Introduction
Bovine tuberculosis (TBb) is a chronic bacterial disease caused by Mycobacterium bovis, a slow-growing obligate intracellular pathogenic bacterium, characterized by producing granulomas in different organs, especially lungs and lymph nodes of different animal species and humans1-4. In cattle, this disease is characterized by formation of granulomas (i.e. tubers) in any body tissue, although lesions are more frequent in lymph nodes [medial retropharyngeal (29.4 %), mediastinal (28.2 %), tracheobronchial (18.0 %), mesenteric (2.9 %), parotid (2.4 %) and the caudal cervical (2.4 %)] and a lower percentage in lungs (8.0 %)5-9.
Bovine tuberculosis affects livestock causing a reduction of about 10 to 20 % of milk and meat production, and limiting markets for animals and their products. So the control of this disease becomes a priority for the national livestock industry, to develop the productive potential of cattle, and allow exportation10,11.
In Mexico, as in many other countries of the world, there is a national program for bovine tuberculosis control and eradication. In beef cattle this program has reduced the prevalence to <0.5% in 85% of the national territory; however, in dairy-cattle areas, where the prevalence is high (≈16%), the program has not been successful12,13.
The role of bacterial genetic variability in the infection’s outcome remains uncertain. Until the early 1990s, when DNA fingerprints were introduced, it was believed that the M. tuberculosis complex was a group of highly genetically conserved bacteria, with limited phenotypic differences that influenced pathogenesis. However, epidemiological data suggest that differences in transmissibility and virulence between strains are related to their genotypes14-18.
M. bovis is an intracellular pathogen that mainly resides in macrophages. Therefore, in vitro macrophage models can be used to determine intracellular survival, which in turn is associated with the virulence of M. bovis strains. For example, Gutiérrez-Pabello and Adams19 demonstrated that virulent strains survive longer than avirulent strains in bovine macrophages. The in vitro model has also been validated for evaluation of the pathogenicity or virulence of mycobacteria with inactivated genes, or for the identification of genes associated with virulence20.
In addition, there are reports of cattle naturally resistant to TBb. According to Qureshi et al21, the classification criteria of resistant animals is based on the ability of macrophages to allow the growth of the avirulent M. bovis BCG strain (Calmette-Guérin Bacillus) in vitro; when this growth is higher than 65 %, they are considered susceptible, and when the bacterial growth is lower than 65 %, they are considered resistant. In addition, these authors showed that macrophages from resistant cattle were significantly superior in the control of intracellular growth of other pathogens such as B. abortus and Salmonella dublin, when compared with macrophages of susceptible animals. These studies conclude that macrophages of resistant and susceptible animals differ in the intracellular control of M. bovis multiplication19. Therefore, the use of the in vitro assessment of bacterial intracellular survival in macrophages from a resistant donor may be considered as a correlate of bacterial virulence.
The genotyping of M. bovis for phylogenetic analysis has been carried out by different methods, Restriction fragment length polymorphisms (RFLP), random amplification of polymorphic DNA - Polymerase Chain Reaction (RAPD-PCR), spoligotyping and interspersed mycobacterial repetitive units - Variable number of tandem repeats (MIRU-VNTR). These methods have demonstrated of a great diversity of strains, with low and high frecuency genotypes22-24.
The random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) method provides results comparable to those of RFLP, but it avoids use of hazardous radioactive materials, is less expensive, and easier and quicker to perform. For example, whereas RFLP may require weeks for results, RAPDPCR can be completed in <5 to 8 hours. In comparison with targeted PCR, RAPD-PCR does not require knowledge of the target sequence to develop primers. Single-stranded, 10-nucleotide oligomers of arbitrary sequence are used individually to perform the PCR25. With the advent of molecular epidemiology, faster and more reliable methods for bTB diagnosis have been developed, which provide information about the genetic structure to establish relationship among strains. The most popular method for M. bovis genotyping is spoligotyping. This method has proved useful in a large number of studies with a wide range of objectives and animal species. In comparison with other methods, it is easy to perform, requires very small amounts of DNA, it is robust and highly reproducible, and it can be applied to clinical specimens. More than that, spoligotyping is very useful in strains of M. bovis with a few copies of the IS6110 insertion element, as it is the case in strains from cattle22. MIRU-VNTR is a molecular tool based on the amplification by PCR of 41 different loci of the Mycobacterium DNA sequence, which function as molecular markers obtaining fast, objective, and high resolution monitoring procedures in outbreaks or epidemiological emergencies23.
