The use of probiotics in animal feed promotes animal health, improves productivity1, and therefore represents a possible strategy for controlling and preventing colonization of the gastrointestinal tract by pathogenic bacteria2. Probiotic potential for lactic acid bacteria has been characterized, and species of the Lactobacillus genus have revealed beneficial effects in vitro and in vivo in the control of diarrhea in calves3.
The major challenge presented by the growing demands for probiotics in the world market is that a probiotic strain must be cultivated in appropriate concentrations in a given product, and cell viability must be maintained throughout shelf life4,5. Probiotic-containing foods have been evaluated as technologies for probiotic delivery, and the incorporation of these microorganisms into fermented milk has resulted in products with high cell viability and functionality1,6).
Either fermented colostrum (first to third days post-partum) or transition milk (until seventh day post-partum) can be used as a milk substitute for artificial feeding, reducing costs and promoting healthy development of calves7,8. These initial lactation secretions have been suggested as substrate for animal probiotic production6, as it does not have commercial value, despite its high protein and vitamin content9,10. In addition, immunoglobulin cells in fermented colostrum have the same viability as colostrum in natura and are capable of transferring passive immunity to newborn calves11. Nevertheless, losses by putrefaction during fermentation have been registered. Those may be associated with the proliferation of pathogenic or spoilage microorganisms7.
Dairy products are considered the best matrix carriers of lactic acid bacteria the main group of probiotic species12. In one preliminary study, it was selected a Lactobacillus strain from the gastrointestinal tract of calf that presented inhibitory effects against Escherichia coli strains that cause calf diarrhea. Additionally, it was observed higher daily weight gains for young female calves fed with fermented milk containing the Lactobacillus sp. strain13. Deeper analysis of this probiotic strain in transition milk and of adequate storage times would be of great relevance since it could reduce the costs of artificial feedings and improve health.
In this study, it was evaluated the potential of four dairy substrates on the growth the Lactobacillus plantarum during two fermentation periods. In addition, assessed cell viability and post-acidification were evaluated, at two different temperatures, over a 60-d storage period in transition milk fermented by this bacterium.
The bacteria strain analyzed was isolated from the feces of a 4-mo-old weaned 3/4 Holstein 1/4 Gyr calf. The bacterium was selected for having greater resistance to both acidic pH and bile salts in vitro, which are important probiotic characteristics, and for demonstrating a stronger antagonistic effect against two Escherichia coli strains that cause calves colibacillosis13.
To perform molecular identification of these bacteria, DNA was extracted, amplified via the polymerase chain reaction (PCR) by usage of primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′), as described by Lane14, and the 16S rRNA gene was sequenced15 by MegaBACE® 1000 (GE Life Sciences, Chicago, USA) automatic sequencer at the Myleus Biotechnology laboratory (Belo Horizonte, Brazil). The 16S rRNA gene sequence was verified by SeqScanner Software® v1.0 (Applied Biosystems, Foster City, USA) and compared against the NCBI database by the BLAST server (http://www.ncbi.nlm.nih.gov/BLAST/). The strain was recognized as Lactobacillus plantarum considering a 99 % similarity threshold. Additionally, the bacteria strain rendered identification scores higher than 2.0 when analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) using MALDI-Biotyper v2.0 software16.
Bacteria were preserved frozen (-18 °C) in tubes containing de Man, Rogosa and Sharpe (MRS) broth with 20% (m/m) glycerol. To activate microorganisms, 0.2 mL of the frozen cultures were added to 10 mL of MRS broth and incubated for 24 h at 37 °C. Subsequently, two successive inoculations were performed in test tubes containing 10 mL of reconstituted skim milk at a concentration of 10% solids-not-fat. For each inoculation, the tubes were incubated for 24 h at 37 °C.
Primarily, the viability of this bacteria was tested in two different fermentation periods times and four different substrate formulations: (1) reconstituted skimmed milk (RSM), which consisted of skimmed milk powder (Molico, Nestlé®) reconstituted in distilled water at a concentration of 10% (m/v) solids-not-fat, (2) transition milk (TM) from Holstein cows on the third day after calving, (3) whole milk (WM) from animals of the same breed and dairy farm as the TM formulation, and (4) a mixture of 50% WM and 50% TM (WTM).
