Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.11 suppl.2 Mérida Mar. 2020 Epub June 30, 2020
https://doi.org/10.22319/rmcp.v11s2.4742
Reviews
Causes and consequences of climate change in livestock production and animal health. Review
a Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias-CENID-FyMA. Querétaro, Querétaro, México.
The accumulation of greenhouse gases in the Earth's atmosphere is causing an unprecedented climate change with serious implications, such as extreme weather events and changes in the function and composition of ecosystems. Due to its importance it is relevant to analyze the impact of climate change on livestock systems. An area that requires special attention is precisely animal health, the emergence and re-emergence of vector-borne diseases in numerous regions of the planet are a clear example of the association between climate change and its effects on the human/animal health interface. The effects on health animal can obey multiple social and environmental factors causing the so-called "diseases of production", which influence the appearance of emerging diseases. However, each region and each livestock system has its own vulnerabilities. These aspects must be taken into account for the design of local and regional risk maps, as well as for the efficient design, implementation and socialization of risk management processes for diseases.
Key words Climate change; Animal diseases; Livestock production; Adaptation measures
La acumulación de gases de efecto invernadero en la atmósfera terrestre está ocasionando un cambio climático con graves implicaciones, como fenómenos meteorológicos extremos, cambios en la función y composición de los ecosistemas. Debido a su importancia, resulta relevante analizar el impacto del cambio climático en los sistemas de producción pecuarios. Un área que requiere especial atención es precisamente la salud animal, la emergencia y reemergencia de enfermedades vectoriales en numerosas regiones del planeta constituye un claro ejemplo de asociación entre cambio climático y efectos sobre la interfaz de la salud humana/animal. Dichas afectaciones a la salud animal pueden obedecer a múltiples factores sociales y medioambientales, provocando las llamadas “enfermedades de la producción”, los cuales influencian la aparición de enfermedades emergentes. Sin embargo, cada región y cada sistema de producción tiene sus propias vulnerabilidades. Estos aspectos deben tomarse en cuenta para diseñar mapas de riesgos locales y regionales; así como diseñar, instrumentar y socializar eficientemente procesos de manejo de riesgos ante enfermedades.
Palabras clave cambio climático; enfermedades animales; producción pecuaria; medidas de adaptación
Introduction
The climate of planet Earth varies according to the epochs and the areas where the observed climate changes generally extend through long periods. However, in recent decades, these changes seem to have accelerated according to certain indicators, such as the increase in temperature, the reduction in the area of Arctic ice and of the continental glaciers, the rising of the mean global level of the ocean, and bio-indicators such as the displacement of the populations of terrestrial and marine animals; as well as the displacement of the stages of agricultural activities. Therefore, climate change goes far beyond global warming and its consequences. Climate change causes more profound implications, such as extreme weather, alteration of the water cycle, ocean acidification, and changes in the role and composition of ecosystems. This whole set of drastic changes causes the formation of destructive natural phenomena such as hurricanes, cyclones or tsunamis. It is predicted that these weather patterns will result in the spread or increase in prevalence of different animal and human diseases, as well as in the extinction of animal and plant species1,2. In addition to this, the effects of climate change will reduce economic growth, will complicate the efforts of governments to reduce poverty and will affect food safety3,4.
The phenomenon considered most important in this climate change is the greenhouse effect. It is originated by the energy coming from the sun, formed by waves of frequencies that pass through the atmosphere with ease, after which the energy transmitted outwards from the earth, being formed by waves of lower frequencies, is absorbed by greenhouse gases (GHGs), thus producing the greenhouse effect5. Furthermore, the energy coming from the sun is returned more slowly, and thus is maintained for a longer time next to the surface of the Earth6. The main GHG emissions associated with the phenomenon of global warming, are carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), hydrofluorocarbons (HFCS), perfluorocarbons (PFCS) and sulfur hexafluoride (SF6)6,7.
Greenhouse gases
Since the industrial revolution in the 18th century, and up until the present, the atmospheric composition of CO2, CH4 and N2or has exceeded the values that were given during the previous 10 000 years. The increase in their concentration has led to the absorption and re-emission of infrared radiation into the atmosphere and the surface of the earth, having generated an increase of the temperature by about 0.6°C during the 20th century. This trend has been attributed to the accumulation of CO2 and other greenhouse gases in the atmosphere derived from human activity8. CO2, for example, participates in the carbon cycle in nature; 1.012 t pass through the natural carbon cycle, in the process of photosynthesis. In addition, it has a collection of the radiation of up to 49 % and has an atmospheric lifetime of between 50 and 200 years9.
Thanks to international agreements such as the Montreal and Kyoto protocols, as well as the recent summits in Copenhagen and Cancun, as well as to the existence of governmental and non-governmental organizations around the world, many countries are taking actions aimed at the mitigation of GHG emissions. In this way, the first action was to determine the GHG inventory that each country emits considering their various socio-economic activities7. The International Protocol The Kyoto Protocol sets limits for the different greenhouse gases and establishes the commitment for developed countries to assess and quantify the concentrations of these gases, and, in particular, to develop techniques for reducing them.
