Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.12 no.1 Mérida ene./mar. 2021 Epub 20-Sep-2021
https://doi.org/10.22319/rmcp.v12i1.5283
Articles
Body distribution of ticks (Acari: Ixodidae and Argasidae) associated with Odocoileus virginianus (Artiodactyla: Cervidae) and Ovis canadensis (Artiodactyla: Bovidae) in three northern Mexican states
a Universidad Autónoma de Nuevo León, Facultad de Ciencias Biológicas, Ave. Universidad S/N, Ciudad Universitaria. 66455 San Nicolás de los Garza, Nuevo León. México.
Ticks are important vectors of medical and veterinary importance pathogens in Mexico; however, the taxonomic studies of abundance, prevalence, intensity, and body distribution in white-tailed deer (Odocoileus virginianus) and bighorn sheep (Ovis canadensis) are limited. This study aimed to fill this knowledge gap in the Mexican states of Sonora, Nuevo León, and Tamaulipas. The area of study included authorized game farms where hunting is practiced. A total of 372 ticks [21 nymphs (5.65 %) and 351 adults (94.35 %); 41% female and 59 % male] were collected from 233 O. virginianus and four O. canadensis. The ticks collected from O. virginianus were identified as Otobius megnini, Rhipicephalus (Boophilus) microplus, and Dermacentor (Anocentor) nitens. Dermacentor hunteri was the only species collected from O. canadensis. Ears were the most infested region (83 females, 70 males, and 21 nymphs, 46.77 %), and the least infested body parts were the legs (10 males and nine females, 5.1 %). This study reports for the first time the abundance, intensity, and prevalence of ticks in O. virginianus in northern Mexico, particularly in the states of Tamaulipas and Nuevo León, since the O. canadensis ticks had already been reported in Sonora. These results show that although ungulates are kept semi-captive, it is essential to control tick infestation by applying acaricide treatments on their preferred adherence sites to avoid the transmission of pathogens.
Key words White-tailed deer; Bighorn sheep; Ticks; Rhipicephalus microplus; Otobius megnini; Dermacentor nitens; Dermacentor hunteri
Las garrapatas impactan como vectores por transmitir patógenos de importancia médica y veterinaria en México, pero los estudios taxonómicos de abundancia, prevalencia, intensidad y preferencia en la distribución corporal de venado cola blanca (Odocoileus virginianus) y borrego cimarrón (Ovis canadensis) son precarios, por lo cual estos fueron los objetivos del presente trabajo en Sonora, Nuevo León y Tamaulipas, México. El área de estudio abarcó ranchos cinegéticos autorizados donde se practica la cacería. Se examinaron 233 O. virginianus y cuatro O. canadensis, recolectándose 372 garrapatas [21 ninfas (5.65 %) y 351 adultos (94.35 %)]; 41 % fueron hembras y 59 % machos. Las garrapatas presentes en O. virginianus fueron Otobius megnini, Rhipicephalus (Boophilus) microplus y Dermacentor (Anocentor) nitens, mientras que Dermacentor hunteri fue la única en O. canadensis. Las orejas fue la región más infestada (83 hembras, 70 machos y 21 ninfas, 46.77 % en total) y la menos infestada fueron las piernas (10 machos y nueve hembras, 5.1 %), con diferencia significativa (P<0.005). Este estudio reporta por primera vez la abundancia, intensidad y prevalencia de garrapatas en O. virginianus en el norte de México y particularmente en los estados de Tamaulipas y Nuevo León, pues solo las garrapatas de O. canadensis habían sido reportadas en Sonora. Estos resultados indican que, aunque los ungulados están en semicautiverio, es importante controlar la infestación por garrapatas de acuerdo a los sitios de adherencia preferidos para aplicar los tratamientos acaridas, debido a la importancia como vectores en la transmisión de patógenos.
Palabras clave Venado cola blanca; Borrego cimarrón; Garrapatas; Rhipicephalus microplus; Otobius megnini; Dermacentor nitens; Dermacentor hunteri
Introduction
Ticks are hematophagous ectoparasites of amphibians, reptiles, birds, and mammals1. As a result of their eating habits, ticks directly decrease their host weight gain and exert traumatic, toxic, infectious, or spoliation actions, in addition to the indirect effects that deteriorate the skin and cause death by dermal diseases2,3. During their life cycle, ticks can horizontally or vertically acquire4 and transmit a wide range of medical and veterinary important pathogens, such as Babesia spp., Borrelia spp., Anaplasma phagocytophilum, and Rickettsia spp.; hence, ticks are considered as vectors of global importance, surpassed only by mosquitoes5.
Wildlife constitutes an important component in the transmission cycle of the vector-host-pathogen triangle, where humans are frequently included as accidental hosts, which makes it a zoonotic cycle6. Therefore, the prevalence of new and reemerging tick-transmitted diseases represents a global public health problem7. The spatial distribution of ticks is mainly influenced by climatic and geographic conditions, the type of vegetation, the agricultural landscape, the population dynamics of their wild hosts3, and the illegal movement of cattle and Odocoileus virginianus for commercialization purposes without meeting the sanitary standards8. These factors facilitate tick dispersion to places where it was not naturally found, increasing the human risk of acquiring diseases associated with these vectors9,10. Previous studies have reported that Rhipicephalus annulatus has a specific preference for deer11, and that Dermacentor spp. and Ixodes spp. specifically adhere to head and neck12.
