Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.11 no.4 Mérida oct./dic. 2020 Epub 02-Mar-2021
https://doi.org/10.22319/rmcp.v11i4.5344
Articles
Genetic improvement of aerial alfalfa biomass and its components: half-sib family selection
a Colegio de Postgraduados. Postgrado en Recursos Genéticos y Productividad. Carretera México-Texcoco km. 36.5, Montecillo, Texcoco, Estado de México, México.
In this study, it was evaluated the genetic variation in aerial biomass (BM) or dry matter yield (DMY) and its components in 400 alfalfa half-sib families (HSF), derived from direct (DC, San Miguel x Oaxaca) and reciprocal crosses (RC, Oaxaca x San Miguel), and the original varieties (SM, San Miguel; O, Oaxaca). The experiment was performed in pots under outdoor conditions in Montecillo, Texcoco, Estado de México, Mexico. Complete plants were cut at a 5 cm height, every five weeks in the fall-winter of 2014-2015, and every four weeks in the spring-summer of 2015. The DMY, AGR (absolute growth rate), RUE (radiation use efficiency), NT (number of tillers per plant), and PH (plant height) were 32, 31, 32, 6, and 36 % higher in DC. The DMY, AGR, RUE, and PH were 30, 28, 30, and 34 % higher in RC than the mean of SM and O varieties. The selection allowed the identification of 13 and 17 % of HSF outstanding in DMY and its components in DC and RC. The DMY of the outstanding HSF of DC was 11% higher than the DMY of the outstanding HSF of RC, indicating maternal genetic effects.
Key words Maternal genetic effects; Heritability; Selection; Dry matter
Se estudió la variación genética en biomasa aérea (BM) o rendimiento de materia seca (RMS) y sus componentes en 400 familias de medios hermanos (FMH) de alfalfa, derivadas de las cruzas directa (CD, San Miguel x Oaxaca) y recíproca (CR, Oaxaca x San Miguel), y las variedades originales (SM, San Miguel y O, Oaxaca). El experimento se realizó en macetas en condiciones de intemperie en Montecillo, Texcoco, Estado de México, México. Se hicieron cortes de plantas completas a 5 cm de altura, cada cinco semanas en otoño-invierno 2014-2015 y cada cuatro semanas en primavera-verano 2015. El RMS, TAC (tasa absoluta de crecimiento), EUR (eficiencia en el uso de la radiación), NT (número de tallos por planta) y AP (altura de planta) fueron 32, 31, 32, 6 y 36 % más altos en la CD, y el RMS, TAC, EUR y AP fueron 30, 28, 30 y 34 % más altos en la CR que la media de SM y O. La selección permitió identificar 13 y 17 % de FMH sobresalientes en RMS y sus componentes en las CD y CR. El RMS de las FMH sobresalientes de la CD fue 11 % mayor que el RMS de las FMH sobresalientes de la CR, indicando la presencia de efectos genéticos maternos.
Palabras clave Efectos genéticos maternos; Heredabilidad; Selección; Materia seca
Introduction
Alfalfa (Medicago sativa L.) is a polymorphic species with wide genetic variability that adapts to various soil and climatic conditions. Alfalfa heritability is complex, mostly because of its autotetraploid meiosis. This species produces a diploid gamete (2n=32), which profoundly affects its phenotypic behavior. Due to its allogamy, alfalfa depends on insects for its pollination and produces some autosterile or autoincompatible plants, and at a lower proportion, plants that produce sterile pollen and ovules1.
The primary aim of selecting new alfalfa varieties is to maximize forage yield with optimal nutritional value, without quality-detrimental compounds, and with high field persistence and minimum use of fertilizers, pesticides, or herbicides2. Forage quality has been rarely included in selection programs; however, given its importance in livestock production, there is interest in obtaining cultivars with high forage quality3. The leaf:stem ratio is an indicator of forage quality due to its positive association with digestibility and forage consumption4, which results from a greater digestibility of leaves in relation to stems5. The selection of high-yield and high-quality forage, using the accumulation of dry matter in the aerial organs and the leaf:stem ratio, could facilitate the identification of genotypes with higher yield and quality in a phenotyping platform under controlled conditions.
The complexity of the hereditary mechanisms of alfalfa and its autotetraploid nature make it difficult to choose the best selection method. However, identifying alleles of individual genetic traits in the phenotype is easier using individual selection methods than inter- or intra-population methods, where the gene flow from one population to another is open and less controlled; also, these methods require a significant number of plants and lower frequencies of favorable genes6. Family selection allows to evaluate the genotype of each plant; the seed of the selected plants is harvested in an open pollinated or polycross population; the seed of each selected plant is kept separately and sown in replicated progeny tests; the worst families are eliminated, and the best families are crossed with each other to allow recombination and production of subsequent generations6.