In a previous study carried out by our group, genotyping of M. bovis strains of cattle from different regions of Mexico, demonstrated the presence of genotypes (spoligotypes and MIRU-VNTR) of high and low frequency22,23,26. These findings led to pose the following questions: Are low frequency strains less virulent than high frequency strains? And if so, is this the reason for the low frequency of some spoligotypes in the population?
Isolation and strain identification are important for disease control. However, little is known about virulence of the circulating strains in cattle populations. Therefore, the aim of this study was to compare the intracellular survival of Mycobacterium bovis strains with high and low frequency of genotypes in Mexican cattle, in macrophages from naturally resistant animals.
Material and methods
This project was approved by the Bioethics Committee of the Natural Sciences Department of the Autonomous University of Queretaro under registry number 47FCN2016.
Molecular characterization of strains
M. bovis strains, previously characterized genetically by spoligotyping and MIRU-VNTR, were used19,22. For the purpose of this study, four strains with a genotype of high frequency and four with genotype of low frequency were selected from a set of strains obtained from cattle in different regions of Mexico (Table 1). High-frequency spoligotypes were first selected and their frequent MIRU-VNTR´s-type were subsequently identified.
Table 1 Spoligotype frequency and VNTR-type of the M. bovis strains used in the study
| Code SB | VNTR | State of origin | ID | Frequency | |
|---|---|---|---|---|---|
| SB0673 | 023404625334 | Coahuila | 777 | 155 | High frequency |
| SB0971 | 433363325242 | Aguascalientes | 833 | 70 | |
| SB0669 | 002060703320 | State of Mexico | 351 | 124 | |
| SB0140 | 242352524244 | Hidalgo | 559 | 71 | |
| SB1495 | 403403625133 | Coahuila | 776 | 1 | Low frequency |
| SB1118 | 452300824234 | State of Mexico | 694 | 1 | |
| SB0290 | 403363525142 | Queretaro | 301 | 1 | |
| SB0131 | 252252323224 | Hidalgo | 906 | 1 |
Inoculum preparation
Experimental M. bovis strains were replicated in Middlebrook 7H9 medium (BBL™ Middlebrook 7H9 Broth Base, Becton, Dickinson Mycrobiology Systems, Cockeysville, MD USA) with OADC enrichment and 0.5 g/l of Tween 80 (Sigma Chemical CO, St. Louis, MO USA); then, incubated at 37 °C under constant stirring for 12 d. For analysis, bacteria were preserved in 1 ml aliquots in RPMI medium (RPMI Medium 1640 Invitrogen™ Corporation Grand Island, NY USA), supplemented with 0.1 mM of essential amino acids, 1 mM of sodium pyruvate, 2 mM of L-glutamine and 20 mM of sodium bicarbonate NaHCO3 (CRPMI), plus 15% of fetal bovine serum (Gibco ™ Invitrogen Corporation Grand Island NY USA) at -70 °C. For each bacterial inoculum, colony forming units (CFU) were determined by serial decuple dilutions seeded in Middlebrook 7H10 agar plates (Bacto® Mycobacteria 7H11 Agar, Difco Laboratories, Detroit MI USA) with OADC enrichment (BBL™ Middlebrook OADC Enrichment, Becton, Dickinson and Company, Sparks, MD USA).
Macrophages derived from bovine monocytes (MDMB)
Peripheral venous blood was collected from the jugular vein of a healthy bovine, previously identified as resistant27, in syringes with acid-citrate-dextrose (ACD) solution. Macrophages were obtained from peripheral blood mononuclear cells (PBMC). PBMC were isolated by gradient centrifugation on a Histopaque -1077 suspension (Sigma-Aldrich, San Luis, USA). Cell pellets were washed and suspended in CRPMI. Cells were grown in Ultra Low Adhesion Plates (Corning® Costar®, USA) at 37 °C with 5% CO2. After 2 h, non-adherent cells were removed and adherent monocytes were cultured in CRPMI, plus 12 % of autologous serum for 12 d to allow their differentiation into macrophages28,29.