In Mexico, a total of 77 tick species has been identified; from these, those with national livestock importance due to their direct and indirect effects are R. (Boophilus) microplus, B. anulatus, Amblyomma cajennense, A. imitador. A. maculatum, A. triste, A. americanum, and Anocentor nitens. However, the most important due to their economic impact are R. microplus and A. cajennense13,14, with losses of US $ 573’608,07615. In Sonora, Nuevo León, and Tamaulipas, O. virginianus and Ovis canadensis have been used to increase income through legalized hunting practices in game farms. In 1996, a conservation program that consisted of constructing an enclosure for the reproduction of economically important species in semi-captivity was implemented in Rancho El Plomito, Sonora, in an area of 961 ha. By 2014, their reproductive population of O. canadensis, O. v. couesi, and O. hemionus was enough for the first repopulations performed by the Organización de Vida Silvestre (OVIS, AC)16. Mexican authorities reformed the operation of game farms by implementing the current system of Wildlife Management and Conservation Units (UMA, acronym in Spanish). This system allows the conservation and management of wildlife in their natural habitat, in addition to the rational use of wildlife or semi-captive populations and specimens17,18. In the studied Mexican states, regulated hunting of these ungulates is allowed, but population studies of ectoparasites, their body distribution, and the presence of species of game importance are scarce3,12. Thus, these results will allow to plan the body areas where proven successful acaricide treatments or devices will be applied for tick control12. This study aimed to i) taxonomically identify the tick species, ii) determine their prevalence, iii) estimate their abundance and intensity, and iv) describe their body distribution in O. virginianus and O. canadensis in game farms in Sonora, Nuevo León, and Tamaulipas. This information helps to understand the potential risk of ticks as vectors of diseases and implement preventive and corrective measures.
Material and methods
Areas of study
This study took place in different areas of Tamaulipas, Nuevo León, and Sonora from 2014 to 2018 in the months from October to February; these months correspond to the legal hunting period in UMAs in situ or ex situ of O. virginianus and O. canadensis17,18. Two areas of study were located in the Sierra Madre Occidental in Sonora: Rancho El Aigame (UMA registration: DGVS-CR-EX-1271-SON), La Colorada municipality (28º 43’ 41” N, 110º 2’0.65” W) at 400 m asl and Rancho El Pitiquito (UMA registration: SEMARNAT-UMA-EX-250-SON) (30º 15’ 0.0’’ N, 112º 22’0.12’’ W)16-18, El Pitiquito municipality, with a predominantly arid and semiarid climate and mean annual precipitation of 450 mm19. In Nuevo León, ticks were collected in Rancho Mamulique (UMA registration: DFYFS-CR-EX-0333-NL), Salinas Victoria municipality (26º 7’ 0.59’’ N, 100º 19’ 0.58’’W), at 464 m asl, with warm arid steppe climate, annual mean temperature of 21-23 °C and annual mean precipitation of 380 mm19. In Tamaulipas, tick collection took place in two localities, Rancho Santa Clara (UMA registration: DGVS-CR-EX-1819-TAM), Nuevo Laredo (27° 33’0.11’’ N, 99° 47’ 59.9’’ W), which has the most arid and extreme climate in the State, ranging from -14 °C during winter and 40 °C during summer, and mean annual precipitation of 472.5 mm19. The second locality was Rancho Los Columpios (UMA registration: DGVS-CR-EX-2066-TAM), Guerrero municipality (26° 33’ 18’’ N, 99° 22’ 0.37’’ W), located on the Río Bravo basin, Tamaulipas. These localities have an arid climate with annual mean precipitation of 440 mm18-20.
Tick collection and taxonomic identification
Ticks were collected from hunted specimens of O. virginianus and O. canadensis during the hunting season through the authorization issued by the Ministry of Environment and Natural Resources of Mexico to each UMA16-18. The utilization rate of these species is oriented to the hunting of male and adult specimens. After acquiring a hunting package, hunters were accompanied by OVIS technicians16. During collection, using sterile forceps, ticks were individually removed from the head (top part), ear, scapula, mid-dorsal neck, and inferior extremities of hunted animals21. Live ticks were transported to the Molecular and Experimental Pathology Laboratory (LPME, FCB, UANL) in 12 ml-vials containing a cotton pad moistened with sterile double-distilled water and labeled with the date, host, stage, locality, and body part from which the tick was collected; vials were stored at 4 °C. Ticks that died during transportation were placed in vials containing absolute ethyl alcohol as a preservative to avoid deterioration of the morphological traits needed for taxonomic identification22.