The Intergovernmental Panel on Climate Change10 (IPCC) has established, through various working groups, models for calculating emissions, suggesting different emission factors according to the level of knowledge and data from each geographical area and agricultural and livestock production. Although these are merely estimates, this constitutes the only consensual model at the global level, because it allows making approximations of the emissions that can be used for comparative purposes between productive systems. In the particular case of Mexico, the inventory of GHG emissions before the United Nations Framework Convention on Climate Change (UNFCCC) is being carried out since 199711. In accordance with this inventory, as shown in Figure 1, the energy sector is the biggest emitter of GHG emissions (20 %), whereas in the case of agriculture and livestock production, it contributes only 6.4 % of the anthropogenic emissions of CH4, CO2 and N2O into the atmosphere. Reports of the Food and Agriculture Organization of the United Nations (FAO) classify the intensive production of cattle as the main contributor to environmental pollution6, enteric fermentation being one of the major sources of CH4. This process has a polluting potential of 23 to 30 times higher than CO2; for example, in the year 2014 it reached a maximum atmospheric concentration of 1.833 ppm, equivalent to 254% of its pre-industrial level12.
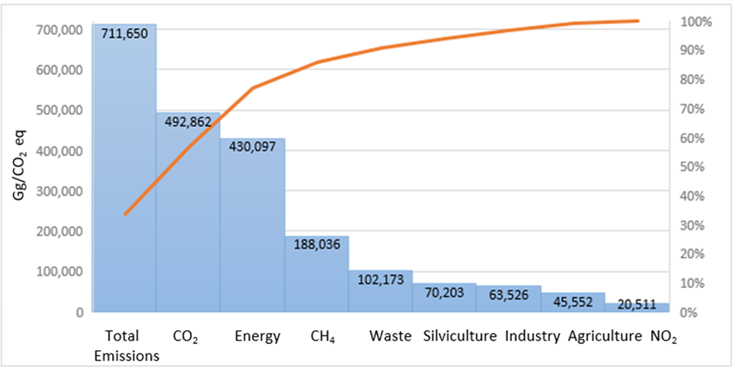
Source: Adapted from IPCC, 201710.
Figure 1: National inventory of greenhouse gas and compound emissions in Mexico
Due to the rapid increase in atmospheric concentrations of CH4 in recent years, as well as to the effects on the climate and on atmospheric chemistry, its emissions should be controlled and reduced11,13. As a result, ruminants are in first place of importance within the stockbreeding, since, in Mexico livestock contributes 84 % of the total CH4 issued by the livestock sector, of which 89 % is generated by the stabled beef and dual-purpose bovine cattle, 10 % by the milk-producing cattle, and 1 % by the rest of the farm animals14.
On the other hand, it is estimated that by the year 2050 the world food production will have to increase by 60 % in order to meet the increasing demand. This production will have to use current agricultural lands under the premise of producing more while using fewer natural resources; hence, the need to create eco-efficient environments for adaptation to climate change15.
Impact of climate change on livestock production
The livestock sector is facing a paradox. On the one hand, it is blamed for the generation of GHG emissions, according to data from the FAO15, since at the global level the production of beef and milk is responsible for the majority of the emissions, as it contributes with 41 and 29%, respectively, of the emissions of the sector. Pork and poultry eggs contribute 9 and 8% of the emissions of the sector. The production and processing of feed and enteric fermentation due to ruminant animals are the two main sources of emissions, responsible for 45 and the 39 % of the emissions of the sector. The storage and processing of manure contribute 10 %. The remaining part is attributed to the processing and transport of livestock products16. On the other hand, food production provides 40 % of the value of the world agricultural production and supports the livelihoods and food safety of almost 1.300 million people in the world17. In many developing countries, livestock is a multifunctional activity; beyond its direct role in the generation of food and income, livestock is a valuable asset, serving as a stock of wealth and a warranty for credits, and constituting an essential safety net in times of crisis18.
Due to its importance, it is relevant to analyze the impact of climate change on livestock production systems. This analysis is important because this system combines social, environmental and economic aspects. The effects of climate change will have a direct impact on the social organization of production units, on food safety and on human and animal health19. From a social perspective, taking into account the local specificities, the effects of climate change on agricultural production will depend, among other factors, on the type of system, which can be either intensive or extensive18.
Intensive systems get 90 % of the cattle feed from external systems; they engage in the production of a single species, driving high densities per surface area unit, and use balanced foods based on cereals; therefore, in these systems, land is not such an important factor as, for example, technology; their production is intended primarily for sale and does not use family labor20. On the contrary, extensive production systems are more closely tied to the natural conditions of the medium and use family labor, and their production is intended mainly for home consumption. The production units are small and run by families, and their economic logic is not to pursue the maximum profit, but rather to seek family welfare21. Therefore, these differences in production systems cause opposite impacts14.
In both production systems, the difference is due to several factors, including the unequal distribution of resources and conditions for the development and deployment of capabilities for decision-making, i.e., to how vulnerable a system is with regard to climate change22. In order to deal with the effects of climate change, adaptive measures are implemented that have to do with environmental, social and ecological adjustments. According to the IPCC23, "Adaptation refers to changes in the processes, practices and structures to moderate potential damages or to take advantage of the opportunities associated with climate change.”
Adaptation involves taking actions aimed at preserving the resilience and increasing the adaptive capacity of agro-ecosystems and the social actors in the agricultural sector18. In this sense, working on climate change adaptation strategies with a family producer is not the same as working with a producer who exports meat. It is estimated that small producers will be most affected, given their low access to technologies, inputs and monetary resources to adopt adaptive measures14,24,25.