Microbicidal assay
Bactericidal assays were carried out according to Qureshi et al21, with some modifications. Bactericidal assays have been useful for classification of cattle resistant or susceptible phenotypes using the avirulent strain M. bovis BCG using 65 % of bacterial replication as a cut-off point. Macrophage monolayers in cell culture in Terasaki plates were infected with M. bovis at a multiplicity of infection (MOI) of 10:1. Subsequently, centrifuged at 200 xg for 10 min and incubated at 37 °C with 5% CO2 for 4 h in a humidified atmosphere to allow phagocytosis. Cells were then washed five times with fresh CRPMI, plus 12 % of autologous serum to remove extracellular bacteria, and incubated again at 37 °C. This was considered as time 0 h. Cells were lysed for analysis at 0 and at 24 h (time 1) after infection. Bacterial phagocytosis was calculated by measuring the serial dilutions of live intracellular bacteria released from macrophages after treatment with 0.5% Tween 20. Bacterial growth was calculated as the ratio between the total number of intracellular bacteria at the end, and the total number of bacteria at the beginning of the assay, expressed as a proportion. Results are the average of three independent experiments, each one with three internal repetitions. Statistical analyzes were carried out with the nonparametric Friedman test and Dunn's multiple comparisons test with 10% significance and the student’s t test to compare UFCs with 5% significance.
Results
The phagocytosis proportion was around 63 % for all strains, indicating that macrophages phagocytized a similar number of bacteria from each strain. Colony forming units (CFU) were counted in the bactericidal assays that showed permissiveness of macrophages for the replication of field strains with low frequency genotypes with higher CFU/ml in the survival time (time 1). In the BCG strain, CFU decreased in survival compared to the phagocytosis time (time 0). The same scenario was observed with high frequency genotype strain the amount of CFU/ml was higher in survival; while in BCG, the amount of CFU/ml decreased (Figure 1).
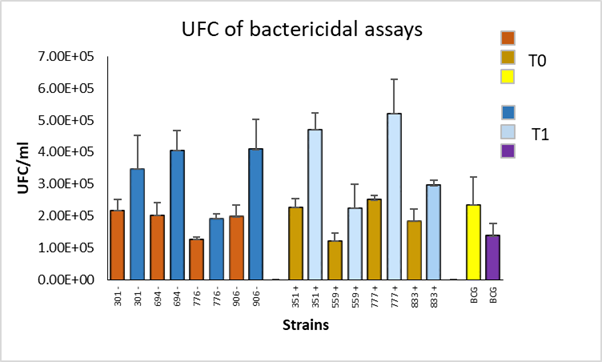
Figure 1 Colony forming units (CFU) of M. bovis strains with high (+) and low (-) frequency genotypes, quantified at 0 (T0) and 24 (T1) hours post-infection of bovine macrophages with a resistant phenotype
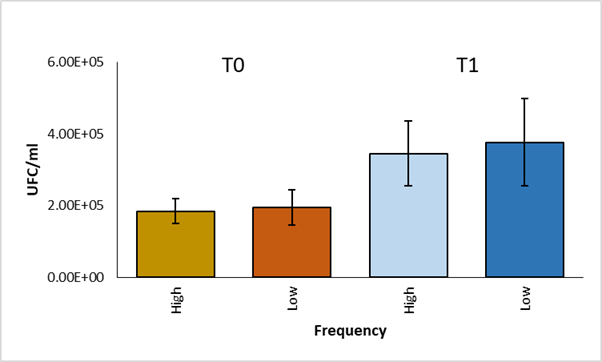
A comparison of the average of CFU per group was made using the student's t test. No significant differences were found (P>0.05) between the high and low frequency groups in phagocytosis and in survival (Figure 2). In addition, within each group, a statistical analysis was performed using the paired student t test to compare the average of CFU between phagocytosis and survival, significant differences were observed (P<0.05) (Figure 3).