The genus, species, sex, and stage of ticks were determined with a stereomicroscope at 10X - 40X (EZ4E, Leica Microsystem, Guadalajara, Jalisco, Mexico) and employing a specific tick taxonomic key23-25. In the taxonomic identification of ticks, the following distinctive structures of each of the species were considered:
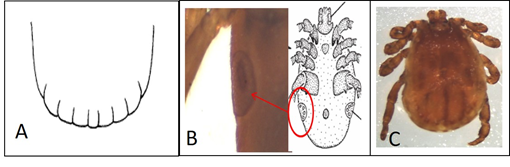
Otobius megnini: Lack of dorsal shield, ventral gnathosoma, rectangular and straight basis capitulum, absent eyes, vestigial or atrophied hypostome, integument with spines, ambulacrum absent at the end of the legs (Figure 1).

A) Dorsal view. Illustration and picture of a nymph. B) Ventral view with details of the anterior gnathosoma, rectangular basis capitulum in the box. C) Integument with spines (the integument is smooth between the spines).
Figure 1 Taxonomic characteristics of Otobius megnini
Rhipicephalus (Boophilus) microplus: Males have ventral plates and a caudal appendage, a shield that covers the dorsal region of males and the anterior dorsal region of females, eyes, a hexagonal basis capitulum, a coxa I with double spines, a dorsally visible regular size and prominent coxa IV, and no festoons (Figure 2).
The Dermacentor species has an anterior gnathosoma with a rectangular and straight basis capitulum, festoons, a big coxa IV in males, and a coxa I with big and paired spurs (Figure 3). D hunteri and D. nitens differ in the number of festoons. D. hunteri has 11 festoons and an ornament with a characteristic pattern, with large spiracular plates forming a ring, rear to leg IV (Figure 4); D. nitens has seven festoons (Figure 5). All illustrations were obtained from tick identification keys24,25.

A) Dorsal shield. B) Anterior gnathosoma, basis capitulum with angular margins. C) Eyes. D) No festoons. E) Ventral plates and caudal appendage. F) Coxa I and IV. G) Female and male adult specimens.
Figure 2 Morphologic characteristics for the taxonomic identification of Rhipicephalus (Boophilus) microplus

A) Dorsal shield. B) Anterior gnathosoma, rectangular and straight basis capitulum. C) Eyes. D) Morphology of coxa I and IV. E) Festoons in posterior end.
Figure 3 Morphologic characteristics for the taxonomic identification of Dermacentor spp

A) 11 festoons. B) Ornamentation (shown in the pattern). C) Complete male specimen.
Figure 4 Morphologic characteristics for the taxonomic identification of Dermacentor hunteri
Statistical analysis
The prevalence (percentage of infested hosts by tick species), intensity (number of ticks/infested hosts by each tick species), abundance (number of ticks by species/hosts), and sex proportion by tick species were calculated26. The significant association between the vector, host location, sex, and locality was determined with a Chi-squared (X2) test at a significance level of 95% of the lower and upper confidence interval (CI). Additionally, the data were analyzed with the Z test to compare the population proportions between stages, species, localities, and hosts with the SPSS program, version 1712.
Results
A total of 237 hosts were inspected, 233 O. virginianus specimens and four O. canadensis specimens; the body infestation percentages were 17.16 % and 100 %, respectively. Of the 372 ticks collected, 5.65 % were nymphs and 94.35 % adults. Four tick species were identified: Rhipicephalus (Boophilus) microplus (Canestrini, 1887), Dermacentor (Anocentor) nitens (Neumann, 1897), and Dermacentor hunteri (Bishopp, 1912) from the Argasidae; and Otobius megnini (Dugès 1883) from the Argasidae family (Table 1).
Table 1 Tick identification by host (Odocoileus virginianus and Ovis canadensis), sex, and locality
| Locality | Host (n/+) | Species | TS/ST (%) | Sex | |
|---|---|---|---|---|---|
| F n/(%) | M n/(%) | ||||
| Sonora | O. virginianus (16/6) | *Otobius megnini | 19/204 (9.3) | NA | NA |
| O. canadensis (4/4) | *O. megnini | 2/204 (0.98) | NA | NA | |
| Dermacentor hunteri | 183/204 (89.7) | 28/(13.7) | 155/(76) | ||
| Nuevo León | O. virginianus (202/28) | Rhipicephalus microplus | 98/151 (64.9) | 84/(55.6) | 14/(9.3) |
| D. nitens | 53/151 (35.1) | 19/(12.6) | 34/(22.5) | ||
| Tamaulipas | O. virginianus (15/6) | R. microplus | 17/17 (100) | 13/(76.5) | 4/(23.5) |
| Total O. virginianus | (233/40) | NA | *19/372 (5.1) | NA | NA |
| 168/372 (45.2) | 116/(41) | 52/(59) | |||
| Total O. canadensis | (4/4) | NA | *2/372 (0.53) | NA | NA |
| 183/372 (49.19) | 28/ | 155/ | |||
TS/ST= Ticks by species/ State total (%); n= number of specimens; *= nymphs; NA= non applicable.
