez CE, Noh S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet Parasitol 2011;180(2):109-125. [ Links ]
23. FAO. Ganadería de América Latina y el Caribe puede jugar rol clave en el logro de los Objetivos de Desarrollo Sostenible. 2016. http://www.fao.org/americas/noticias/ver/es/c/421098/. [ Links ]
24. Donadeu M, Nwankpa N, Abela-Ridder B, Dungu B. Strategies to increase adoption of animal vaccines by smallholder farmers with focus on neglected diseases and marginalized populations. PLoS Negl Trop Dis 2019;13(2). [ Links ]
25. Rodríguez V. La investigación científica en Ecuador es cinco veces más cara por costos de reactivos y equipos. 2019. https://www.primicias.ec/noticias/tecnologia/investigacion-cientifica-ecuador-cuesta-cinco-veces-mas/. [ Links ]
26. Dong J, Ismail N, Walker DH. Molecular testing in emerging infectious diseases. En: Coleman W, Tsongalis G, editores. Diagnostic molecular pathology. Londres, Reino Unido: Elsevier Academic Press; 2016:179-200. [ Links ]
Received: March 09, 2020; Accepted: August 10, 2020











 texto en
texto en 