Due to its polymorphic nature, the wide variability in alfalfa yield and its components offers excellent selection opportunities. However, although alfalfa's genetic variability favors selection by forage yield, in some cases, the phenotypic expression can be inhibited by a low heritability due to non-additive genetic effects3. In alfalfa, the high impact of non-additive genetic variation in forage yield is attributed to the high intra-locus interaction caused by its autotetraploid nature (which also includes tri- and tetra-allelic interactions) and the interactions of complementary genes that involve favorable alleles with additive effects in linkage blocks7. By eliminating non-additive genetic effects, the evaluation is based on additive effects and, therefore, on heritable genetic effects, which can manifest in highly productive genotypes with superior additive alleles2,8,9. However, the complexity of genetic inheritance, selection could be performed with different strategies, such as paternity testing in diverse germplasm, introgression of quantitative traits, and genomic selection10.
In Mexico, the germplasm in commercial use includes traditional low productivity varieties and new, generally introduced, varieties with low field adaptation and persistence. The new challenges posed by climate change and the increasing demand for high-quality forage require new germplasm with greater adaptation to stressful environments, productivity, forage quality, and durability under field conditions. This study aimed to evaluate the variability of aerial biomass or dry matter yield and its components in 400 half-sib families derived from the segregating populations of San Miguel x Oaxaca and Oaxaca x San Miguel, and the original populations of San Miguel and Oaxaca, to perform the first familiar selection cycle in pots under outdoor conditions.
Material and methods
Localization
The experiment was performed in plastic pots during the fall-winter of 2014-2015 and the spring-summer of 2015 at Colegio de Postgraduados, Texcoco, Estado de México (19° 29’ N, 98° 54’ W, 2,250 masl). The climate is humid subtropical (Cb(wo)(w)(i´)g), with rains during summer, annual precipitation of 637 mm, and temperature of 15 °C11.
Plant material
It was used a total of 200 half-sib families (HSF) from the commercial varieties of San Miguel and Oaxaca (certified seeds from Casa Cobos S.A. de C.V., Central de Abastos, Ciudad de México, Mexico) and 200 HSF from the segregating populations of San Miguel (female progenitor) x Oaxaca (male progenitor) (direct cross) and Oaxaca (female progenitor) x San Miguel (male progenitor) (reciprocal cross). The seeds from segregating populations were obtained by the genetic cross between the San Miguel and Oaxaca varieties (direct cross) and the Oaxaca and San Miguel varieties (reciprocal cross), using pollinator insects (bees) under field conditions (Figure 1). The San Miguel and Oaxaca varieties were chosen for the crossings due to their notorious durability under field conditions in an experiment designed to study the productive behavior of five commercial varieties of alfalfa (San Miguel, Oaxaca, Moapa, Valenciana, and Cuf-101) during March 200012, this experiment was conducted until the winter-spring cycle of 2006 at Colegio de Postgraduados, Montecillo, Texcoco, Estado de México.
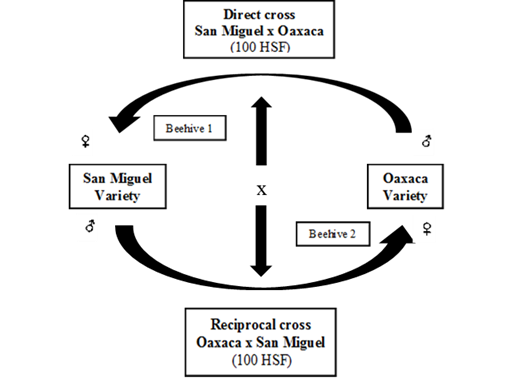
Figure 1: Genetic crossing system between two alfalfa varieties using pollinator insects under field conditions. Montecillo, Municipio de Texcoco, Estado de México
Seed production took place in the period from January to May 2006. Seeds were harvested from all the plants in each cross's population and used to establish the first family selection cycle6. A styrofoam seed tray with 100 cells was used for each population. Five seeds of the same size were sown I n each cell. The soil used had a sandy loam texture with 41.6 % of field capacity (FC), 28.2 % of permanent wilting point (PWP), 9.3 % of organic matter , 0.019 % of nitrogen, 4.8 ppm of phosphorus, 4 mmol L-1 of potassium, 1.5 dS m-1 of electric conductivity, and pH of 6.9. Seeds were sown on June 6, 2014; when the seedlings presented the first trifoliate leaf (15 days after sowing, das), the most vigorous seedling was chosen from each cell, and it was transplanted into a plastic pot with a soil capacity of 3 kg. The HSF of each population were randomly assigned to the plastic pots in a completely randomized experimental design. It was fertilized with the 60-140-00 dose at 15 and 240 das, with urea as a nitrogen source and calcium triple superphosphate as a phosphorus source. At 98 das, a uniformization cut was made in the plants. During the experiment, soil humidity was maintained close to FC by applying water every other day.