Each formulation (25 mL) was dispensed into test tubes, and 0.3% (m/v) sodium citrate was added to each tube as a stabilizer. The tubes were then autoclaved at 121 °C for 15 min. Subsequently, the tubes were cooled to room temperature (25 ± 2 °C), and 2% (v/v) of L. plantarum culture (8 log CFU·mL-1) was added to each formulation, as first concentrations were of 6.6 log CFU mL-1. The tubes were agitated and incubated in a BOD incubator at 37 °C for 24 and 48 h. The production flowchart for each formulation is shown in Figure 1.
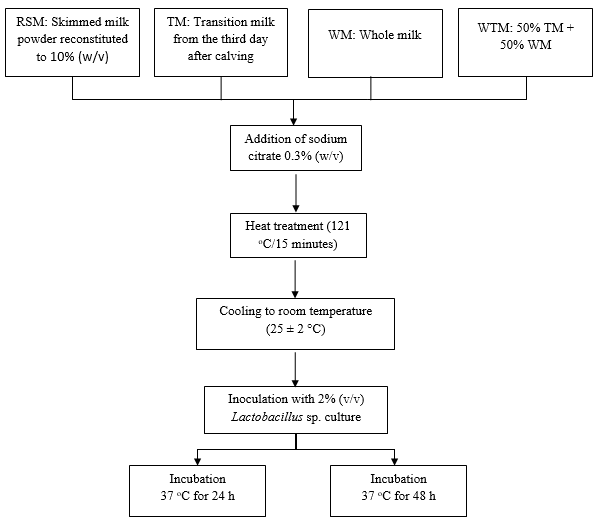
Figure 1 Flowchart of the production process for four milk products, fermented by Lactobacillus plantarum, with probiotic potential
In a second trial, bacteria viability and post-acidification of fermented transition milk which were prepared as described above and were evaluated, though, incubated at 37 °C for only 24 h. After fermentation, samples were stored at 4 and 25 °C for microbiological and physicochemical analyses, as shown in Figure 2.
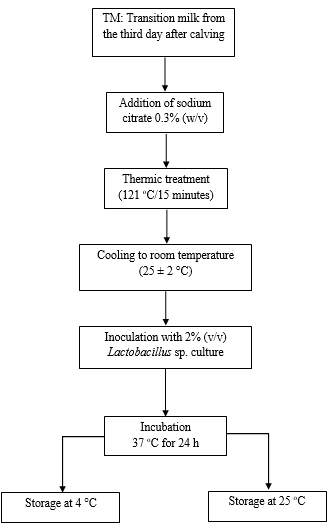
Figure 2 Flowchart of the production process for transition milk, fermented by Lactobacillus plantarum, with probiotic potential
Viable bacterium counts for the four fermentations were performed immediately after the 24 h or 48 h of incubation. For the fermented transition milk stored at two different temperatures, viable cell counts were evaluated at 0, 10, 20, 30, 40, 50, and 60 d of storage.
Samples of the fermented substrates were serially diluted to 10-7 in sterile 0.1% (m/v) peptone water, and 1-mL aliquots were transferred to sterile Petri dishes. MRS agar medium (HiMedia, Mumbai, India) was then added, and the material was homogenized by pour plate method. Plating was performed twice for all analyses. The plates were incubated in at 37 °C for 72 h under aerobic conditions, and colony-forming units (CFUs) were counted. Results were expressed as log CFU per mL of fermented substrate.
The pH of fermented substrates was measured by a digital potentiometer with a combined glass electrode (Hanna brand, model pH21). The titratable acidity, expressed as % (m/v) of lactic acid, was determined by acid-base titration. Both analyses were performed after 0, 10, 20, 30, 40, 50, and 60 d of storage.
Data were analyzed by analysis of variance, and the significance of differences between means was assessed by the Tukey test at the 95% confidence level (P<0.05). Regression analyses were performed to describe cell viability, pH, and titratable acidity as a function of storage time at each storage temperature. For both experiments, a factorial scheme was used, with four replicates for each condition, and the experimental design was completely randomized. The analyses were carried out by the Statistical Analysis System v9.4 (SAS, 2014).