For example, the impact of climate change on the extensive systems translates into reduced availability of food, a consequence of the decline in agricultural production and the inadequacy of conditions for a livestock production that requires large amounts of pasture land to maintain the cattle, which, in sum, results in a diet that is poor in nutrients for the most vulnerable populations. The conditions become all the more severe because the dependence of producers on the natural cycles of production, and even the geographic location of the lands where they dwell places them in a situation of vulnerability19.
Within this context, an aspect that requires special attention is related to animal health. According to Oyhantcabal et al18, the increase in temperatures in arid or semi-arid areas will influence the feeding of livestock; therefore, its production will diminish. This will result in a situation of physiological stress; in close relationship, problems of access to and need for water will appear, an inconvenient to be shared with humans. Thus, the absence of food and water can trigger diseases in the animals that affect their productivity. The emergence and reemergence of vector-borne diseases in many regions of the planet is a clear example of association between climate change and effects on the interface of human/animal health (12,26. In response to the intensified frequency of extreme events, the number of climate-related deaths and diseases may increase, since their effects on animal health may be due to multiple environmental factors that cause the so-called "production diseases"18,27.
Taking as reference the model of convergence to classify the factors that influence the emergence and reemergence of diseases, among a number of social and economic factors, the climate factor stands out28. According to Oyhantcabal et al18, the relationships can be simplified or can be broken down further, taking into consideration that the social and ecological factors interact with each other, instead of each one acting on its own. Certain scientists, like Black and Nunn29, refer to the system as complex, calling him socio-ecological system or eco-social approach of health. Studies investigating trends in emerging infectious diseases have confirmed that these are almost always caused by socio-economic, environmental and ecological factors, so that new approaches are required to supplement traditional methods30,31. It is important to specify that the purpose of the models is to help understand the relationships between the factors and to improve the capacity for adaptation and anticipation for the future29.
Thus, the socio-economic and environmental factors influence the occurrence of emerging diseases, which represent a threat to global health27,32,33. However, what is worrying is that the distribution of resources for surveillance measures is not risk-based but is related to the increased capacity and availability of resources in each country. However, each region and each production system has its own vulnerabilities; these aspects must be taken into account in designing maps of local and regional risks, as well as designing, implementing and efficiently socialize risk management processes in the face of diseases34.
Impact of climate change on animal health
There is a vast literature on the contribution of agricultural activities to the generation of GHG emissions and, hence, to climate change; however, the effects of climate change on animal diseases have received very little attention(31,33, 35-37), despite their direct relationship to poverty and their impact on public health. Animal diseases have always appeared and evolved, changing for various reasons; however, the rapid changes in habitat distributions can alter the behavior. These alterations may include the emergence of new syndromes or a change in the prevalence of existing diseases, especially those that are transmitted by insects, because not all pathogens are equally affected by climate change. For some species it can mean an increase in area of influence, while for other can mean a decrease32,38,39.
Climate change can affect infectious diseases through own factors of the pathogen, the vector, the guest, epidemiology and other indirect factors3,40. Microorganisms have the ability to mutate in order to adapt to environmental changes. For example, RNA (ribonucleic acid) viruses have high rates of mutation due to their rapid replication and lack of DNA correction (proof-reading) mechanisms33. Another example that illustrates this rapid adaptation to climate change was observed with the virus that causes the Venezuelan equine encephalitis in Mexico; a single amino acid substitution in a membrane glycoprotein allowed its adaptation to another vector, the Ochlerotatus (Aedes) taeniorhynchus mosquito41. This vector became more abundant in the regions of the Pacific coast of Mexico after the deforestation of 80 years destroyed the habitat of the Culex taeniopus mosquito, identified as one of the main vectors of the virus at that time41-43. Thus, climate change affects not only the geographical distribution and abundance of vectors, but also the interaction between the pathogen and the vector, through its transmission to new vectors. Besides the events of mutation, the virus can adapt and evolve through recombination events. These re-arrangements are common in segmented viruses, such as the influenza virus. In addition to this, climate change may reduce the available habitats, forcing the species to live in smaller areas. This favors the exchange of pathogens between animal species of various types, a phenomenon that favored the spread of the highly pathogenic avian influenza virus (H5N1)44.
Many animal diseases of importance are associated with insects and arthropods such as mosquitoes, flies and ticks, which serve as vectors. Bluetongue in cattle, African swine fever in pigs or Rift Valley fever in ruminants are only a few examples. Some diseases are not zoonotic, but their impact on the livestock industry can be devastating due to the loss of trade opportunities and to the costs of monitoring45. These diseases can reach new territories through the spread of the vector to new geographical areas. This is considered to have occurred in the case of the bluetongue virus in United Kingdom in the year 200627. The geographical distribution of the vectors is highly dependent on environmental variables such as temperature, humidity and wind. For example, the extrinsic incubation period, defined as the period between which a vector that is fuelled by a host is able to transmit the infection to another susceptible host, extends to low temperatures40. It has also been observed that the bluetongue virus is transmitted more efficiently by C. imicola at temperatures of 28 to 30 °C, being less efficient at temperatures close to 10 °C. In this way the warm temperatures favor the transmission of certain diseases42. In the same way, the feed rate of arthropods augments at higher temperatures, which increases the exposure of livestock to pathogens, favoring their dissemination21. The abundance of mosquitoes and midges is increased during periods of heavy rainfall that favor the formation of puddles or bodies of water that are ideal for oviposition. In Africa, for example, there have been outbreaks of Rift Valley fever in the warm phase of El Niño27. In particular, climate change may open territory that was previously uninhabitable for arthropod vectors, as well as increase the rate of reproducibility and stings (mosquitoes)/bites (ticks) and shorten the incubation period of pathogens3,33. Many arthropods that feed on blood, such as ticks, spend most of their life cycle in the environment. Their development, survival and population dynamics depend on factors such as the availability of a host, the vegetation and the climate, among others46-48.