Variables
Cuts were made every five weeks in the fall-winter 2014-2015 period and every 4 wk in the spring-summer 2015 period from day 98; the cuts were made 5 cm above ground level. The plant's morphological composition was evaluated in a subsample of four complete secondary tillers from each plant and cut in all the HSF, separating the leaves (leaflets and petioles) from the tillers. The subsample and the rest of the secondary tillers were dried at 65 °C until constant weight and were then weighted.
Plant height (PH, cm) was measured in all the HSF before the cutting with a 1 m long wooden ruler (graduated in cm) from the soil surface to the stem apex. The number of secondary tillers (NT) per plant was determined in each cut in all the HSF. The dry matter yield (DMY, g of DM plant-1) or aerial biomass was obtained by adding the dry weight of the subsample of the four stems and the dry weight of the rest of the plant. The leaf:stem ratio (L:S) was calculated with the dry weight data from the leaves (LDW) and secondary tillers (TDW) (L:S = LDW / TDW). The absolute growth rate (AGR, g of DM plant-1 d-1) was calculated by dividing the DMY by the number of days (t) elapsed between one cut and the next (AGR = DMY/t). The radiation use efficiency (RUE, g of DM MJ-1) was calculated by dividing the DMY by the amount of photosynthetically active radiation (PAR, MJ MJ m-2 d-1) accumulated (PAR= incident global radiation (cal cm-2 d-1) x 0.5 x 0.04148) between one cut and the next (RUE= DMY / PAR)13. The incident global radiation data were obtained from the meteorological station of the Universidad Autónoma Chapingo, Chapingo, Estado de México, located 4 km away from the experiment location.
Air temperature and rain
The maximum (TM) and minimum (Tm) air temperature data were recorded daily at 0800 h with a Six's thermometer (Taylor, model 5458P) placed 2 m above ground level. The TM ranged from 23.5 to 29.9 °C and the Tm from 4.0 to 12.6 °C during the experiment. Daily rainfall data were determined with a weekly accumulation rain gauge placed next to the plants. The total rain precipitation was 1,042 mm; the lowest levels occurred from November 2014 to January 2015; this did not affect plant growth as plants were irrigated as necessary.
Statistical analysis
The analysis of variance for all variables was performed using the Windows software SAS, Version 9.114 and the statistical model:
Y ijk = µ + Pop i + Fam(Pop) ik + Cutoff j + (Pop*Cutoff) ij + Cutoff*Fam(Pop) ijk + E ijk ;
Where:
Y ijk represents the value of the response variable in the population i of the cutoff level j and k of the HSF;
µ is the general mean, Pob i is the Population effect at level i = 1, 2, 3, and 4;
Fam(Pop) ik is the effect of population nested half-sib families at level i and k;
Cutoff j is the effect of the cutoff date at level j = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and 11;
(Pop*Cutoff) ij is the effect of the Population x Cutoff interaction at level i and j;
Cutoff*Fam(Pop) ijk is the effect of the Cutoff x population nested HSF interaction at level i, j, k;
E ijk is the experimental error.
The Cutoff*Fam(Pob) ijk component could not be separated from the error because there was no source of variation for replications in the analysis of variance.
Genetic and environmental effects
Genetic and environmental effects were calculated through phenotypic variance (
Results and discussion
Selection parameters
Family selection increased 32, 31, 32, 6, and 36 % the DMY, AGR, RUE, NT, and PH in the San Miguel x Oaxaca population; and 30, 28, 30, and 34 % the DMY, AGR, RUE, and PH in Oaxaca x San Miguel compared to the progenitors’ mean. Meanwhile, the L:S ratio decreased 48 % in San Miguel x Oaxaca and 51 % in Oaxaca x San Miguel (Table 1). The gain in DMY observed in this study was higher than that reported in progeny testing; the HSF and CSF (full-sib families) derived from open pollination (OP1) of a diallel cross (F1) produced only 8 and 9 % higher yield of green matter and 5 and 12 % higher number of tillers per plant than the original varieties18. The results of familial selection versus mass selection in alfalfa have indicated similar percentages for DMY (16 and 14 %) and raw fiber (-1.6 vs -1.5 %)19.