The viability of microorganisms in probiotic foods is the main determinant of the functionality of these products. In this study, the viable cell counts of the probiotic cultures were greater than 8.40 log CFU·mL-1 (Table 1). There was no significant difference in the concentration of L. plantarum in the four dairy substrates after the same incubation period. Transition milk enabled viable growth of probiotic cells to the same concentrations as whole milk did, suggesting it should be chosen as a growth substrate since it has no commercial value for the dairy industry.
Table 1 Lactobacillus plantarum viable cell counts (log CFU.mL-1) in reconstituted skimmed milk (RSM), transition milk (TM), whole milk (WM), and 50% TM + 50% WM (WTM) after 24 and 48 h fermentation at 37 °C
| Fermentation time | Substrate | |||
|---|---|---|---|---|
| RSM | TM | WM | WTM | |
| 24 h | 8.77Aa | 8.67Aa | 8.91Aa | 8.62Aa |
| 48 h | 8.58Aa | 8.82Aa | 8.40Ab | 8.64Aa |
Different uppercase letters for values in the same row and different lowercase letters for values in the same column indicate significant differences (P<0.05).
Coefficient of variation: 2.61 %.
For the WM formulation, there was a significant decrease (30.9 %) in viable cell counts between 24 h and 48 h of fermentation. In contrast, for the RSM, TM, and WTM formulations, the L. plantarum viable cell counts were similar after 24 h and 48 h of fermentation, indicating that only 24 h of fermentation is required for the microorganism to grow to high concentrations in these substrates.
In another study, the growth of five probiotic strains in UHT milk supplemented with tryptone and fructose was studied, and all strains reached maximum viable cell counts of 8.7 to 9.2 log CFU·mL-1 after 6-16 h of incubation. However, three strains exhibited a decrease of 0.4 to 1.1 log CFU·mL-1 in viable cell count between 24 h and 72 h of incubation17. The fermentation of six probiotic strains over a 48-h period at different temperatures was also evaluated having UHT milk as a substrate. A temperature of 37 °C and an incubation time of 12-24 h yielded the highest growth and viable cell counts (8.65 to 9.21 log CFU·mL-1) for all evaluated strains of Lactobacillus spp.18.
The substrate the strain and its adaptation to the culture medium, strongly influence the rate of fermentation and the duration of the cell growth phase. Faster growth causes faster nutrient consumption and acid production, which has a negative environmental impact, leading to rapid progression to the decline phase. In general, the duration of fermentation is determined by pH; fermentation continues until the pH reaches 4.5-4.6. In the production of yogurt that have varied probiotic species, faster fermentation was reported in whole milk than in skimmed milk19. In other study, the total fermentation time for whole milk varied between 16 and 31 h, depending on the species of Lactobacillus spp. evaluated20.
In this study, all formulations yielded viable cell counts above 6 log CFU·g-1 (Table 1), which is the minimum viable cell count required for products of Lactobacillus spp. to function as probiotics21. Manufacturers of probiotic cultures also recommend a minimum viable cell count of 6 log CFU·g-1 in milk fermented by these bacteria22. The results of this study were similar to those reported by Coman et al23, who reported viable cell counts above 8 log CFU·mL-1 of L. rhamnosus and Lactobacillus paracasei, individually or combined at the end of the fermentation of whole milk.
Lasting viability and stability during storage are considered fundamental prerequisites for probiotic products. We therefore measured cell viability, pH, and titratable acidity in fermented transition milk over a 60-day storage period at 4 °C and 25 °C. As expected, each parameter was dependent on storage temperature (Table 2). After 40 days’ storage, the viable cell counts of fermented transition milk stored at 25 °C were significantly lower than those of the product stored at 4 °C. Likewise, storage of the fermented transition milk at 25 °C yielded a significantly lower pH after 10 d and a significantly higher titratable acidity after 20 d, compared with storage at 4 °C.