Regarding sex, 41 % of ticks were female and 59 % male (Table 2). However, most ticks in O. virginianus were female (116/168, 69 %), only 31% were male (52/168). On the contrary, in O. canadensis, D. hunteri specimens were mainly male (155/183, 84.7 %), females represented 15.3 % (28/183). There was a significant association between the male and female proportion of ticks between O. virginianus and O. canadensis (X2= 104.57, g.l.= 1, P<0.05). A significant association was also observed in the female and male proportion between the four tick species in O. virginianus (X2= 39.92, g.l.= 1, P<0.05), and between males and females by locality (X2= 105.01, g.l.= 2, P<0.05). These results were consistent with the population proportions between stages, species, localities, and hosts since it was confirmed with the Z test, which showed a significant association (Z> 1.2, IC 95%).
Table 2 Percentage of the body distribution of the identified tick species
| Body region | Sex | R. microplus n/(%) | D. nitens n/(%) | O. megnini n/(%) | D. hunteri n/(%) | Total n/(%) |
|---|---|---|---|---|---|---|
| Head | ♀ | 21 (5.7) | 6 (1.6) | NF | 7 (1.9) | 34 (9.1) |
| ♂ | 5 (1.3) | 5 (1.3) | NF | 31 (8.3) | 41 (11.1) | |
| Ear | ♀ | 58 (15.6) | 17 (4.6) | NF | 8 (2.2) | 83 (22.3) |
| ♂ | 11 (2.9) | 11 (2.9) | NF | 48 (12.9) | 70 (18.8) | |
| N | NF | NF | 21 (5.7) | NF | 21 (5.7) | |
| Neck | ♀ | 9 (2.4) | 4 (1.1) | NF | 5 (1.3) | 18 (4.8) |
| ♂ | 1 (0.3) | 5 (1.3) | NF | 29 (7.8) | 35 (9.4) | |
| Back | ♀ | 4 (1.1) | NF | NF | 6 (1.6) | 10 (2.7) |
| ♂ | NF | NF | NF | 31 (8.3) | 31 (8.3) | |
| Legs | ♀ | 6 (1.6) | 2 (0.5) | NF | 2 (0.5) | 10 (2.7) |
| ♂ | NF | 3 (0.8) | NF | 16 (4.3) | 19 (5.1) | |
| Total by species (n/%) |
115 (30.9) | 53 (14.2) | 21 (5.7) | 183 (49.2) | 372 (100) | |
N= nymphs; n= number of specimens; NF= not found.
In Sonora, 54.8 % (204/372) of the ticks from both hosts were collected. In O. virginianus, 9.3 % (19/204) were O. megnini nymphs. O. canadensis had 0.98 % (2/204) of O. megnini nymphs and 89.7 % of D. hunteri (Table 1). In Nuevo León, 40.6 % (151/372) of ticks were collected from O. virginianus; two different species were identified: R. microplus (98/151, 64.9 %) and D. nitens (34/151, 35.1 %). In Tamaulipas, only 17 specimens of R. microplus were collected from O. virginianus; this represents 4.56 % (17/372) of the total adult ticks collected in the three localities.
Ticks, from all four species, were more abundant on ears, with a total of 174 ticks (46.77 %) (22.3 % females, 18.8 % males, and 5.7 % nymphs), and the upper part of the head (9.1% females and 11.1% males), followed by the neck, scapula, and, lastly, inferior extremities. Without considering the host or locality, there was a significant association between the different tick species and their body distribution (X2= 46.18, g.l.= 8, P<0.05); even O. megnini nymphs were located exclusively on the ears. Also, the females and males from different species were significantly associated with body location (X2 = 13.25, g.l.= 4, P<0.05).
In O. virginianus, R. microplus was the most prevalent (30.9%), abundant (0.49), and intense (2.88) species; in O. canadensis, D. hunteri was the most outstanding species (Table 3).
Table 3 Prevalence, abundance, and intensity of infestation by tick species
| Species | O. virginianus (40/233) | O. canadensis (4/4) | ||||
|---|---|---|---|---|---|---|
| Prev (%) | Abund X̅ | Intens | Prev (%) | Abund X̅ | Intens | |
| R. microplus | 115 (30.9) | 0.49 | 2.88 | -- | -- | -- |
| D. nitens | 53 (14.2) | 0.23 | 1.33 | -- | -- | -- |
| O. megnini | 19(5.1) | 0.082 | 0.48 | 2 (0.54) | 0.5 | 0.5 |
| D. hunteri | -- | -- | -- | 183 (49.2) | 45.8 | 45.8 |
Prev= prevalence; Abund= abundance; Intens= intensity.