Table 1 Selection parameters and determination of the selection pressure (p) for dry matter yield (DMY), absolute growth rate (AGR), radiation use efficiency (RUE), leaf:stem ratio (L:S), number of tillers (NT), and plant height (PH) in two segregating populations. 2014 -2015 cycle
| Variables | Populations | µ1 | µ2 | D | σf | i | p |
|---|---|---|---|---|---|---|---|
| DMY (g of DM plant-1) | San Miguel x Oaxaca | 8.03 | 5.45 | 2.58 | 1.58 | 1.63 | 13% |
| Oaxaca x San Miguel | 7.78 | 5.45 | 2.33 | 1.58 | 1.47 | 17% | |
| AGR (g of DM plant-1 d-1) | San Miguel x Oaxaca | 0.26 | 0.18 | 0.08 | 0.05 | 1.6 | 13% |
| Oaxaca x San Miguel | 0.25 | 0.18 | 0.07 | 0.05 | 1.4 | 17% | |
| RUE (g of DM MJ-1) | San Miguel x Oaxaca | 0.84 | 0.57 | 0.27 | 0.17 | 1.59 | 13% |
| Oaxaca x San Miguel | 0.81 | 0.57 | 0.24 | 0.17 | 1.41 | 17% | |
| L:S | San Miguel x Oaxaca | 0.97 | 1.44 | -0.47 | 0.21 | -2.24 | - |
| Oaxaca x San Miguel | 0.95 | 1.44 | -0.49 | 0.21 | -2.33 | - | |
| NT | San Miguel x Oaxaca | 18 | 17 | 1 | 5.05 | 0.2 | - |
| Oaxaca x San Miguel | 17 | 17 | 0 | 5.05 | 0 | - | |
| PH (cm) | San Miguel x Oaxaca | 58 | 37 | 21 | 5.08 | 4.13 | - |
| Oaxaca x San Miguel | 56 | 37 | 19 | 5.08 | 3.74 | - |
µ1= mean of the 100 HSF derived from San Miguel x Oaxaca and Oaxaca x San Miguel; µ2 = mean of the 200 HSF derived from the original varieties San Miguel and Oaxaca; D = selection differential; (f = phenotypic standard deviation, i = selection intensity; p = selection pressure.
The values obtained for the selection parameters (D, σf, i, and p) indicated that 13 % of the HSF derived from San Miguel x Oaxaca and 17 % of the HSF derived from Oaxaca x San Miguel had higher DMY, AGR, and RUE per plant than the rest of the HSF (Table 1). The mean (µ1) of DMY, AGR, and RUE for the 13 San Miguel x Oaxaca families and the 17 Oaxaca x San Miguel families was higher than the mean (µ2) for the HSF derived from the original varieties of San Miguel and Oaxaca. However, the L:S ratio, number of tillers, and plant height behaved differently than the DMY. The mean L:S ratio of San Miguel x Oaxaca and Oaxaca x San Miguel HSF was lower than the mean of San Miguel and Oaxaca. The mean NT of the HSF derived from San Miguel x Oaxaca was greater than the mean of San Miguel and Oaxaca. The mean NT of Oaxaca x San Miguel HSF was similar to the mean of San Miguel and Oaxaca. The mean PH of the HSF derived from the two segregating populations was much higher than the mean in San Miguel x Oaxaca (Table 1).
The intrapopulation individual selection is effective in identifying superior genotypes using progeny testing6. The high average values of the L:S ratio, NT, and PH observed in the segregating populations did not allow the detection of outstanding HSF for these plant characteristics (Table 1).
Dry matter yield and its components
The analysis of variance detected 13 outstanding HSF, based on their DMY, AGR, and RUE, in the San Miguel x Oaxaca population (Table 2) and 17 HSF in the Oaxaca x San Miguel population (Table 3); this research also present data for the five families with the lowest DMY in these populations (Table 2 and 3). The 13 outstanding HSF of the San Miguel x Oaxaca population produced 53 % higher DMY than the average on the original varieties and 82 % higher DMY than the mean of the HSF with the lowest yield (Table 2). The outstanding HSF derived from the Oaxaca x San Miguel population produced 47 and 68 % higher DMY than the mean of the original varieties and the families with the lowest yield, respectively (Table 3).
