Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.11 no.3 Mérida jul./sep. 2020 Epub 05-Feb-2021
https://doi.org/10.22319/rmcp.v11i3.5019
Articles
Effect of the internal size of the hive on brood, honey, and pollen production in Apis mellifera colonies in the central Mexican plateau
a Universidad Autónoma del Estado de Hidalgo. Centro de Investigaciones Biológicas. Km 4.5 carretera Pachuca-Tulancingo s/n, 42184, Hidalgo, México.
This study aimed to analyze the effect of the internal size on the strength (brood area) and productivity (honey and pollen areas) of the honeybee hives during the winter season in a semiarid region of the central Mexican plateau. Four Jumbo hive frames (45 x 28 cm) were used inside brood chambers with three internal sizes (52.2, 42.3, and 23.9 L), each chamber contained the same number of honeybees. Simultaneously, it was recorded the temperature inside the hives to determine if the brood chamber temperatures varied with the volume. The Jumbo hive, which is the largest hive, is the most used in the central Mexican plateau and showed the lowest strength and productivity values, as well as the lowest internal temperature. These results show that Jumbo hives can decrease the honey and pollen productivity for the central Mexican plateau beekeepers, which is why it is necessary to implement a practice or mechanism that allows maintaining strong beehives for the spring harvest.
Key words Apis mellifera; Hive; Brood chamber; Winter; Temperature; Honey
El objetivo del estudio fue analizar el efecto del tamaño interno de la colmena sobre la fortaleza (superficie con cría) y reservas de alimento (superficie con miel y polen) de abejas melíferas en la temporada invernal en una región semiárida del altiplano central mexicano. Se utilizaron cuatro bastidores de colmenas tipo Jumbo (45 x 28 cm) dentro de cámaras de cría con tres tamaños internos (52.2, 42.3 y 23.9 L), que contenían el mismo número de abejas melíferas. De manera simultánea se registraron las temperaturas dentro de las colmenas para determinar si la temperatura de la cámara de cría variaba con el volumen. La colmena de mayor tamaño, que corresponde al tipo Jumbo y la más utilizada en el altiplano central mexicano, presentó los valores más bajos de fortaleza y reservas de alimento, así como la menor temperatura interna. Estos resultados muestran que la utilización de colmenas tipo Jumbo puede repercutir en una disminución de la productividad de miel y polen para los apicultores del altiplano central de México, por lo que es necesario implementar alguna práctica o mecanismo que permita mantener las colonias de abejas fuertes para la cosecha de primavera.
Palabras clave Apis mellifera; Colmena; Cámara de cría; Invierno; Temperatura; Miel
Introduction
The climate plays an important role in the activity and behavior of social insects1. For example, the flight activity of Apis mellifera has a positive linear response to the ambient temperature from 14 to 22 °C2,3, and above 22 °C, the foraging activity decreases until it stops at 35 °C3. The egg-laying by the queen bee of this species starts at 24 °C, and at around 33 °C it reaches its maximum capacity, subsequently decreasing4. Additionally, the choice of flowers by the honey bee depends on several factors, mainly the floral availability, that is, it depends on the plant species whose flowering coincides with the foraging period, and this flowering depends on the climatic conditions5,6.
Bees storage large amounts of honey and pollen to provide energy and proteins to the brood, as well as for thermoregulation purposes, which allows maintaining the brood nest temperature between 32 and 36 °C for the correct development of larvae7,8, despite having external temperatures that fluctuate between -20 and 48 °C. To maintain a stable internal temperature, bees employ active and passive mechanisms. Among the active mechanisms, physical activity can increase (e.g., contraction of wing muscles) and decrease (e.g., wing-fanning) the temperature1,7,8. Passive mechanisms include brood nest selection and moving the brood to regions with a more favorable temperature1,8.