Discussion
Rhipicephalus (Boophilus) microplus, also known as cattle tick, was the most prevalent species in O. virginianus in Nuevo León and Tamaulipas because it inhabits the same hunting territory. This tick is considered of high incidence in livestock production systems due to the significant economic losses it causes worldwide27; additionally, it is a vector of Babesia bovis, B. bigemina, and Anaplasma marginale. This study considers that O. virginianus plays an important role as a natural reservoir of these diseases in the areas of study, as it has been previously reported in Texas, USA, which shares borders with Nuevo León and Tamaulipas27. During insecticide treatment, female ticks and larvae scape to favorable habitats to survive; this facilitates the upsurge of infestations in cattle and ungulates28. However, the O. virginianus specimens analyzed in Sonora were free of R. microplus; SADER/SENASICA reported that the Animal and Plant Health Inspection Service (APHIS) of the United States Department of Agriculture (USDA) declared Sonora was free from this tick17. In Nuevo León and Tamaulipas, efforts to eradicate R. microplus continue despite the program operating a permanent quarantine zone in south Texas, USA, along the Mexican border28. In northern Mexico, O. virginianus is handled in game farms due to the income received from hunting permits, furs, and meat for human consumption. In these farms, semi-captive O. virginianus specimens share the same feeding, drinking, and transportation areas as cattle, representing a possible infestation risk factor and complicating the eradication of this tick29,30.
The prevalence of R. microplus in northern Mexico was 31%, which is lower than in Yucatán, Mexico, where 97 % was reported in Cervus elaphus, an important host for this tick species; R. microplus not only feeds from its host, it also completes its nymph developing cycle3. This species was also the most common in O. v. yucatanensis and Mazama temama, with a 28.4 % frequency and an intensity of 25.2 ticks per animal31. In contrast, R. annulatus was exclusively reported in C. elaphus with a prevalence of 7.9 % in Cádiz, Spain11.
In Nuevo León, D. nitens has not been previously reported in O. virginianus; however, it was able to collect specimens from this species. There are several reports of this tick in different Mexican states in hosts such as cattle, horses, dogs, mules, and rodents32. The veterinary importance of this ectoparasite is that female specimens of D. nitens transmit Babesia caballi to their offspring transovarially, and all stages are competent for this disease, besides being the etiologic agent of equine piroplasmosis33.
In Sonora, this study reported nymphs of the spinous ear tick (O. megnini) in O. virginianus (5.1 %) and O. canadensis (0.53 %); the adult stage is not an ectoparasite34. Although this tick has been reported from the southwest of the USA to the south of Mexico and South America, it has not received the same importance as other ixodid ticks. O. megnini can have multiple blood ingestions and deposit batches of eggs that represent a danger in the veterinary and clinical fields, as it has a predilection for the ear canal, which can result in otoacariasis, with complications of external otitis, ear pain in 90 % of the cases, and other signs of internal otitis, such as facial and respiratory paralysis. This tick affects the people that have a close contact with livestock animals, be it cows, mules, goats, rabbits, and sheep35. This ectoparasite can affect the host in several ways, such as severe irritations, weight loss, and offspring behavior36. In ungulates and other hosts, when a tick or nymph feeds, it causes blood loss, which attracts other insects, causing stress to the hosts37. Additionally, these ticks act as rickettsial vectors, responsible for spotted fever and Coxiella burnetii (Q fever)38.
In Sonora, ticks were more abundant in bighorn sheep. From the total amount of collected ticks, 49.19% belonged to the D. hunteri species; males had a higher proportion with 41.6%. D. hunteri was almost exclusively collected from this host, similar to previous reports. This tick has also been reported in Baja California, in wild populations of O. canadensis32,39. In California, USA, bighorn sheep populations were seropositive to Anaplasma spp40; it is even considered as a primary vector of A. ovis (Lestoquard, 1924)41,42 and Rickettsia spp., which suggests the importance of this ectoparasite in the epidemiology of these diseases42.
Based on the body region, when considering the total amount of ticks, the four identified tick species preferred the ears, followed by the head. In Capreolus capreolus, 61% of ticks preferred the head area12,21; in cattle, 32.02 % preferred ears and head; in sheep, 48.08 % also preferred the head, which included ears. These previous reports are similar to what was observed in this study. Ectoparasites prefer the head and ears because of the skin thickness in these areas, where the skin is relatively thin and vascularized43,44.
In O. canadensis, males had similar proportions in each body region, with an increased number in ears and head. A study in Mexican tropical regions showed similar results; the Ixodidae tick distribution in sheep infestation was 26.50 % in head and neck45. Previous reports have stated that tick density in O. virginianus can vary depending on the collection season and the age of the ungulates12,46. In this study, ticks were collected during the fall-winter season, when hunting is allowed, and ticks are in their adult stage, except O. megnini, which parasitic stage is nymphal. Ixodes and Dermacentor ticks preferred younger ungulates due to their habits and thinner skin in Capreolus capreolus. Adult ticks had no preference for the sex of the host, but they did for their body mass12,46. Although in south Texas, where there is a quarantine area for eradicating ticks in cattle, ticks have not been eradicated due to unregulated movements of illegal cattle and the dispersal of wildlife animals such as O. virginianus in Mexico27.