It is clear that climate change alters, directly or indirectly, the distribution and incidence of a broad range of diseases. However, the complexity of the host-pathogen relationships and their interaction with the environment makes it difficult to accurately predict the occurrence or modification of these diseases30. An example illustrated by Gallana et al.49 demonstrates the complexity of the process: Arctic warming has allowed the white-tailed deer (Odocoileus virginianus) and moose (Cervus canadensis) to expand their territories to the north, so that they now coexist with the musk ox and the caribou. The white-tailed deer and the moose harbor parasites that are new to the ox and the caribou, and therefore they do not have a natural resistance to the new parasites, which renders them more susceptible. Now the musk ox and the caribou are being infected with new parasites and, in addition, they are dealing with a higher parasite load, due to the increase in temperature that favors the life cycle, threatening their survival49,50.
Tick-borne diseases and climate change
Ticks are the most important disease vectors after mosquitoes. They are hematophagous ectoparasites which feed on the blood of both animals and humans. This condition, gives them the ability to transmit a wide variety of pathogens such as viruses, bacteria and protozoa (flavivirus, erlichiosis, anaplasmosis, babesiosis, ricketsiosis, among others). Unfortunately, in Mexico there is no routine diagnosis of tick-borne diseases in animals or people; however, according to the Official Mexican Norm NOM-017-SSA2-201251, it is mandatory to notify the occurrence of spotted fever caused by Rickettsia rickettsii (R. rickettsii) in humans. Likewise, it is estimated that there are clinical cases of patients infected with Anaplasma and Ehrlichia chaffeensis, Ehrilichia canis52. Despite the risk, it has not yet been possible to control tick infestations and, therefore, the diseases they transmit; for this reason, those areas where this vector is distributed still entail a risk to animal and human health53. Recent evidence indicates that climate change has a direct or indirect effect in tick-borne diseases; the increase in temperature impacts their distribution and frequency. In addition to the effects of deforestation, land use change, among other factors, also have an impact on the hosts, the vectors and the pathogens54,55.
Some studies in Europe and the United States of America documented changes in the distribution of ticks associated with climate change. In Sweden, the expansion of the tick Ixodes ricinus has been reported in much of the territory56, but mainly in the north, where the distribution of the tick doubled in 26.8 % of the territory in a period of 18 yr. Another study performed in Russia reported an increase in the abundance of I. ricinus in the eastern region of that country57.
The ticks of the genus Ixodes are the primary vectors of Lyme disease in North America, and their distribution depends largely on climate changes58,59. The abiotic environment is crucial to their survival because much of their life cycle takes place in the vegetation; therefore, the climate is a determining factor in the distribution and establishment of tick populations59,60. Lyme disease is the main emerging zoonosis transmitted by ticks in the United States of America and Europe. In Mexico the first reports were associated to infection close to parks in the City of Mexico, La Marquesa and Nevado de Toluca; later, cases were reported in the states of Nuevo León and Tamaulipas61. To date, the distribution of ticks infected with Borrelia burgdorferi is very broad, covering regions from the Yucatan Peninsula and all the way to the north of the country. For this reason, it is considered that climate change will be of great importance in the distribution of this tick in future years61.
Temperature affects the activity of nymphs and adults of tick62; for example, the species Ixodes ricinus can survive in temperatures of 14.4 to 18.9°C for a period of exposure of 24 h. Considering the high degree of tolerance to low temperatures, it is believed that climate change could increase the niche of this and other ticks in Europe or in nearby areas26. Other species can withstand low temperatures, since they are well adapted to survival in sub-zero temperatures, as is the case of Dermacentor reticulatus, vector of canine babesiosis62. According to some reports, the adaptation of ticks to climate change will not be the same in all regions, as it will depend largely on the species concerned. Through the model of ecological niche for I. ricinus in Europe, an expansion of habitat of the 3.8% was predicted to occur throughout that continent. The expansion of the habitat would encompass Scandinavia among other regions, while there would be a reduction of habitats in the Alps, Italy and a part of Poland63. Climate change is also expected to affect the reproductive capacity of Ixodes scapularis in Canada and the United States of America1. The effect of climate change on tropical areas could adversely affect some species, affecting the optimum habitat and forcing them to colonize new places; in this way, it is estimated that the gradual increase in temperature will force the tropical bont tick, Ambylomma variegatum, to colonize new areas where there is prolonged drought in Zimbabwe64.