Table 2 Viable cell count, pH, and titratable acidity (expressed as % lactic acid) of transition milk fermented by Lactobacillus plantarum and stored for 60 d at 4 and 25 °C
| Time (days) | Viable cell count (log CFU·mL-1) |
pH | Lactic acid (%) |
|||
|---|---|---|---|---|---|---|
| Temperature | Temperature | Temperature | ||||
| 4 °C | 25 °C | 4 °C | 25 °C | 4 °C | 25 °C | |
| 0 | 8.89Aa | 8.89Aa | 5.70Aa | 5.70Aa | 0.37Aa | 0.37Ac |
| 10 | 8.72Aa | 8.56Aa | 5.52Aab | 5.01Bb | 0.42Aa | 0.73Ab |
| 20 | 8.79Aa | 8.02Aab | 5.47Aab | 4.78Bb | 0.53Aa | 0.90Bab |
| 30 | 8.50Aa | 7.63Abc | 5.36Ab | 4.85Bb | 0.55Aa | 0.98Bab |
| 40 | 8.71Aa | 7.63Bbc | 5.36Ab | 4.86Bb | 0.53Aa | 1.03Bab |
| 50 | 8.67Aa | 6.88Bc | 5.43Aab | 4.89Bb | 0.48Aa | 1.14Ba |
| 60 | 8.48Aa | 5.65Bd | 5.37Ab | 4.95Bb | 0.55Aa | 1.19Ba |
Different uppercase letters for values in the same row and different lowercase letters for values in the same column, for each parameter, indicate significant differences (P<0.05).
Coefficients of variation: 4.47% (Viable cell count); 2.36% (pH); 19.41% (lactic acid).
The viable cell count was 8.48 log CFU·mL-1 after 60 d of storage at 4 °C and did not change significantly (P>0.05) over the storage period. However, when the product was stored at 25 °C, there was a significant reduction (P<0.05) in cell viability of more than 1 log cycle after 30 d of storage. However, the decrease was more pronounced after 60 d of storage, showing viable cell count of 5.65 log CFU·mL-1 at the end of the storage period (Table 2). The decrease in cell viability at this storage temperature could be justified by lower pH and higher acidity. Using regression analysis, we inferred that the viable cell count of the product remained within acceptable limits (>6.5 log CFU·mL-1) for more than 60 d under refrigeration and for up to 50 d at 25 °C (Table 2 and Figure 3). For a probiotic product, stability of the viable cell count over its shelf life is essential, and stability of the probiotic at room temperature is particularly relevant because it allows producers to save energy while storing the transition milk for artificial feeding of calves.
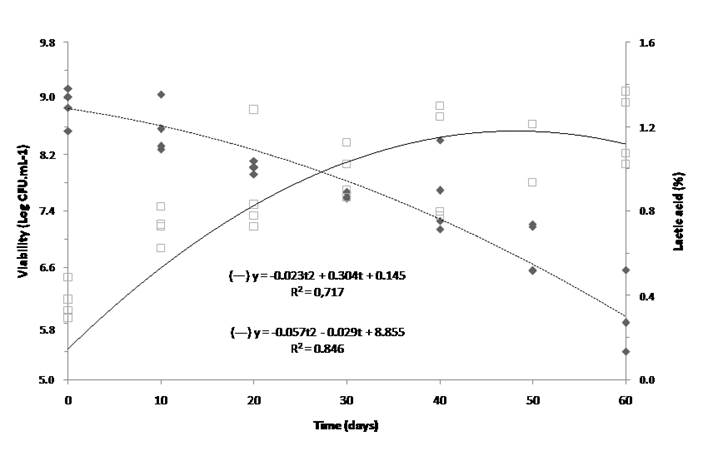
Figure 3 Quadratic regression of Lactobacillus plantarum viability (---) and acidity (-) of fermented transition milk as a function of storage time (up to 60 d) at 25 °C. R2: coefficient of determination; y: viability or acidity; t: storage time
Different storage times under refrigeration have been used previously to evaluate the viability of probiotic microorganisms in fermented products. In the development of a milk fermented by L. plantarum, there was a decrease of 1.2 log CFU·mL-1 in viable cell counts when the product was stored for 70 d at 10 °C, which was a satisfactory result24. Analysis conducted with colostrum and transition milk silages showed that appropriately fermented samples had an average Lactobacillus spp. concentration of 5.15 log CFU·mL-1 after 33 days’ storage at 25 °C7.