Overall, A. mellifera wild bees choose a brood nest based on different characteristics like the size, height, entrance orientation, and internal volume9,10,11. For example, A. mellifera worker bees choose brood nests with volumes between 15 and 100 L, although the most recurring size is 35 L11. The characteristics that bees choose are important as the brood nest protects against adverse temperatures and provides thermal stability for bees8,12,13. In beekeeping, humans determine the place and size of the brood nest; this space is known as a hive14. In Mexico, in general, beekeepers use two types of technified hives: Jumbo and Langstroth15. The internal space of the brood chamber of these hives is 52.1 and 41.7 L, respectively16.
The technified hives have mobile structures that allow increasing the space for egg-laying by the queen, increasing or decreasing the size of the colony when the external environmental factors are adverse17. Therefore, the proper management of the space can influence the survival of the colonies during critical times13,18.
Recently, multiple changes in meteorological events have propitiated erratic gales and ground frosts, which does not favor optimal conditions for the development of bee flora19,20,21. Therefore, it is necessary to evaluate how the internal space of the hives used in Mexico affects the maintenance of populations during winter (when temperatures drop and bees must warm the brood nest using active mechanisms), and determine the effect of the hive size on bees and some of their products such as honey and pollen, under the specific conditions of each apicultural region. Therefore, this study aimed to determine the effect of the internal size of the hive on the production of offspring and the storage of honey and pollen, as well as the internal temperature of the brood nest in A. mellifera colonies during winter.
Material and methods
Area of study
This study was performed in the locality of Huitzila, Tizayuca municipality, in the state of Hidalgo (19°47’50’’ - 19°53’50’’ N; 99°02’ - 98°54’ W) at an altitude of 2,260 masl22 within the central Mexican plateau. This region has a humid subtropical climate with rainfall during summer, an annual mean temperature of 15.1 °C, and annual mean precipitation of 627 mm. The coldest month is January, with minimum and mean temperatures of 1.4 and 11.5 °C, respectively; the warmest month is May, with minimum and mean temperatures of 8.7 and 17.8 °C, respectively23.
Preparation of bees
On December 2016, at the end of the nectar and pollen flows, a total of 10 colonies showing a homogeneous development were selected, from these colonies we extracted approximately 120,000 adult bees24 for the experiment, from which 10,000 worker bees were assigned homogeneously to each of the 12 colonies used in the experiment. The number of bees was determined by immobilizing them in a growth chamber (Shel Lab, model LI15) at -2 °C for 10 min.
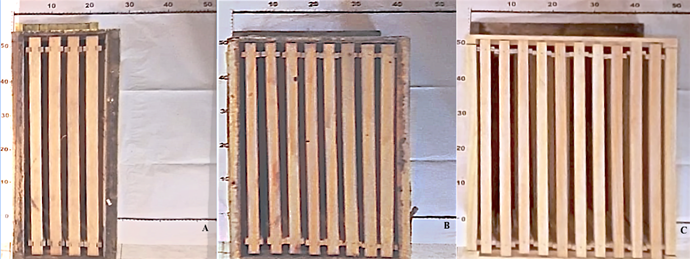
Each of the 12 groups of 10,000 bees was placed inside 12 hives with different internal volumes and the capacity to house several Jumbo type frames (45 x 28 cm) (Figure 1). It was used four hives with external measurements of 51 x 30 x 21 cm to house four frames, four hives with external measurements of 51 x 30 x 34 cm to house eight frames, and four 51 x 30 x 41 cm hives to house 10 frames; the internal volumes were 23.9 L (experimental hive), 42.3 L (Langstroth hive), and 52.2 L (Jumbo hive), respectively. The hives were built of 2 cm thick pine wood and treated with paraffin. To each colony of 10,000 bees, we placed four frames with wax foundation and a newly fertilized queen bee from the same breeding stock and from the same batch of a heterogeneous mixture of the Italian and Carniolan species, which allowed the colonies to start with the same initial conditions (queen bees were sisters). Each hive was supplied with 600 ml of syrup with a sugar to water ratio of 1:1 and 150 g of a protein supplement (Apitir plus, Tirtécnica company) every eight days25,26. The supply of syrup and protein supplement was maintained throughout the experiment.