There are studies that correlate the presence of Mediterranean fever with an increase mediated by global warming in the number of tick bites in dogs65. Also, in the north of Russia climate change has been the catalyst for the expansion of the habitat of Ixodes persulcatus and for the incidence of tick-borne encephalitis66. In contrast, there are studies which indicate that, in spite of climate change, the distribution of some ticks will not be affected in a major way. In a predictive model using a maximum entropy approximation, by geographical correlation data and climatic variables, it was determined that the habitat for the distribution of I. scapularis infected with Borrelia burgdorferi between Texas and Mexico should remain relatively stable over the next 33 yr30. The inconsistency between these studies will give rise to the controversy over whether climate change will impact or not the vectors and the diseases they transmit31. Other studies, for example, mention that effects associated to climate change are undoubtedly involved in the increase of various diseases. The meta-analysis of more than 200 effects on 61 species of parasites suggests that a decrease in biodiversity may increase the human and animal diseases, as well as decrease agricultural and forestry production67. Thus, although the relationship between the rate of development of ticks and the temperature is not yet clear68, the influence of climate change not only in the redistribution of disease vectors but in the life of any organism that inhabits the earth is unquestionable69.
As a consequence of the adaptation of these vectors to new climates, the risk of infectious diseases transmitted by these vectors may be potentiated. A comprehensive understanding of the climatic effects requires multidisciplinary study that allows the analysis of the ecosystem of the pathogens and their vectors, in order to identify whether they have the potential to affect human and animal populations under a scenario of climate change. For this reason, mappings are being conducted at the National Laboratory of Genomics in Health (LANGESA) in Hidalgo, Mexico, for the purpose of determining the distribution and frequencies of the vectors and reservoirs in the country. This information will allow to determine the impact of climate change on the tick-borne diseases in Mexico.
Impact of climate change on other diseases
As mentioned, various animal diseases are affected by climate change, either directly or indirectly and vector-borne diseases are the most studied. However, diseases associated with flooding or standing water such as leptospirosis, anthrax, cryptosporidiosis, fascioliasis, among others, also require special attention35. Table 1 lists are some of them.
Table 1 Main animal diseases affected by climate change
| Classification | Disease | Causal agent | Vector | Zoonosis |
|---|---|---|---|---|
| Vector-borne diseases |
Bluetongue | Bluetongue virus (Orbivirus) | Culicoides midge | No |
| African horse sickness | Ahsv (Orbivirus) |
Culicoides midge Occasional transmission by mosquitoes (Culex, Anopheles, Aedes spp.) and ticks (Hyl, Rhipicephalus) has also been reported. |
No | |
| Rift Valley fever | Rift Valley fever virus (Phlebovirus) |
Mosquitoes (Aedes spp.) |
Yes | |
| West Nile virus infection |
West Nile Virus (Flavivirus) |
Mosquitoes (Culex spp.) |
Yes | |
| Venezuelan equine encephalitis |
Venezuelan equine encephalitis virus (Alphavirus) |
Mosquitoes (Aedes spp., Culex spp.) |
Yes | |
| Chagas Disease |
Trypanosoma cruzi |
Bed bugs of the subfamily Triatominae |
Yes | |
| Leishmaniasis | Protozoa of the genus Leishmania | Sandfly of the genus Lutzomyia |
Yes | |
| Several authors | Babesiosis | Protozoa of the genus Babesia |
Ticks of the genus Ixodes |
Yes |
| Dirofilariosis | Nematode Dirofilaria immitis. |
Mosquitoes (Aedes, Anopheles, Culex) | Yes | |
| Lyme Disease | Bacterium Borrelia burgdorferi |
Ticks of the genus Ixodes |
Yes | |
| Diseases associated with flooding or stagnant water. |
Anthrax | Bacteria, Bacillus anthracis |
Does not apply | Yes |
| Leptospirosis | Bacterium Leptospira interrogans |
Does not apply | Yes | |
| Cryptosporidiosis | Coccidia, Crystosporidium spp. |
Does not apply | Yes | |
| Fasciolasis | Fluke, Fasciola hepatica. |
Snails of the genus Lymnaea |
Yes |
The list of diseases in Table 1 aims to summarize those diseases that deserve special attention because of their impact on the public and livestock health. The increase in temperature, humidity and rainfall may increase the prevalence of vector-borne diseases. However, there are other diseases that can generate outbreaks associated with the increase of humidity by excessive rains or floods48. The temperature, relative humidity and soil moisture favor the germination of the spores of anthrax; while the heavy rains can activate them. Anthrax outbreaks have been associated with the alternation of heavy rains, drought and high temperatures39,70. Leptospirosis and cryptosporidiosis are diseases with epidemic potential after heavy rains71. Finally, the prevalence of diseases of global distribution like haemoncosis and fasciolasis may be increased; the larvae of Haemonchus contortus can survive for months on earth under appropriate conditions of temperature and humidity. Likewise, the formation of puddles or water bodies and the increase of rainfall favor the survival of the snail that transmits Fasciola hepatica. These diseases cause significant economic losses, due to the decrease in the production parameters of livestock. In addition to this, it is expected that the increase in the prevalence of these diseases will favor the development of resistance to antiparasitic drugs that will render them difficult to control.
Conclusions
Human influence on global warming is clear; recent climate changes require rethinking of the manner in which the stockbreeding sector is acting and implement more sustainable systems that will maintain the resilience of the cattle system; this will improve the supply of products and services derived from this industry, decreasing the impact on the environment and, consequently, on the emergence and reemergence of animal and human diseases. This implies a major challenge for developing countries that still have pending, among other things, the reduction of poverty in which an important part of its population lives. Therefore, it is clear that interventions aimed to promote and facilitate adaptation to climate change must not be divorced from social, cultural and health interventions.