Table 2 Dry matter yield (DMY), absolute growth rate (AGR), and radiation use efficiency (RUE) of the statistically superior HSF and the five HSF with the lowest yield derived from San Miguel x Oaxaca, 11 successive cutoffs on average. 2014 -2015 cycle
| Family number | Genealogy | DMY | AGR | RUE |
|---|---|---|---|---|
| Statistically superior HSF | ||||
| 282 | Family-82 | 13.8 | 0.45 | 1.45 |
| 264 | Family-64 | 12.1 | 0.39 | 1.26 |
| 268 | Family-68 | 11.9 | 0.39 | 1.22 |
| 260 | Family-60 | 11.7 | 0.38 | 1.22 |
| 212 | Family-12 | 11.7 | 0.38 | 1.21 |
| 201 | Family-1 | 11.6 | 0.38 | 1.21 |
| 253 | Family-53 | 11.2 | 0.36 | 1.17 |
| 300 | Family-100 | 11.1 | 0.36 | 1.16 |
| 248 | Family-48 | 10.9 | 0.36 | 1.14 |
| 252 | Family-52 | 10.9 | 0.35 | 1.15 |
| 219 | Family-19 | 10.9 | 0.36 | 1.14 |
| 250 | Family-50 | 10.9 | 0.36 | 1.13 |
| 237 | Family-37 | 10.8 | 0.36 | 1.13 |
| Mean | 11.5 | 0.38 | 1.20 | |
| Lowest yield HSF | ||||
| 276 | Family-76 | 2.5 | 0.08 | 0.26 |
| 266 | Family-66 | 2.5 | 0.08 | 0.26 |
| 257 | Family-57 | 2.2 | 0.07 | 0.24 |
| 220 | Family-20 | 2.1 | 0.07 | 0.22 |
| 272 | Family-72 | 2.1 | 0.07 | 0.21 |
| Mean | 2.3 | 0.07 | 0.24 | |
| Standard deviation (σf) of the 200 half-sib families derived from Oaxaca and San Miguel |
1.6 | 0.05 | 0.17 | |
| Mean (µ2) of the 200 half-sib families derived from San Miguel and Oaxaca |
5.4 | 0.18 | 0.57 | |
DMY (g DM plant-1); AGR (g DM d-1); RUE (g DM MJ-1).
Table 3 Dry matter yield (DMY), absolute growth rate (AGR), and radiation use efficiency (RUE) of the statistically superior HSF and the five HSF with the lowest yield derived from Oaxaca x San Miguel, 11 successive cutoffs on average. 2014 -2015 cycle
| Family number | Genealogy | DMY | AGR | RUE |
|---|---|---|---|---|
| Statistically superior HSF | ||||
| 646 | Family-46 | 11.4 | 0.37 | 1.21 |
| 619 | Family-19 | 11.1 | 0.37 | 1.16 |
| 694 | Family-94 | 11.0 | 0.35 | 1.15 |
| 661 | Family-61 | 10.7 | 0.35 | 1.11 |
| 631 | Family-31 | 10.4 | 0.34 | 1.09 |
| 639 | Family-39 | 10.3 | 0.33 | 1.08 |
| 697 | Family-97 | 10.3 | 0.34 | 1.08 |
| 605 | Family-5 | 10.2 | 0.33 | 1.07 |
| 690 | Family-90 | 10.2 | 0.33 | 1.07 |
| 630 | Family-30 | 10.1 | 0.33 | 1.06 |
| 652 | Family-52 | 10.1 | 0.33 | 1.06 |
| 649 | Family-49 | 9.9 | 0.33 | 1.02 |
| 685 | Family-85 | 9.8 | 0.32 | 1.02 |
| 673 | Family-73 | 9.7 | 0.32 | 1.01 |
| 609 | Family-9 | 9.7 | 0.31 | 1.01 |
| 623 | Family-23 | 9.6 | 0.31 | 1.00 |
| 682 | Family-82 | 9.5 | 0.31 | 1.00 |
| Mean | 10.2 | 0.33 | 1.07 | |
| Lowest yield HSF | ||||
| 687 | Family-87 | 4.2 | 0.13 | 0.43 |
| 688 | Family-88 | 4.2 | 0.14 | 0.42 |
| 683 | Family-83 | 2.8 | 0.09 | 0.29 |
| 678 | Family-78 | 2.7 | 0.09 | 0.27 |
| 641 | Family-41 | 2.6 | 0.09 | 0.26 |
| Mean | 3.3 | 0.11 | 0.26 | |
| Standard deviation (σf) of the 200 half-sib families derived from Oaxaca and San Miguel |
1.6 | 0.05 | 0.17 | |
| Mean (µ2) of the 200 half-sib families derived from San Miguel and Oaxaca |
5.4 | 0.18 | 0.57 | |
DMY (g DM plant-1); AGR (g DM d-1); RUE (g DM MJ-1).
The DMY of the outstanding HSF derived from the San Miguel x Oaxaca (direct cross) population (Table 2) was 11 % higher than the DMY of the outstanding HSF derived from the Oaxaca x San Miguel (reciprocal cross) population (Table 3). However, the San Miguel x Oaxaca HSF with the lowest yield produced 30 % lower DMY (Table 2) than the Oaxaca x San Miguel HSF with the lowest yield (Table 3).
The different genetic behavior, regarding DMY and its components, between the HSF derived from direct (San Miguel x Oaxaca) (Table 2) and reciprocal (Oaxaca x San Miguel) (Table 3) crosses could indicate the presence of DNA molecules in cytoplasmic organelles (chloroplasts and mitochondria), also known as cytoplasmic inheritance or maternal genetic effects, that express in reciprocal crosses by showing different results with respect to the direct cross16. The selection of the outstanding progeny derived from parental populations with cytoplasmic genetic effects on DMY inheritance is important to maximize genetic gains in productivity20.