Experiment
Four replications were used for each hive size, giving a total of 12 experimental units arranged in a completely randomized design, at a distance of 2 m between rows and 1 m between hives in the same row17. The internal temperature of the hives was recorded using 24 data loggers (Thermochron iButton, model DS1921G). In each hive, two data loggers were placed in the third frame, one in the center and the second in the head to determine if the internal temperature varied with the brood chamber volume. Additionally, the ambient temperature in the shade was recorded using 12 dataloggers. Temperatures were recorded every 60 min during the experiment.
The experiment started the first week of December and ended on April 28, at the beginning of flowering. The brood, honey, and pollen areas were quantified using a plastic laminate grid in centimeters24 (Figure 2). The number of adult bees was not determined in order to maintain social cohesion24. The brood, honey, and pollen areas on both sides of each frame were recorded on only three dates (February 2, March 7, and April 28) to preserve social cohesion24.
Statistical analysis
A one-way analysis of variance (ANOVA) was used to determine if there are significant differences between treatments (hive type) in the brood, honey, and pollen areas for each sampling date. The mean temperatures at the center and head throughout the experiment were also compared with an ANOVA, and assumptions were verified using the Kolmogorov-Smirnov test for normality and the Levene test for homogeneity of variances, and if not met, a non-parametric ANOVA (Kruskal-Wallis) was used. Turkey's multiple comparisons test was applied when significant differences were obtained (P˂0.05). The statistical analyses were performed with the statistical program SigmaStat 3.527. Average ± Standard Error is reported.
Results
The brood area of A. mellifera on the first sampling date (February 2) was 1125.73 ± 136.65 cm2 for the 23.9 L hives, 1,016.75 ± 364.64 cm2 for the 42.3 L hives, and 1,398.63 ± 334.67 cm2 for the 52.2 L hives; these values were not significantly different from each other (F=0.44, g.l.=2,11, P>0.05) (Figure 3). The brood area of A. mellifera on the second sampling date (March 7) was 1,610.25 ± 83.37 cm2 for the 23.9 L hives, 1,654.75 ± 473.37 cm2 for the 42.3 L hives, and 1,692.75 ± 68.03 cm2 for the 52.2 L hives; these values were not significantly different from each other (H =0.50, g.l.=2, P>0.05). The brood area was significantly different between the hive types at the end of the experiment (April 28) (F=23.88, g.l.=2,11, P˂0.001). The brood area in hives with an internal volume of 23.9 L was on average 3,077.25 ± 81.81 cm2, and in the 42.3 L hives, 2,906.2 ± 94.6 cm2; both values were significantly higher than the value in the colonies developed in 52.2 L hives, whose brood area was 2,331.2 ± 59.5 cm2 (P˂0.01). The brood area in the 23.9 L hives was not significantly different from that in the 42.3 L hives (P>0.05).
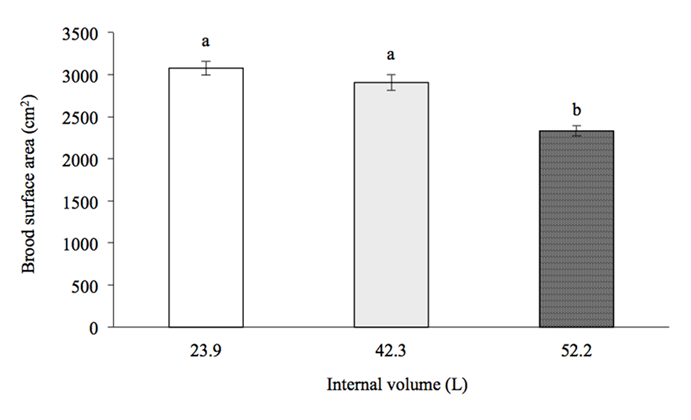
ab Values with different letters indicate significant differences (P˂0.05).