Conclusions and implications
This study reports three tick species in O. virginianus (O. megnini, R. microplus, and D. nitens) and two species in O. canadensis (O. megnini and D. hunteri) in game farms from northern Mexico. These species play an important role in pathogen epizootiology47; for this reason, it is essential to identify potential vectors of diseases and tick-associated pathogens in ungulates of game importance and implement control measures. The most and least infested body regions were ears and legs, respectively, due to skin thickness. One of the main strategies for tick eradication is knowing their host specificity (O. virginianus or other ungulates); this information helps recommend applying specific acaricides or ivermectins based on the type of soil or pasture48. It is also important to consider the life cycle of the tick since it could change its susceptibility to the insecticide37. Consequently, knowing the tick distribution in northern Mexico and their abundance and intensity in O. virginianus and O. canadensis will help implement preventive or control measures in game farms and livestock, as well as in the importation or hunting of these ungulates. This information will also prevent the development of new vectors of infectious diseases that could represent a public health or zoonotic problem.
Acknowledgments
To OVIS, S.A. DE C.V. for their participation in tick collection. To CONACyT for the scholarship granted to the first author during her Master’s degree.
REFERENCES
1. Klompen JSH, Black WC, Keirans JE, Oliver Jr JH. Evolution of Ticks. Annu Rev Entomol 1996;41:141-161. doi:10.1146/annurev.en.41.010196.001041. [ Links ]
2. García-Vázquez Z. Garrapatas que afectan al ganado bovino y enfermedades que trasnmiten en México. 1er. Simposium de Salud y Producción de Bovinos de Carne en la Zona Norte-Centro de México 2010;1-9. http://biblioteca.inifap.gob.mx:8080/jspui/handle/123456789/3281 Consultado 5 Jun, 2019. [ Links ]
3. Rodríguez-Vivas RI, Ojeda-Chi MM, Rosado-Aguilar JA, Trinidad-Martínez IC, Torres-Acosta JFJ, Ticante-Perez V, et al. Red deer (Cervus elaphus) as a host for the cattle tick Rhipicephalus microplus (Acari: Ixodidae) in Yucatan, Mexico. Exp Appl Acarol 2013;60:543-552. doi: 10.1007/s10493-013-9672-z. [ Links ]
4. Koneman E, Koneman AS: Diagnostico microbiologico/Texto y atlas en color. 6ta ed. Buenos Aires, Argentina: Editorial Médica Panamericana; 2008. [ Links ]
5. Claerebout E, Losson B, Cochez C, Casaert S, Dalemans AC, De Cat A, et al. Ticks and associated pathogens collected from dogs and cats in Belgium. Parasit Vectors 2013;6:183. doi:10.1186/1756-3305-6-183. [ Links ]
6. Sosa-Gutiérrez G, Vargas M, Torres J. Gordillo-Pérez G. Tick-borne rickettsial pathogens in rodents from Mexico. J Biomed Sci Eng 2014;7:884-889. doi:10.4236/jbise.2014.711087. [ Links ]
7. CDC. Centers for Disease Control and Prevention. New & Emerging Tickborne diseases: Agents, clinical features & surveillance. 2018. https://www.cdc.gov/ticks/diseases/trends.html#2013-video Accessed 12 Sept, 2018. [ Links ]
8. Bush JD, Stone NE, Nottingham R, Araya-Anchetta A, Lewis J, Hochhalter C, et al. Widespread movement of invasive cattle fever ticks (Rhipicephalus microplus) in southern Texas leads to shared local infestations on cattle and deer. Parasit Vectors 2014;7:188. doi:10.1186/1756-3305-7-188. [ Links ]
9. Shaw MT, Keesing F, McGrail R, Ostfeld RS. Factors influencing the distribution of larval blacklegged ticks on rodent hosts. Am J Trop Med Hyg 2003;68:447-452. [ Links ]
10. Blagburn BL, Dryden MW. Biology, treatment, and control of flea and tick infestations. Vet ClinNorth Am Small Anim Pract 2009;39:1173-1200. doi:10.1016/j.cvsm.2009.07.001. [ Links ]
11. Ruiz-Fons F, Fernandez de Mera IG, Pelayo-Acevedo UH, Höfle U, Vicente J, De la Fuente J, et al. Ixodid ticks parasitizing Iberian red deer (Cervus elaphus hispanicus) and European wild boar (Sus scrofa) from Spain : Geographical and temporal distribution Vet Parasitol 2006;140:133-142. doi:10.1016/j.vetpar.2006.03.033. [ Links ]
12. Vor T, Kiffner C, Hagedorn P, Niedrig M, Ru F. Tick burden on European roe deer (Capreolus capreolus). Exp Appl Acarol 2010;51:405-417. doi:10.1007/s10493-010-9337-0. [ Links ]