Literatura citada
1. Ogden NH, Lindsay LR. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol 2016;32(8):646-656. https://doi.org/10.1016/j.pt.2016.04.015 [ Links ]
2. Tercera Comunicación Nacional de Cambio Climático ante la Convención Marco de las Naciones Unidas sobre Cambio Climático. IDEAM. Instituto de Hidrología, Meteorología y Estudios Ambientales. México 2017. http://documentacion.ideam.gov.co/cgibin/koha/opacdetail.pl?biblionumber=38147. [ Links ]
3. Patz JA, Epstein PR, Burke TA, Balbus JM. Global climate change and emerging infectious diseases. JAMA, 1996;275(3):217-223. [ Links ]
4. Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, et al. White LL. (eds.). IPCC: Climate Change 2014: Impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2014. [ Links ]
5. Rosenzweig C, Hillel D. Climate change and the global harvest: potential impacts of the greenhouse effect on agriculture. Oxford University Press; 1998:324. [ Links ]
6. Seinfeld JH, Pandis SN. Atmospheric chemistry and physics from air pollution to climate change. New York. John Wiley and Sons, Incorporated; 1998. [ Links ]
7. Protocolo de Kyoto de la Convención Marco de Las Naciones Unidas sobre el cambio climático. Naciones Unidas. 1998. [ Links ]
8. Hansen JE, Sato M. Trends of measured climate forcing agents. Proc Nat Acad Sci. United States of America 2001;98(26);14778-14783. [ Links ]
9. Watson RT, Rodhe H, Oeschger H, Siegenthaler U. Greenhouse gases and aerosols. In: Climate change: the IPCC Scientific Assessment. Houghton JT, et al, editors. Cambridge: Cambridge University Press; 1990. [ Links ]
10. IPCC (Intergovernmental Panel on Climate Change) 2016. https://www.ipcc.ch/ . Consultado 10 Feb, 2017. [ Links ]
11. Convención Marco de las Naciones Unidas sobre el Cambio Climático y su Protocolo de Kioto. (CMNUCC ) (CMNUCC ) https://www.gob.mx/semarnat/acciones-y-programas/convencion-marco-de-las-naciones-unidas-sobre-el-cambio-climatico-y-su-protocolo-de-kioto-cmnucc?idiom=es Consultado 7 Feb, 2017. [ Links ]
12. FAO. Food and Agriculture Organization of the United Nations. La ganadería a examen. Estado mundial de la agricultura y la alimentación. Roma. 2009. http://www.fao.org/docrep/012/i0680s/i0680s.pdf. [ Links ]
13. OMM (Organización Meteorológica Mundial). 2015. Boletín sobre los gases de efecto invernadero. https://www.wmo.int/media/es/content/las-concentraciones-de-gases-de-efecto-invernadero-vuelven-batir-un-r%C3%A9cord . Consultado 4 Feb, 2017. [ Links ]
14. FAO. Food and Agriculture Organization of the United Nations. La larga sombra del ganado: problemas ambientales y opciones. Roma. 2009. http://www.fao.org/docrep/011/a0701s/a0701s00.htm. [ Links ]
15. FAO. Food and Agriculture Organization of the United Nations. Tackling Climate Change Through Livestock. A global assessment of emissions and mitigation opportunities Roma. 2013. http://www.fao.org/docrep/018/i3437e/i3437e00.htm. [ Links ]
16. Grain. Campo y crisis climática. Soberanía Alimentaria. Biodiversidad y Culturas. Barcelona. 2010. [ Links ]
17. Barbier EB. Agricultural expansion, resource booms and growth in Latin America: Implications for long-run economic development. World Develop 2004;32(1):137-157. [ Links ]
18. Oyhantçabal W, Vitale E, Lagarmilla P. El cambio climático y su relación con las enfermedades animales y la producción animal. En: Compendio de los temas técnicos presentados ante la Asamblea mundial de los delegados o a las Comisiones regionales de la OIE-2009, Paris: Organización Mundial de Sanidad Animal (OIE); 2010:169-177. [ Links ]
19. Lorente-Saiz A. Ganadería y cambio climático: una influencia recíproca. GeoGraphos. Revista Digital para Estudiantes de Geografía y Ciencias Sociales 2010;1(3):1-22. http://web.ua.es/revista-geographos-giecryal. [ Links ]
20. Bravo-Ortega C, Lederman D. Agriculture and national welfare around the world: Causality and international heterogeneity since 1960. World Bank Policy Research Working Paper. 2005. [ Links ]
21. Ardila A, Wilson V. El sector pecuario frente al cambio climático: una realidad incómoda. Rev Cienc Anim 2012;(5):107-120. [ Links ]
22. Oswald Ú. Cambio climático, conflictos sobre recursos y vulnerabilidad social. En Delgado GC, Gay C (coordinadores). México frente al cambio climático. Retos y oportunidades, UNAM, México. 2010:51-83. [ Links ]
23. IPCC. Intergovernmental Panel on Climate Change Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland. 2014. http://www.ipcc.ch/report/ar5/wg3/ [ Links ]
24. Fischer G, Shah M, Tubiello FN, Van-Velhuizen H. Socio-economic and climate change impacts on agriculture: an integrated assessment, 1990-2080. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 2005;360(1463):2067-2083. https://doi.org/10.1098/rstb.2005.1744. [ Links ]