Figure 3 Brood area (average ± standard error) in three hive sizes at the end of the study period
The honey area on the first sampling date was 980.38 ± 263.64 cm2 for the 23.9 L hives, 952.75 ± 201.76 cm2 for the 42.3 L hives, and 992.50 ± 93.93 cm2 for the 52.2 L hives (F=0.01, g.l.=2,11, P>0.05) (Figure 4). The honey area on the second sampling date was 905.75 ± 198.38 cm2 for the 23.9 L hives, 465.25 ± 167.03 cm2 for the 42.3 L hives, and 621.50 ± 184.88 cm2 for the 52.2 L hives (F =1.48, g.l.=2,11 P>0.05). The honey area at the end of the study period was significantly different between the hive types (F=8.80, g.l.=2,11, P˂0.01). The average of the honey area in the 23.9 L hives (1,424 ± 56.9 cm2) was significantly higher than that in the 52.2 L hives (849.5 ± 94.4 cm2, P˂0.05), but it did not differ significantly from the value in the 42.3 L colonies (1,056 ± 19.7, P>0.05). The honey area in the 42.3 and 52.2 L hives was similar (P>0.05).

ab Values with different letters indicate significant differences (P˂0.05).
Figure 4 Honey area (average ± standard error) in three hive sizes at the end of the study period
The pollen area on the first sampling date was 136.38 ± 26.67 cm2 for the 23.9 L hives, 242.75 ± 147.59 cm2 for the 42.3 L hives, and 112.50 ± 13.14 cm2 for the 52.2 L hives (H =0.34, g.l.=2, P>0.05). The pollen area on the second sampling date was 252.75 ± 77.03 cm2 for the 23.9 L hives, 146.5 ± 35.11 cm2 for the 42.3 L hives, and 192.5 ± 43.19 cm2 for the 52.2 L hives (F =0.94, g.l.=2,11 P>0.05). The pollen area at the end of the study period was significantly different between the hive types (F =9.12, g.l.=2,11, P˂0.01) (Figure 5). The pollen area at the end of the study period was significantly larger in the 23.9 L hive (227.0 ± 37.0 cm2) than in the 42.3 L (87.7 ± 29.9 cm2) and 52.2 L (56.2 ± 21.3 cm2) hives (P˂0.05). The pollen area in the 42.3 and 52.2 L hives was not significantly different (P>0.05) (Figure 5).

ab Values with different letters indicate significant differences (P˂0.05).
Figure 5 Pollen area (average ± standard error) in three hive sizes at the end of the study period
The average temperature in the center of the hives was relatively stable throughout the day during the experiment, despite the fact that the ambient temperature varied on average between 7 and 33 °C (Figure 6). The temperature in the center of the 23.9 L hives was 33.6 ± 1.0 °C, 33.4 ± 0.8 °C in the 42.3 L hives, and 33.7 ± 0.9 °C in the 52.2 L hives (F=0.04, g.l.=2,11, P>0.05). The average temperature in the head varied throughout the day in the three hive types and was significantly higher in the 23.9 L (23.4 ± 0.5 °C) and 42.3 L (23.8 ± 0.6 °C) hives than in the 52.2 L hives (21.4 ± 0.4 °C) (F=6.92, g.l.=2,11, P˂0.05) (Figure 7). The head temperature in the 23.9 and 42.3 L hives was not significantly different (P>0.05).

The values represent the average ± standard error.