13. Martínez Arzate SG. Análisis filogenético molecular de la secuencia de la proteína Bm86 de la garrapata Rhipicephalus (boophilus) microplus de Colima, México [tesis maestria]. Toluca, Estado de México. Universidad Autónoma del Estado de México; 2014. [ Links ]
14. SENASICA. Situación actual del control de la garrapata Boophilus spp. 2016. https://www.gob.mx/senasica/documentos/situacion-actual-del-control-de-la-garrapata-boophilus-spp . Consultado 20 Sept, 2019. [ Links ]
15. Rodríguez Vivas RI, Grisi L, Pérez de León A, Silva Villela H, Torres Acosta J, Fragoso H, et al. Potential economic impact assessment for cattle parasites in Mexico. Review. Rev Mex Cienc Pecu 2017;8(1):61-74. [ Links ]
16. OVIS. PROGRAMAS.2011. http://ovis.org.mx/programas/ Consultado 10 Nov, 2017. [ Links ]
17. SEMARNAT. Reglamento de la ley general de vida silvestre. Diario Oficial de La Federación; 2014; DOF 09-05-2014. 1-52. http://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/agenda/DOFsr/DO2008.pdf . Consultado 15 Abr, 2018. [ Links ]
18. SEMARNAT. Registros de unidades de manejo para la conservación de la vida silvestre (UMA). 2019. https://datos.gob.mx/busca/dataset/registros-de-unidades-de-manejo-para-la-conservacion-de-la-vida-silvestre-uma/resource/8815e80b-1779-469f-80b8-b4a4756f61c3. [ Links ]
19. INEGI. Cuentame. 2017. http://www.inegi.org.mx/ mx/ http://www.cuentame.inegi.org.mx/monografias/informacion/son/territorio/div_municipal.aspx?tema=me&e=26 . Consultado 21 Mar, 2018. [ Links ]
20. OCDE. (2018). Nuevo Laredo, Tamaulipas | OCDE Mexico. http://www.ocdemexico.org.mx/Tamaulipas/Nuevo-Laredo/ . Consultado Mar 25, 2018. [ Links ]
21. Warwick BT, Bak E, Baldassare J, Gregg E, Kioko J, Saning K, et al. Abundance estimations of ixodid ticks on Boran cattle and Somali sheep in Northern Tanzania. Int J Acarol 2016;42:12-17. doi:10.1080/01647954.2015.1109708. [ Links ]
22. Amerasinghe F, Breisch N, Azad A. Distribution, density, and Lyme disease spirochete infection in Ixodes dammini (Acari: Ixodidae) on white-tailed deer in Maryland. J Med Entomol 1992;29:54-61. [ Links ]
23. Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J Med Entomol 1989;26:435-448. [ Links ]
24. Delabra G, Fragoso H, Franco R, Martínez F, Ortiz M, Ortiz A, et al. Manual de identificación de las especies de garrapatas de importancia en México. México: Dirección General de Salud Animal. Secretaría de Agricultura, Ganadería y Desarrollo Rural. 1996. [ Links ]
25. Walker AR, Bouattor A, Camicas J, Estrada-Pena, Horak IG, Latiff A, et al. Ticks of domestic animals in Africa, a guide to identification of species. Edinburgh Scotland, U.K: Bioscience Reports. 2014. [ Links ]
26. Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997;83(4):575-583. [ Links ]
27. Busch JD, Stone NE, Nottingham R, Araya-Anchetta A, Lewis J, Hochhalter, et al. Widespread movement of invasive cattle fever ticks (Rhipicephalus microplus) in southern Texas leads to shared local infestations on cattle and deer. Parasit Vectors 2014;7:188. [ Links ]
28. Wang HH, Teel PD, Grant WE, Schuster G, Pérez de León AA. Simulated interactions of white-tailed deer (Odocoileus virginianus), climate variation and habitat heterogeneity on southern cattle tick (Rhipicephalus (Boophilus) microplus) eradication methods in south Texas, USA. Ecol Modell 2016;342:82-96. [ Links ]
29. Cantú-Martinez M, Salinas-Meléndez JA, Zarate-Ramos J, Ávalos-Ramírez R, Martínez-Muñoz A, Segura-Correa J. Prevalence of antibodies against Babesia bigemina and B. bovis in white-tailed deer (Odocoileus virginianus texanus) in farms of northeastern Mexico. J Anim Vet Adv 2008;7:121-123. [ Links ]
30. Medrano C, Boadella M, Barrios H, Cantú A, García Z, De la Fuente J, et al. Zoonotic pathogens among white-tailed deer, northern Mexico, 2004-2009. Emerg Infect Dis 2012;18:1372-1374. [ Links ]
31. Ojeda-Chi M, Rodriguez-Vivas RI, Esteve-Gasent MD, Pérez de León A, Modarellid J J, Villegas-Perez S. Molecular detection of rickettsial tick-borne agents in white-tailed deer (Odocoileus virginianus yucatanensis), mazama deer (Mazama temama), and the ticks they host in Yucatan, Mexico. Ticks Tick Borne Dis 2019;10:365-370. doi: 10.1016/j.ttbdis.2018.11.018 [ Links ]