Probiotic fermented milk can be stored for several weeks with minimal viability loss if acidification can be minimized by refrigeration25. In this study, the pH of the product decreased significantly after 30 days’ storage at 4 °C, reaching a value of 5.36 after that period (Table 2). When stored at 25 °C, the pH of the product dropped from 5.70 to 5.01 after 10 d of storage. According to the regression equations, the fermented transition milk would have reached their minimum pH values after 44 and 38 d when stored at 4 and 25 °C, respectively (Figure 4).
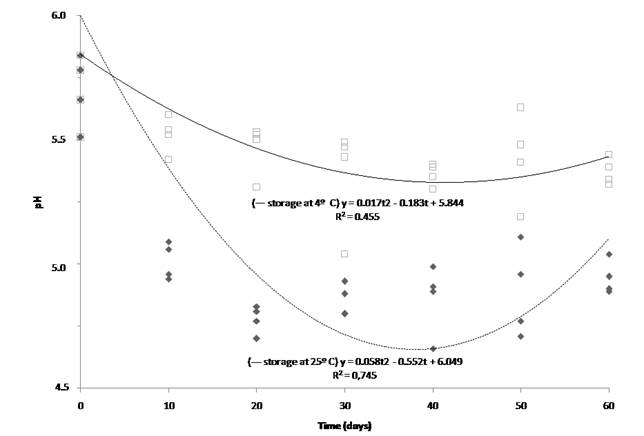
Figure 4 Quadratic regression of the pH of the fermented transition milk as a function of storage time (up to 60 days) at 25 °C (---) and 4 °C (-). R2: coefficient of determination; y: pH; t: storage time
PH affects protein conformation, enzyme activity, and acid dissociation, and is therefore the most important parameter to characterize milk acidity and dairy products. Coman et al23 showed that, after fermentation and storage for 4 wk at 4 °C, whole milk fermented by L. paracasei and L. rhamnosus reached minimum pH values of 5.60 and 4.31, respectively26. Another fact that should be taken into consideration is nutrient availability; for example, milk fermented by L. plantarum showed pH values of 5.81 for the control sample (without added nutrients) and 3.82 for samples with added nutrients (amino acids, vitamins, minerals, and nucleotides) after 72 h of fermentation27.
PH reduction causes a passive flow of protons into microbial cells, which actively export protons. The uncontrolled influx of protons should decrease cell internal pH, inhibiting the synthesis of cellular components and cell multiplication. Undissociated lactic acid can penetrate the cell membrane and contribute to acidification of bacterial cytoplasm28.
Initial and final pH, along with other factors such as organic acid production and exposure to different temperatures during storage, can affect cell viability during fermentation. The growth of undesirable microorganisms is reduced in products with a pH below 5.026. In this study, the pH of the fermented transition milk at 25 °C remained below those levels from 20 to 50 d (Figure 4).
Ferreira et al29 observed a rapid decrease in pH when colostrum was fermented naturally at 32.5 °C; products with pH values below 4.5 were obtained after 35 d of fermentation. In previous studies, bovine colostrums, from the second milking after calving, presented a mean 5.41 for the pH after 33 days of fermentation at 25 °C7.
In this study, the titratable acidity of the fermented transition milk did not change significantly (P>0.05) over the storage period when stored under refrigeration. However, when the product was stored at 25 °C, the titratable acidity almost doubled after 10 d, increasing from 0.37 to 0.73 % of lactic acid; another significant increase in acidity occurred only after 50 d, reaching 1.14 %. Regression analysis indicated that the fermented transition milk stored at 25 °C reached maximum values of titratable acidity after 56 d (Figure 4). These results show that the stability and viability of the product could be influenced by acidity, since the strain used in this study is acid-sensitive.
The strain of L. plantarum with probiotic potential evaluated showed satisfactory growth in each of the milk-based formulations tested, yielding high concentrations of viable cells (> 8 log CFU·mL-1). Transition milk of the third day after calving represents a useful substrate for the growth of this bacterium and can be stored for up to 50 d at room temperature. Therefore, fermentation of transition milk by the L. plantarum strain shows to be a viable method for production those probiotics.











 text in
text in 