Figure 6 Ambient and internal (center and head of the third frame) temperatures in three hive sizes
Discussion
The results obtained in this study demonstrate that the colony development expressed as the brood area at the end of the experiment was lower when it was on the Jumbo type brood chamber, the larger one (Figure 3). Similarly, the honey and pollen area were lower in the hives with larger internal volumes (52.2 and 42.3 L) than the 23.9 L experimental hives (Figure 4,5). These results coincide with those reported by Abd-Elmawgood et al15, who compared three internal hive sizes (38, 31, and 24 L) and obtained the best response (increased amount of brood, pollen, and honey) with the smaller hives. As in our work, they also found that the differences in the amount of brood, pollen, and honey between hives of different internal sizes manifested in late winter and early spring. Similarly, Ballesteros et al28 reported that royal jelly production was higher in rearing hives with six frames than in hives with eight and ten frames.
It was also found that the temperature in the center of the brood nest of A. mellifera was not significantly different concerning the size of the hive, indicating that regardless of the size of the hive, bees can thermoregulate and maintain the brood at temperatures around 33 °C for their offspring to develop properly7,8. However, the head temperature was significantly lower in the Jumbo hives (52.2 L) than in the 42.3 and 23.9 L hives (Figures 6,7). These results show that bees can more efficiently conserve the heat generated during the heating of the brood nest in the smaller brood chambers, which had already been suggested15,28.
The lower percentage of brood, honey, and pollen areas found in the larger hives may be related to different factors. Bees have probably consumed more honey in the hives with a lower internal temperature to obtain the necessary metabolic energy to thermoregulate29,30. Although the heat production was not directly quantified, we did observe that in the Jumbo hives the worker bees remained aggregated around the brood nest for a longer time than in the smaller hives, which suggests that the worker bees in the larger hives invested more time in thermoregulation than in brood production31 or foraging2,3,18. Additionally, a higher temperature inside the smaller hives could facilitate the nest construction since the wax elasticity increases, and the energy expenditure to mold it decreases as the temperature increases32,33. Finally, it is necessary to consider that the colony development also depends on the adults present during the winter season17; however, in this study, it was not quantified the adults to avoid affecting the social cohesion of the colonies24, but it is necessary to consider these data in future studies.
It has been suggested that it is necessary to use hives that allow increasing the internal temperature of the European beehives during the winter season34,35,36. In this study, it was compared two hives commonly used in Mexico (Jumbo and Langstroth) and a smaller experimental hive. The results show that the experimental hive provides better thermal conditions and increases in strength (greater amount of brood) and productivity of the honeybee. However, the thermal and internal space requirements of the A. mellifera hive can vary between breeds and climates37-40; therefore, it is necessary to perform studies under the specific conditions of each apicultural region.
Conclusions and implications
The best response of strength (greater amount of brood) and honey reserves were recorded when the hive with the smallest internal size (23.9 L) was used. The Jumbo hive, used in the central Mexican plateau, showed the lowest honey, pollen, and brood values at the end of winter. This can decrease the honey and pollen production of beekeepers that use this type of hive, which is why it is convenient to implement a practice or mechanism that allows maintaining strong bee colonies for the spring harvest. For example, bees can be fed water and nectar and pollen substitutes, and the internal size of the hive can be reduced using space reducers. Winter is traditionally considered a season in which the queen bee stops laying eggs, and the thermal and feeding requirements of the colony decrease. However, during this study, performed in the Tizayuca municipality, it was recorded ambient temperatures higher than 24 °C for more than six hours a day during the winter, which allowed the queen bee to maintain its egg-laying activity during this season. This different behavior requires better monitoring by the beekeeper to maintain the bee colonies with enough food reserves during the winter to avoid their weakening.
Acknowledgments
To the Consejo Nacional de Ciencia y Tecnología (CONACYT). for the scholarship awarded to AHC during the performance of this study. We also thank the Centro de Investigaciones Biológicas, and the Programa Anual de Investigación (2016) of the Universidad Autónoma del Estado de Hidalgo, as well as REFAMA (REFAMA CONACYT code 251272 "Red Temática Biología, Manejo y Conservación de Fauna Nativa en Ambientes Antropizados” for the support given during this study. Finally, we thank Irina Zuria and two anonymous reviewers for their valuable contributions to improving the manuscript.