25. Mendelsohn R. The impact of climate change on agriculture in developing countries. J Nat Resour Policy Res 2008;1(1):5-19. https://doi.org/10.1080/19390450802495882 [ Links ]
26. Porretta D, Mastrantonio V, Amendolia S, Gaiarsa S, Epis S, Genchi C, et al. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasites & Vectors 2013;(6):271. https://doi.org/10.1186/1756-3305-6-271. [ Links ]
27. Lubroth J. Climate change and animal health. En: FAO: Building resilience for adaptation to climate change in the agriculture sector. Roma. 2012:63-70. [ Links ]
28. King L. What is one health and why is it relevant to food safety? Workshop Improving Food Safety Through One Health, Forum on Microbial Threats; Washington, DC: Institute of Medicine; 2011. [ Links ]
29. Black P, Nunn M. Repercusiones de los cambios climáticos y medioambientales en las enfermedades animales emergentes y reemergentes y en la producción animal. Conf. OIE 2009:27-39. [ Links ]
30. Feria-Arroyo TP, Castro-Arellano I, Gordillo-Pérez G, Cavazos AL, Vargas-Sandoval M, Grover A, et al. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasites & Vectors 2014:7-199. https://doi.org/10.1186/1756-3305-7-199 [ Links ]
31. Gilbert L. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia 2010:162 (1):217-225. https://doi.org/10.1007/s00442-009-1430-x [ Links ]
32. Shuman EK. Global climate change and infectious diseases. New England J Medicine 2010;362(12):1061-1063. https://doi.org/10.1056/NEJMp0912931 [ Links ]
33. Shope R. Global climate change and infectious diseases. Environmental Health Perspectives 1991;(96):171-174. [ Links ]
34. McBean G. Climate change: Global risks, challenges and decisions. Eos Trans. AGU, 2012;93(18):182. [ Links ]
35. Colwell DD, Dantas-Torres F, Otranto D. Vector-borne parasitic zoonoses: emerging scenarios and new perspectives. Vet Parasitol 2011;182(1):14-21. https://doi.org/10.1016/j.vetpar.2011.07.012. [ Links ]
36. Mills JN, Gage KL, Khan AS. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environment Health Perspect 2010;118(11):1507-1514. https://doi.org/10.1289/ehp.0901389 [ Links ]
37. Cumming GS. Comparing climate and vegetation as limiting factors for species ranges of African ticks. Ecology 2002;83(1):255-268. https://doi.org/10.2307/2680136. [ Links ]
38. Wu X, Lu Y, Zhou S, Chen L, Xu B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environment Int 2015;86:14-23. [ Links ]
39. Rossati A. Global warming and its health impact. Int J Occupat Environment Med 2017;8(1-963):7-20. [ Links ]
40. Baylis M, Githeko AK. The effects of climate change on infectious diseases of animals. Report for the Foresight Project on Detection of Infectious Diseases, Department of Trade and Industry. UK: UK Government. 2006. [ Links ]
41. Brault AC, Powers AM, Ortiz D, Estrada-Franco JG, Navarro-Lopez R, Weaver SC. Venezuelan equine encephalitis emergence: enhanced vector infection from a single amino acid substitution in the envelope glycoprotein. Proc Nat Acad Sci. United States of America 2004;101(31),11344-11349. https://doi.org/10.1073/pnas.0402905101. [ Links ]
42. Gale P, Drew T, Phipps LP, David G, Wooldridge M. The effect of climate change on the occurrence and prevalence of livestock diseases in Great Britain: a review. J Appl Microbiol 2009;106(5):1409-1423. https://doi.org/10.1111/j.1365-2672.2008.04036.x [ Links ]
43. Lindahl JF, Grace D. The consequences of human actions on risks for infectious diseases: a review. Infection Ecology & Epidemiology 2015:5. https://doi.org/10.3402/iee.v5.30048 [ Links ]
44. Forrest HL, Webster RG. Perspectives on influenza evolution and the role of research. Anim Health Res Rev 2010;11(1):3-18. https://doi.org/10.1017/S1466252310000071 [ Links ]
45. Thornton PK, Steeg J. Van de Notenbaert A, Herrero M. The impacts of climate change on livestock and livestock systems in developing countries: A review of what we know and what we need to know. Agric Syst 2009;01(3):113-127. http://dx.doi.org/10.1016/j.agsy.2009.05.002 [ Links ]
46. Dantas-Torres F, Figueredo LA, Otranto D. Seasonal variation in the effect of climate on the biology of Rhipicephalus sanguineus in southern Europe Parasitology 2011;138(4): 527-536. https://doi.org/10.1017/S0031182010001502 [ Links ]
47. Estrada-Peña A, Gray JS, Kahl O, Lane RS, Nijhof AM. Research on the ecology of ticks and tick-borne pathogens--methodological principles and caveats. Frontiers Cellular Infection Microbiol 2013;3:29. https://doi.org/10.3389/fcimb.2013.00029 [ Links ]
48. Jore S, Vanwambeke SO, Viljugrein H, Isaksen K, Kristoffersen AB, Woldehiwet Z, et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasites & Vectors 2014;7:11. https://doi.org/10.1186/1756-3305-7-11 [ Links ]