32. Guzmán-Cornejo C, Robbins RG, Guglielmone AA, Montiel-Parra G, Rivas G, Pérez TM. The Dermacentor (Acari, Ixodida, ixodidae) of Mexico: Hosts, geographical distribution and new records. ZooKeys 2016;569:1-22. doi:10.3897/zookeys.569.7221. [ Links ]
33. Schwint ON, Knowles DP, Ueti MW, Kappmeyer LS, Scoles GA. Transmission of Babesia caballi by Dermacentor nitens (Acari: Ixodidae) is restricted to one generation in the absence of alimentary reinfection on a susceptible equine host. J Med Entomol 2008;45:1152-1155. doi:10.1603/0022-2585(2008)45[1152:TOBCBD]2.0.CO;2. [ Links ]
34. Nava S, Mangold J, Guglielmone AA. Field and laboratory studies in a Neotropical population of the spinose ear tick, Otobius megnini. Med Vet Entomol 2009;23:1-5. doi:10.1111/j.1365-2915.2008.00761.x [ Links ]
35. Cakabay T, Gokdogan O, Kocyigit M. Human otoacariasis : Demographic and clinical outcomes in patients with ear-canal ticks and a review of literature. J Otol 2016;11:111-117. doi:10.1016/j.joto.2016.06.003. [ Links ]
36. Niebuhr CN, Mays SE, Breeden JB, Lambert BD, Kattes DH. Efficacy of chemical repellents against Otobius megnini (Acari :Argasidae) and three species of ixodid ticks. Exp Appl Acarol 2014;64:99-107. doi:10.1007/s10493-014-9799-6. [ Links ]
37. Almada Resende JDS, Daemon E, De Olivera Montero CM, Maturano R, Prata DA, Rodrigues AFS. Toxicity of solvents and surfactants to Amblyomma cajennense (Fabricius, 1787) (Acari:Ixodidae) and Dermacentor nitens (Neumann, 1897) (Acari : Ixodidae) larvae. Exp Parasitol 2012;131:139-142. doi:10.1016/j.exppara.2012.03.002. [ Links ]
38. Diyes GCP, Rajakaruna RS. Seasonal dynamics of spinose ear tick Otobius megnini associated with horse otoacariasis in Sri Lanka. Acta Trop 2016;159:170-175. doi:10.1016/j.actatropica.2016.03.025. [ Links ]
39. Crosbie P, Goff W, Stiller D, Jessup D. The distribution of Dermacentor hunteri and Anaplasma sp. in desert bighorn sheep (Ovis canadensis). Med Vet Entomol 1997;83:31-37. [ Links ]
40. De la Fuente J, Atkinson MW, Hogg JT, Miller DS, Naranjo V, Almazán C, et al. Genetic characterization of Anaplasma ovis strains from bighorn sheep in Montana. J Wildl Dis 2006;42:381-385. doi:10.7589/0090-3558-42.2.381. [ Links ]
41. Stiller D, Crosbie PR, Boyce WM, Goff WL. Dermacentor hunteri (Acari: Ixodidae): an experimental vector of Anaplasma marginale and A. ovis (Rickettsiales:Anaplasmataceae) to calves and sheep. J Med Entomol 1999;36:321-324. [ Links ]
42. Yabsley MJ, Davidson WR, Stallknecht DE, Varela AS, Swift PK, Devos JC, et al. Evidence of tick-borne organisms in mule deer (Odocoileus hemionus) from the Western United States. Vector Borne Zoonotic Dis 2005;5:351-362. [ Links ]
43. Bloemer SR, Zimmerman RH, Fairbanks K. Abundance, attachment sites, and density estimators of lone star ticks (Acari:Ixodidae) infesting white-tailed deer. J Med Entomol 1988;25:95-300. doi:10.1093/jmedent/25.4.295. [ Links ]
44. L’Hostis M, Diarra O, Seegers H. Sites of attachment and density assessment of female Ixodes ricinus (Acari:Ixodidae) on dairy cows. Exp Appl Acarol 1994;18:681-689. doi:10.1007/BF00051535 [ Links ]
45. Coronel-Benedett KC, Ojeda-Robertos NF, Gonzalez-Garduño R, Martinez- Ibañez F, Rodriguez-Vivas RI. Prevalence , intensity and population dynamics of hard ticks (Acari :Ixodidae ) on sheep in the humid tropics of Mexico. Exp Appl Acarol 2018:74:99-105. [ Links ]
46. Kiffner C, Lödige C, Alings M, Vor T, Rühe F. Body-mass or sex-biased tick parasitism in roe deer (Capreolus capreolus). A GAMLSS approach. Med Vet Entomol 2011;25:39-44. doi:10.1111/j.1365-2915.2010.00929.x [ Links ]
47. Han S, Hickling GJ, Tsao JI. High Prevalence of Borrelia miyamotoi among adult blacklegged ticks from white-tailed deer. Emerg Infect Dis 2016;22: 22-24. [ Links ]
48. Pound JM, George JE, Kammlah DM, Lohmeyer KH, Davey RB. Evidence for role of white-tailed deer (Artiodactyla: Cervidae) in epizootiology of cattle ticks and Southern cattle ticks (Acari:Ixodidae) in reinfestations along the Texas/Mexico border in South Texas: A Review and Update. J Econ Entomol 2010;103:211-218. doi:10.1603/EC09359 [ Links ]
Received: February 26, 2019; Accepted: November 20, 2019











 texto en
texto en