REFERENCES
1. Jones CV, Oldroyd BP. Nest thermoregulation in social insects. Adv Insect Physiol 2007;33:153-171. [ Links ]
2. Burril RM, Dietz A. The response of honey bees to variations in solar radiation and temperature. Apidologie 1981;12(4):319-328. [ Links ]
3. Reyes CJL, Cano RP. Manual de polinización apícola. Programa nacional para el control de la abeja africana-Instituto interamericano para la cooperación agrícola. Manual no. 7. Distrito Federal, México: Secretaría de Agricultura, Ganadería, Pesca y Alimentación (SAGARPA); 2003. [ Links ]
4. Dunham WE. Temperature gradient in the egg-laying activities of the queen bee. Ohio J Sci 1930;30(6):403-410. [ Links ]
5. Yuca-Rivas R. Variación intranual en el espectro polínico de la miel producida en Huarán (Cusco, Perú). Ecol Apl 2016;15(1):27-36. [ Links ]
6. Cho LH, Gynheung A. The control of flowering time by environmental factors. Plant J 2017;90:708-719. [ Links ]
7. Heinrich B. The hot-blooded insects: strategies and mechanisms of thermoregulation. Alemania, Berlin: Springer-Verlag; 1993. [ Links ]
8. Winston ML. The biology of the honey bee. Cambridge, Massachusetts, EUA: Harvard University Press; 1987. [ Links ]
9. Seeley TD, Morse RA. The nest of the honey bee (Apis mellifera L.). Insectes Soc 1976;23(4):495-512. [ Links ]
10. Seeley TD, Morse RA. Nest site selection by the honey bee, Apis mellifera. Insectes Soc 1978;25(4):323-337. [ Links ]
11. Seeley TD. Measurement of nest-cavity volume by the honey bee (Apis mellifera). Behav Ecol Sociobiol 1977;2(2):201-227. [ Links ]
12. Szabo TI. The Thermology of wintering honeybee colonies in 4-colony packs as affected by various hive entrances. J Apic Res 1985;24(1):27-37. [ Links ]
13. Toomemaa K, Mand M, Williams IH. Wintering of honey bee colonies in cylindrical nest cavities versus oblong box-hives in a North European climate. J Apic Res 2016;54(4):105-111. [ Links ]
14. Ros PJM. Iniciación a la apicultura. Murcia, España: Comunidad Autónoma de la Región de Murcia; 2009. [ Links ]
15. Romero NJM. Diseño de colmena [tesis maestría]. Ciudad de México, México; Universidad Nacional Autónoma de México; 2017. [ Links ]
16. SAGARPA. Manual de buenas prácticas pecuarias en la producción de miel. Distrito Federal, México: Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA);2010. [ Links ]
17. Jean-Prost P, Le Conte Y. Apicultura: conocimiento de la abeja, manejo de la colmena 4ª ed. Barcelona, España: Mundi-Prensa; 2006. [ Links ]
18. Abd-Elmawgood BH, Al-Rajhi MA, El-Ashhab AO. Effect of the internal size and thermal insulation of the hive on bee colonies strength and productivity. Egyptian J Agric Res 2015;93:185-195. [ Links ]
19. Hoover SER, Hoover TM. Impact of environmental change on honeybees and beekeeping. In: Gupta R, et al editors. Beekeeping for poverty alleviation and livelihood security. Nueva York, EUA: Springer; 2014:463-480. [ Links ]
20. Reddy PVR, Verghese A, Rajan VV, Potential impact of climate change on honeybees (Apis spp.) and their pollination services. Pest Manag Hortic Ecosyst 2012;18:121-127. [ Links ]
21. Contreras EF, Pérez AB, Echazarreta CM, Cavazos AJ, Macías MJO, Tapia GJM. Características y situación actual de la apicultura en las regiones Sur y Sureste de Jalisco, México. Rev Mex Cienc Pecu 2013;4(3):387-398. [ Links ]