The outstanding San Miguel x Oaxaca HSF exhibited a higher AGR= 0.2 g of DM d-1 than the progenitors’ mean and 0.3 g of DM d-1 than the HSF with the lowest DMY (Table 2). The outstanding Oaxaca x San Miguel HSF showed a higher AGR= 0.15 g of DM d-1 than the mean of San Miguel and Oaxaca and an AGR= 0.22 g of DM d-1, higher than the mean of the HSF with the lowest DMY (Table 3). The AGR differences between the outstanding HSF and those with the lowest DMY were similar to the ones reported in other studies12. The AGR is an important component of the DMY. In a previous study, the San Miguel, Oaxaca, and Moapa varieties produced a higher seasonal AGR and DMY than the Cuf-101 and Valenciana varieties in Montecillo, Texcoco, Edo. de México12. Additionally, AGR is significantly related to the accumulation of dry matter and the leaf area index under field conditions; this is not the case for the number of leaves per stem21.
The RUE of the outstanding San Miguel x Oaxaca HSF was 52 % higher than the average of the original varieties and 82% higher than the mean of the HSF with the lowest DMY (Table 2). Similarly, the RUE of the outstanding Oaxaca x San Miguel HSF was 47 % higher than the progenitors’ mean and 78 % higher than the mean of the HSF with the lowest DMY (Table 3). Radiation use efficiency (RUE) determines the plant's capacity to capture solar radiation and transform it into biomass. Several studies have determined that the RUE of alfalfa is approximately 1.13 g of DM MJ-1 in biomass production22. The average RUE increased linearly from 0.60 to 1.60 g of DM MJ-1 as the air temperature increased from 6 to 18 °C23.
Regarding PH, NT, and L:S ratio, it is important to consider their importance for DMY and forage quality in the selection of new varieties since, in this study, the selection by high DMY resulted in tall plants with a low L:S ratio and NT. However, it was identified some HSF with high DMY, L:S ratio (HSF-201), and NT (HSF-219, HSF-250, HSF-260, and HSF-282) in the San Miguel x Oaxaca population (Table 2) and high DMY and L:S ratio (HSF-652, HSF-673, and HSF-685), and NT (HSF-605, HSF-623, HSF-631, HSF-639, HSF-652, HSF-673, HSF-685, and HSF-690) in the Oaxaca x San Miguel population (Table 3). The selection by DMY and other plant morphological traits depends on the number of genes that control the trait of interest (additive, dominant, or epistatic genes); qualitative traits (controlled by one or few genes) are easier to select than quantitative traits (controlled by numerous genes). It is also important to consider the heritability level and negative genetic interactions that by improving one genetic trait inhibit the expression of another, as well as the appropriate general steps such as the aims, variability creation/collection, selection, evaluation, and release of cultivars, use of methods and techniques based on the species reproduction (autogamy, allogamy, or clonal propagation)24.
Genetic, environmental, and heritability effects
The genetic variance, for DMY and its components, was higher in the direct cross (San Miguel x Oaxaca) than in the reciprocal cross (Oaxaca x San Miguel); the values of genetic variance for the San Miguel and Oaxaca varieties were intermediate between both crosses (Table 4). Genetic variance derives from the contribution of segregating genes and their interactions with other plant genes; therefore, the effective selection of genetically superior individuals requires that: 1) the phenotypic variation is appropriate in the original population, and 2) the heritability is sufficiently high for an effective selection. Overall, an increase in heritability and phenotypic variance is considered to increase genetic gain through selection25.
Table 4 Genetic (
| Variables | San Miguel | San Miguel x Oaxaca | Oaxaca | Oaxaca x San Miguel | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
h2b |
|
|
h2b |
|
|
h2b |
|
|
h2b | |
| DMY | 2.9 | 4.4 | 0.40 | 5.8 | 7.1 | 0.45 | 1.5 | 4.0 | 0.27 | 2.9 | 7.8 | 0.27 |
| AGR | 0.003 | 0.01 | 0.34 | 0.006 | 0.01 | 0.42 | 0.001 | 0.01 | 0.21 | 0.003 | 0.01 | 0.23 |
| RUE | 0.03 | 0.05 | 0.41 | 0.06 | 0.08 | 0.43 | 0.02 | 0.04 | 0.28 | 0.03 | 0.09 | 0.27 |
| L:S | 0.04 | 0.06 | 0.41 | 0.02 | 0.02 | 0.51 | 0.04 | 0.07 | 0.35 | 0.01 | 0.02 | 0.36 |
| NT | 18.2 | 37.4 | 0.33 | 31.9 | 29.0 | 0.52 | 11.6 | 37.5 | 0.24 | 10.5 | 33.1 | 0.24 |
| PH | 20.1 | 55.2 | 0.27 | 45.1 | 40.0 | 0.53 | 21.8 | 49.0 | 0.31 | 30.9 | 29.5 | 0.51 |
The DMY, NT, and PH showed greater genetic variance than the other yield components, both in the crosses and in the progeny varieties. One of the major aspects of genetic improvement programs is the heritability (h 2) of useful plant traits present in the available genetic variability. In the direct cross, heritability ranged from moderate to high in DMY and its components and from low to moderate in the reciprocal cross. PH was the exception; this trait showed a greater heritability than the other traits (Table 4). Heritability measures the genotype contribution to the total phenotypic variance, which can theoretically vary from zero, when there is no genetic variation, to one, when the total variation is genetic in origin. However, the previously described broad-sense heritability (h 2 b) must be distinguished from narrow-sense heritability (h 2 n), which represents the quotient between the additive variance, instead of the total genetic variance, and the phenotypic variance26.