49. Gallana M, Ryser-Degiorgis MP, Wahli T, Segner H. Climate change and infectious diseases of wildlife: Altered interactions between pathogens, vectors and hosts. Current Zoology 2013;59(3):427-437. https://doi.org/10.1093/czoolo/59.3.427 [ Links ]
50. Black PF, Butler CD. One Health in a world with climate change. Revue Scientifique et Technique (International Office of Epizootics) 2014;33(2):465-473. [ Links ]
51. Secretaría de Salud.2013 Norma Oficial Mexicana NOM-017-SSA2-2012, para la vigilancia epidemiológica. [ Links ]
52. Sosa-Gutiérrez CG, Solórzano-Santos F, Walker DH, Torres J, Serrano CA, Gordillo-Pérez G. Fatal monocytic ehrlichiosis in woman, México, 2013. Emerg Infect Diseases 2016;22(5):871-874. https://doi.org/10.3201/eid2205.151217 [ Links ]
53. De la Fuente J, Kocan KM. Strategies for development of vaccines for control of ixodid tick species. Parasite Immunol 2006;28(7):275-283. https://doi.org/10.1111/j.1365-3024.2006.00828.x [ Links ]
54. Parham PE, Waldock J, Christophides GK, Hemming D, Agusto F, Evans KJ, et al. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Philosoph Transact Royal Soc of London. Series B, Biological Sci 2015;370:(1665). https://doi.org/10.1098/rstb.2013.0551 [ Links ]
55. Medlock JM, Leach SA. Effect of climate change on vector-borne disease risk in the UK. Lancet Infectious Diseases 2015;15(6):721-730. https://doi.org/10.1016/S1473-3099(15)70091-5. [ Links ]
56. Jaenson TG, Hjertqvist M, Bergström T, Lundkvist Å. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden(a). Parasites & Vectors 2012;5:184. https://doi.org/10.1186/1756-3305-5-184 [ Links ]
57. Korotkov Y, Kozlova T, Kozlovskaya L. Observations on changes in abundance of questing Ixodes ricinus, castor bean tick, over a 35-year period in the eastern part of its range (Russia, Tula region). Med Vet Entomol 2015;29(2):129-136. https://doi.org/10.1111/mve.12101 [ Links ]
58. Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Medic Entomol 1998;35(5):629-638. [ Links ]
59. Bertrand MR, Wilson ML. Microclimate-dependent survival of unfed adult Ixodes scapularis (Acari:Ixodidae) in nature: life cycle and study design implications. J Medic Entomol 1996;33(4):619-627. [ Links ]
60. Fish D. Population ecology of Ixodes damini. In: Ecology and environmental management of Lyme disease. New Brunswick, NJ: Rutgers University Press; 1993:25-42. [ Links ]
61. Gordillo-Pérez G, Vargas M, Solórzano-Santos F, Rivera A, Polaco OJ, Alvarado L, et al. Demonstration of Borrelia burgdorferi sensu stricto infection in ticks from the northeast of Mexico. Clinical Microbiol Infection: European Soc Clinical Microbiol Infect Diseases 2009;15(5):496-498 https://doi.org/10.1111/j.1469-0691.2009.02776.x [ Links ]
62. Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, et. al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites & Vectors 2013;6(1):1. https://doi.org/10.1186/1756-3305-6-1 [ Links ]
63. Boeckmann M, Joyner, TA. Old health risks in new places? An ecological niche model for I. ricinus tick distribution in Europe under a changing climate. Health & Place 2001;30:70-77. https://doi.org/10.1016/j.healthplace.2014.08.004. [ Links ]
64. Estrada-Peña A, Horak IG, Petney T. Climate changes and suitability for the ticks Amblyomma hebraeum and Amblyomma variegatum (Ixodidae) in Zimbabwe (1974-1999). Vet Parasitol 2008;151(2-4):256-267. https://doi.org/10.1016/j.vetpar.2007.11.014 [ Links ]
65. Parola P, Socolovschi C, Jeanjean L, Bitam I, Fournier PE, Sotto A, et al. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl Trop Dis. 2008;2(11):e338. [ Links ]
66. Tokarevich NK, Tronin AA, Blinova OV, Buzinov RV, Boltenkov VP, Yurasova ED, Nurse J. The impact of climate change on the expansion of Ixodes persulcatus habitat and the incidence of tick-borne encephalitis in the north of European Russia. Glob Health Action. 2011;4:8448. doi: 10.3402/gha.v4i0.8448. [ Links ]
67. Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc Natl Acad Sci USA. 2015;112(28):8667-71. Doi: 10.1073/pnas.1506279112. [ Links ]
68. Estrada-Peña A, Ayllon N, De la Fuente J. Impact of climate trends on tick-borne pathogen transmission. Frontiers Physiol 2012;3:64. https://doi.org/10.3389/fphys.2012.00064 [ Links ]
69. Shevenell AE, Ingalls AE, Domack EW, Kelly C. Holocene Southern ocean surface temperature variability west of the Antarctic Peninsula. Nature 2011; 470(7333):250-254. https://doi.org/10.1038/nature09751 [ Links ]
70. Parker R. Anthrax and livestock. Guide B-120. En Cooperative Extension Service, College of Agriculture and Home Economics. Las Cruces, Nuevo Mexico. University of New Mexico. 2002. [ Links ]
71. McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence 2015;6(6):543-547. https://doi.org/10.4161/21505594.2014.975022 [ Links ]
Received: January 08, 2018; Accepted: June 04, 2018











 text in
text in 