22. SPDRMH. Secretaría de planeación y desarrollo regional y metropolitano del estado de Hidalgo, México. http://intranet.e-hidalgo.gob.mx/NormatecaE/Archivos/archivo6405.pdf . Consultado 30 Ago, 2017. [ Links ]
23. DCM. Datos Climáticos Mundiales. https://es.climate-data.org/ . Consultado 4 Jun, 2017. [ Links ]
24. Delaplane KS, Steen JVD, Guzman-Novoa E. Standart methods for estimating strength parameters on Apis mellifera colonies. J Apic Res 2013;52(1):1-12. [ Links ]
25. Cervantes GER. Incidencia de la alimentación suplementaria en la producción y productividad de la apicultura (Apis mellifera) [tesis licenciatura]. Colimbuela, Ecuador; Universidad Técnica del Norte; 2010. [ Links ]
26. Martínez GEG, Pérez LH. La producción de miel en el trópico húmedo de México: avances y retos en la gestión de la innovación 1ª ed. Texcoco, México: Universidad Autónoma Chapingo; 2013. [ Links ]
27. Systat Software. SigmaStat 3.5. Chicago, EUA: Systat Software; 2006. [ Links ]
28. Ballesteros HH, Vásquez RE. Determinación de jalea real en colmenas de recría de diferentes dimensiones. Revista Corpoica 2007;8(1):75-78. [ Links ]
29. Southwick E. Metabolic energy of intact honey bee colonies. Com Biochem Physiol 1982;71(2):277-281. [ Links ]
30. Seeley TD, Visscher PK. Survival of honeybees in cold climates: the critical timing of colony growth and reproduction. Ecol Entomol 1985;10(1)81-88. [ Links ]
31. Vogt FD. Thermoregulation in bumblebee colonies. I. Thermoregulatory versus brood-maintenance behaviors during acute changes in ambient temperature. Physiol Zool 1986;59(1):55-59. [ Links ]
32. Hepburn HR. Honeybees and wax, an experimental natural history. Heidelberg, Alemania: Springer; 1986. [ Links ]
33. Karihaloo BL, Zhang K, Wang J. Honeybee combs: how the circular cells transform into rounded hexagons. J R Soc Interface 2013;10(86):20130299. [ Links ]
34. Abou-Shaara HF, Oways AA, Ibrahim YY, Basuny NK. A review of impacts of temperature and relative humidity on various activities of honey bees. Insectes Soc 2017;64(4):455-462. [ Links ]
35. Wineman E, Lensky Y, Mahrer Y. Solar heating of honey bee colonies (Apis mellifera L.) during the subtropical winter and its impact on hive temperature, worker population and honey production. Am Bee J 2003;43(7):565-570. [ Links ]
36. Erdogan Y, Dodologlu A, Emsen B. Some physiological characteristics of honeybee (Apis mellifera L.). housed in heated, fan wooden and insulated beehives. J Anim Vet Adv 2009;8(8):1516-1519. [ Links ]
37. Gould JL. Why do honey bees have dialects? Behav Ecol Sociobiol 1982;10(1):53-56. [ Links ]
38. Schmidt JO, Hurley R. Selection of nest cavities by Africanized and European honey bees. Apidologie 1995;26(6):467-475. [ Links ]
39. Schneider S, Blyther R. The habitat and nesting biology of the African honey bee Apis mellifera scutellata in the Okavango River Delta, Botswana, Africa. Insectes Soc 1988;35(2):167-181. [ Links ]
40. Hoover SER, Hoover TM. Beehives in the world. In: Gupta R, Reybroeck W, van Veen J, Gupta A editors. Beekeeping for poverty alleviation and livelihood security. Nueva York, EUA: Springer ; 2014:125-170. [ Links ]
Received: August 16, 2018; Accepted: June 09, 2019











 texto en
texto en