The DMY and forage quality, represented by the L:S ratio, are primary objectives for alfalfa improvement. The heritability for DMY, L:S ratio, and the rest of the growth components was higher in San Miguel x Oaxaca than in Oaxaca x San Miguel. However, the data confirm genotypes with high DMY, L:S, and remaining growth components in both populations (Tables 2 and 3). These results differ from those obtained for DMY and L:S ratio in alfalfa HSF and lines (S1), in which forage yield and L:S ratio were independent due to the lack of L:S variability in the materials selected because of their high DMY9. However, these results indicate that the selection allowed the identification of HSF with high values for DMY and other desirable traits, such as L:S ratio, NT, AGR, and RUE (Tables 2 and 3), which may favor a continuous response to selection26.
The combination of the nuclear gene inherited traits that result from the additive genetic variance and the cytoplasmic inheritance or maternal genetic effects, due to the presence of DNA molecules in mitochondria and chloroplasts, can contribute to maximize the genetic gain in DMY and increase the selection efficiency in alfalfa and other cultivated species with maternal genetic effects expressed in the progenies phenotype. The breeder hardly needs to deal with genetic traits carried by one or the other of said organelles; however, sometimes, these organelles can be carriers of exceptional important traits for genetic improvements, such as the cytoplasmic male sterility that resides in the mitochondria27.
Conclusions and implications
The selection allowed the identification of some outstanding HSF with high DMY and L:S ratio in both populations; these HSF could be used to form a new synthetic variety or broad genetic base population to continue with subsequent selection cycles. The direct cross (San Miguel x Oaxaca) showed higher genetic variance and heritability for DMY and its components than the reciprocal cross (Oaxaca x San Miguel). The outstanding HSF derived from the direct cross produced higher DMY than the HSF derived from the reciprocal cross, which indicates that the San Miguel variety used as a female progenitor has a better genetic behavior than the Oaxaca variety progenies. In the future, obtaining new and improved varieties of alfalfa could be more practical by making direct and reciprocal crosses in breeding programs to identify the progenitor with the best behavior as female cytoplasm, that allows maximizing dry matter yield through selection.
Literatura citada
1. Busbice TH, Hill RR (Jr.), Carnahan HL. Genetics and breeding procedures. In: Hanson CH editor. Alfalfa science and technology. American Society of Agronomy. Number 15, Series Agronomy. Madison, WI, USA;1972:283-318. [ Links ]
2. Brummer EC, Bouton JH, Casler MD, McCaslin MH, Wadron BL. Grasses and legumes: Genetics and plant breeding. In: Wedin WF, Fales SL editors. Grassland: Quietness and strength for a new American agriculture. Am Soc Agron. Madison WI, USA;2009:157-171. [ Links ]
3. Annicchiarico P, Barrett B, Brummer EC, Julier B, Marshall AH. Achievements and challenges in improving temperate perennial forage legumes. Crit Rev Plant Sci 2015;34(1-3):327-380. [ Links ]
4. Lemaire G, Allirand JM. Relation entre croissance et qualité de la luzerne: interaction génotype-mode d’ exploitation. Fourr 1993;134:183-198. [ Links ]
5. Marten GC, Buxton DR, Barnes RF. Feeding value (Forage quality). In: Hanson AA, et al editors. Alfalfa and alfalfa improvement. Agronomy Monograph 29, ASA, Madison, WI. USA;1988:463-491. Doi:10.2134/agronmonogr29.c14. [ Links ]
6. Rumbaugh MD, Caddel JL, Rowe DE. Breeding and quantitative genetics. In: Hanson AA, et al editors. Alfalfa and alfalfa improvement. Agronomy Monograph 29, ASA, Madison, WI. USA;1988:777-808. Doi:10.2134/agronmonogr29.c25. [ Links ]