Statistical analysis using Student's t test
Figure 2 Comparison of CFU averages of high and low frequency strains at time 0 and time 1
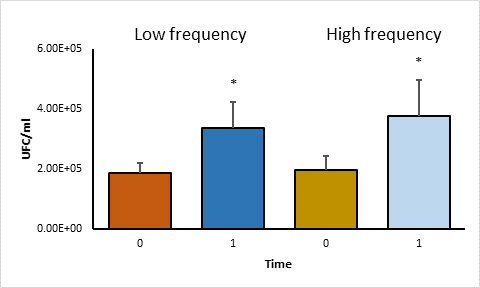
Statistical analysis using the Paired Student t test. Asterisks represent statistical significance (P<0·05).
Figure 3 Comparison of CFU averages of time 0 and time 1 in high and low frequency strains
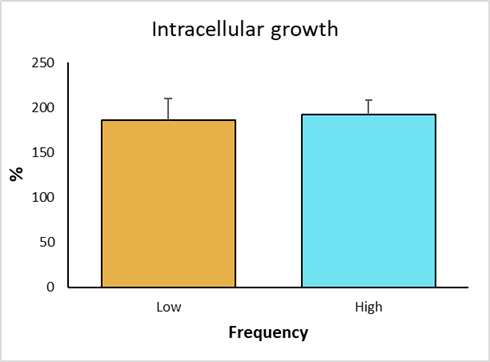
The intracellular growth of strains with low frequency genotype was obtained. Strain 906 was the one with the highest survival score, with 214 %; whereas the strain 776 had the lowest survival score, with 153 %. Strains 694 and 301 showed 205 % and 172 % intracellular growth, respectively (Figure 4). In the high frequency groups, strain 777 had the highest growth, with 208 %; while the lowest growth corresponded to strain 833, with 169 %. Strains 351 and 559 had a growth of 207 % and 187 %, respectively. The control strain (BCG), showed a growth of 57 %, validating the trial, because it confirms the resistant bovine phenotype.
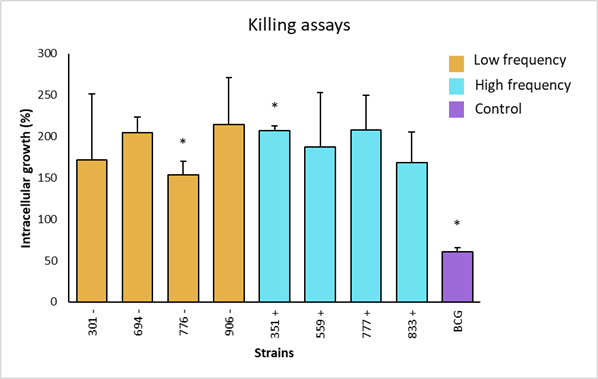
Asterisks represent statistical significance (P<0.1).
Figure 4 Intracellular growth of M. bovis strains and statistical analysis by non-parametric Dunn's multiple comparisons test of the survival of low and high frequency strains and avirulent M. bovis in bovine macrophages with resistant phenotype
In individual comparisons of strains for intracellular growth, a significant difference was observed (P<0.01) between the low frequency strain 776 and the high frequency strain 351. Strain 776 (SB1495) was collected from a bovine from the state of Coahuila, while strain 351 (SB0669) was from a bovine in the state of Mexico. There was also a significant difference between the low frequency strains 776 (SB1495) and 694 (SB1118). Additionally, a significant difference was observed between the attenuated BCG strain and the analyzed field strains (P<0.01) (Figure 4). Finally, intracellular increase averages of high versus low frequency strains were compared, where no significant differences were observed (P>0.05) (Figure 5).
Discussion
It has been shown that mycobacteria genotype is one of the factors that contribute to the severity of the disease, and that it may play a role in the emergence of drug resistance, susceptibility, host response and transmissibility. However, the genetic factors that determine the association of different mycobacterial lineages with different levels of disease severity remain unknown30,31. The high frequency of strain genotypes can be indicative of the movement of infected cattle without an adequate sanitary control measures. On the other hand, it is probable that the low frequency of some genotypes is due to the lack of representativeness in the sampling, that is, the number of isolates analyzed is rather a consequence of their availability due to a low prevalence in the region; therefore, it is probable that the little or null presence of certain genotypes in some regions is rather a consequence of the small amount of isolates provided, and not to a real absence22.