7. Bingham ET, Groose RW, Woodfield DR, Kidwell KK. Complementary gene interactions in alfalfa are greater in autotetraploids than diploids. Crop Sci 1994;34(4):823-829. [ Links ]
8. Katepa-Mutondwa FM, Christie BR, Michaels TE. An improved breeding strategy for autotetraploid alfalfa (Medicago sativa L.). Euphytica 2002;123(1):139-146. [ Links ]
9. Annicchiarico P. Alfalfa forage yield and leaf/stem ratio: narrow-sense heritability, genetic correlation and parent selection procedures. Euphytica 2015;205(2):409-420. Doi:10.1007/s10681-015-1399-y. [ Links ]
10. Li X, Brummer EC. Applied genetics and genomics in alfalfa breeding. Agron 2012;2:40-61. Doi:10.3390/agronomy2010040. [ Links ]
11. García E. Modificaciones al sistema climático de Köppen. 5a. ed, Instituto de Geografía. Serie de libros No. 6. Universidad Nacional Autónoma de México. México, DF. México;2004. [ Links ]
12. Rivas-Jacobo MA, López-Castañeda C, Hernández-Garay A, Pérez-Pérez J. Efecto de tres regímenes de cosecha en el comportamiento productivo de cinco variedades comerciales de alfalfa (Medicago sativa L.). Téc Pecu Méx 2005;43(1):79-92. [ Links ]
13. The international system of units (SI) - Conversion factors for general use. In: Butcher K, Crown L, Gentry EJ, Hockert C editors. Weights and Measures Division, Technology Services, NIST, Special Publication 1038. Department of Commerce, USA;2006. [ Links ]
14. SAS. The SAS System release 9.1 for windows. Institute Inc., Cary, North Carolina, United States. 2009. [ Links ]
15. Márquez SF. Genotecnia vegetal. Tomo I. México, DF: AGT Editor, SA; 1992. [ Links ]
16. Hartl DL, Freifelder D, Snyder LA. Cytoplasmic inheritance. In: Basic genetics. Jones and Bartlett Publishers, Inc. Portola Valley, CA, USA;1988:179-193. [ Links ]
17. Falconer DS. Introducción a la genética cuantitativa. Traducción de Fidel Márquez Sánchez, PhD. C.E.C.S.A. México, DF; 1984. [ Links ]
18. Milić D, Katić S, Boćanski J, Karagić Đ, Mikić A, Vasiljević S. Importance of progeny testing in alfalfa breeding (Medicago sativa L.). Genetika 2010;42(3):485-492. [ Links ]
19. Bakheit BR, Ali MA, Helmy AA. Effect of selection for crown diameter of forage yield and quality components in alfalfa (Medicago sativa L.). Asian J Crop Sci 2011;3(2):68-76. Doi:10.3923/ajcs.2011.68.76. [ Links ]
20. Rebetzke GJ, Richards RA, López-Castañeda C. Nuclear and maternal gene action affect selection for early vigour in wheat. In: Langridge P, et al editors. Proc. Aust Plant Breed Conf. Adelaide, South Australia; 1999:146-147. [ Links ]
21. Villegas-Aparicio Y, Hernández-Garay A, Pérez-Pérez J, López-Castañeda C, Herrera-Haro JG, Enríquez-Quiroz JF, et al. Patrones estacionales de crecimiento de dos variedades de alfalfa (Medicago sativa L.). Téc Pecu Méx 2004;42(2):145-158. [ Links ]
22. Khaiti M, Lemaire G. Dynamics of shoot and root growth of lucerne after seeding and after cutting. Europ J Agron 1992;1(4):241-247. [ Links ]
23. Brown HE, Moot DJ, Teixeira EI. Radiation use efficiency and biomass partitioning of lucerne (Medicago sativa) in a temperate climate. Europ J Agron 2006;25(4):319-327. [ Links ]
24. Aquaah G. Conventional plant breeding principles and techniques. In: Al-Khayri J, et al editors. Advances in plant breeding strategies: Breeding, biotechnology and molecular tools 2015:115-158. Doi:10.1007/978-3-319-22521-0_5. [ Links ]
25. Brewbaker JL. Agricultural genetics. New Jersey, United States of America: Prentice-Hall, Inc.; 1964. [ Links ]
26. Hill J, Becker HC, Tigerstedt PMA. Quantitative and ecological aspects of plant breeding. London, England: Chapman and Hall; 1998. [ Links ]
27. Cubero JI. Introducción a la mejora genética vegetal. 3.a ed. España: Ediciones Mundi-Prensa; 2013. [ Links ]
Received: April 16, 2019; Accepted: September 27, 2019











 texto en
texto en 