The proportion of phagocytosis was no different between strains; however, statistical differences were found between field strains and the attenuated M. bovis BCG strain. BCG showed less survival, 65 %, confirming that macrophages came from a bovine with resistant phenotype, and the virulence of field strains, as described by Qureshi et al21.
In another study28, in addition to classifying cattle as resistant or susceptible, the authors suggested that the difference in bacterial adhesion to resistant or susceptible cattle cells could be associated with cell wall components in Brucella abortus, which has mechanisms other than M. bovis to evade the immune response and elimination by macrophages. On the other hand, Gutiérrez-Pabello and Adams19, carried out bactericidal assays using a field strain and found similar results to ours regarding the difference in survival with respect to the attenuated BCG strain, with a growth of 165 % in resistant bovine macrophages. Macrophages obtained from cattle with resistant genotype, produce a higher amount of nitric oxide (NO) compared to susceptible bovine macrophages. In bovines, NO plays an important role in the elimination of intracellular pathogens by macrophages27,29.
Virulent M. bovis has different ways of evading the bactericidal mechanisms of the innate immune system. One of the most known and important mechanisms is the "respiratory burst" for the elimination of phagocytized bacteria, although M. bovis has the ability to evade it32. In addition, M. bovis evades elimination by interrupting the maturation of phagosomes, preventing their fusion with the lysosomes and also appears to inhibit the presentation of antigens by reducing the expression of MHC II molecules33,34.
Statistical differences were found in three of the eight field strains, between high and low frequencies, and within the same low frequency group. This suggests the existence of a difference between the virulence of these strains and that the frequency of the genotype in cattle is not related to strain virulence using this methodology. One limitation in the present study was the number of strains due to the lack of a representative quantity. Previous studies have tried to associate spoligotypes with virulence in cattle30, relating the spoligotype to the number of injuries and the injury degree in the carcass. Results showed that SB0273 and SB0520 spoligotypes had high virulence and low frequency. On the other hand, Aguilar León et al36, attempted to relate virulence to different genotypes in a model of progressive pulmonary tuberculosis in mice, finding that the genotypes of wild animals are more virulent than those isolated from humans or livestock; however, it is proposed that M. bovis is highly virulent in the mouse model35,36.
In Northern Ireland herds, differences in the pathogens genotype have been identified by the size of the outbreak and the proportion of cases with visible lesions in the carcass, suggesting that closely related M. bovis genotypes may vary in terms of transmissibility. Genotypes have differences in the formation of visible lesions, immune response patterns and virulence levels; however, the genetic factors that determine the association of different genotypes of mycobacteria with different degrees of disease severity remain largely unknown. In Mycobacterium tuberculosis (M. tuberculosis), it has been found that among its 6 main genotypes there is variation in immunogenicity, virulence and pathology, together with evidence of a host-pathogen coevolution in the regions where these genotypes are established37.
The great genetic diversity of M. tuberculosis complex (CMTB) strains supports an important natural scenario, where these strains (particularly M. bovis) have diversified, offering an interesting epidemiological and evolutionary context in which new genotypes can emerge and diversify in terms of adaptation to the host under a range of environmental and human factors38.
In high incidence countries, the appearance of an epidemiologically successful strain has been attributed to virulence due to the characteristics encoded in the bacterial genome; however, these characteristics are related to genes other than genotyping39. The above is because these genotyping methods cover only a small part of the approximately 4,000 genes contained in 4.4 Mb of the mycobacterial genome, and are not directly related to virulence40.
Conclusions and implications
Results from this study contribute to understand the host-pathogen interaction. Intracellular bacteria attempt to survive the hostile environment presented by the immune response where one of the main effectors is the macrophage. The results contribute to validate the microbicidal assay, as a tool to partially evaluate the virulence of intracellular bacterial pathogens, as well as suggest that bacterial genotypes are not directly related to virulence.











 texto en
texto en