Introduction
Parasitic diseases are one of the most important causes of economic losses for the livestock industry in tropical and subtropical production systems. These diseases have a huge prevalence impact related to epidemiological chain factors such as the presence of vectors and environmental changes1. Ivermectin is a popular macrocyclic lactone since its introduction to the market2,3. This drug is a mixture of two homologs in a ratio of 80 % 22,23-dihydroavermectin B1a (H2B1A) and no more than 20 % of 22,23-dihydroavermectin B1b (H2B1b)4. The maximum residue limits (MRL) in the European community have been determined to utilize the B1A portion as a marker5. The outstanding use in veterinary medicine is derived from the spectrum activity for the control of nematodes and arthropods, generating a remarked interest for research development. Additionally, the macrocyclic lactones group has also been used in agriculture for pest control and in human medicine mainly for onchocerciasis treatment6,7.
From a pharmacokinetic point of view, studies have shown that the main organ for Ivermectin residues detection is the liver in almost all the evaluated species8,9,10. Therefore, due to the huge usage in the field, consumption of viscera and possible toxicity, this organ was chosen as the matrix to carry out this study. For detecting residues, several methodologies have been developed, liquid chromatography (HPLC) and mass spectrometry are the most recommended due to their high sensitivity and selectivity, however, they also present certain disadvantages such as complex extraction process and expensive equipment is needed11. Another semi-quantitative-methodologies such as Competitive ELISA are used as screening method in several matrices. Some advantages are related to a low cost, fastness with a relative good sensitivity and specificity in comparison to HPLC4,12.
The purpose of this investigation corresponded to determine the presence of ivermectin residues in bovine livers from the Bogota, D.C. savanna using the competitive ELISA technique, correlating the presence of residues presence with gender and age variables. This study also describes the histopathological changes of the evaluated samples.
Material and methods
Study location
Samples were obtained from Zipaquira´s slaughterhouse, which has an average of 200 animals slaughtered daily. This slaughterhouse is classified as type II, according to INVIMA (Decree 1036 of 1991) and the Ministry of Health and is also certified by ISO 9001: 2008 and NTCGP1000: 2009 normativity.
Sample size
Considering an error of 10 % and a confidence level of 99 %, the number of analyzed livers corresponded to 89. The animals were chosen randomly in the suspension area and subsequently identified. Once they were in the evisceration area, approximately 100 g of liver were taken in sterile bags for the ELISA test. Each sample was marked, duly labeled, refrigerated, transported and later stored at -20 °C until their analysis. At the same time, data corresponding to origin, breed, gender, and age of the evaluated animals were obtained.
In the standardization phase the liver of a bovine was included as a negative control. This animal certainly did not receive any previous treatment with ivermectin. Prior authorization from the Bioethics Committee of the U.D.C.A, an 8-d-old calf was euthanized using Euthanex® (60 mg/kg, I.V) and liver samples were taken for analysis. The pregnant cow belonged to the university and did not receive antiparasitic treatment with macrocyclic lactones during pregnancy.
Procedure
Competitive ELISA test was used for residue detection and quantification. This test uses rabbit polyclonal antibodies against ivermectin and the analysis process for each sample was carried out considering the kit provider information13, briefly the amount of active ingredient in the samples was expressed as equivalents of ivermectin (ng/ml). In the case of the liver, the values that were obtained from the calibration curve were multiplied by a factor of 5 in order to express the concentration in ng/g. The equivalents of ivermectin corresponded to the maximum percentage of absorbance of each extract read from the calibration curve of the seven standards (Table 1). The determination coefficients obtained in each calibration curve were considered to calculate the specific residues concentration.
Table 1 Concentration of the ivermectin in the standard solutions
| Standard | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| ng/ml | -- | 1.25 | 2.5 | 5 | 10 | 25 | 50 |
Each sample was thawed and homogenized with a food processor. The obtained material was subjected to an extraction process and therefore residue quantification based on the kit supplier parameters. Samples were considered as residually positives when they exceeded the kit’s reported limit of detection (LOD) of 8 ppb. The maximum residue limit (MRL) for the liver corresponded to 100 ppb, thus considering the reported values in Resolution 1382 of 2013 of the Ministry of Health and Social Protection. The samples were analyzed in duplicate at the U.D.C.A microbiology laboratory.
Histopathological analysis
In addition to the sample taken for ELISA analysis, 5 g of liver were obtained for histopathological evaluation, conserving them in 10% formaldehyde. Samples were processed by hematoxylin and eosin technique in the U.D.C.A pathology laboratory. Three zones of the hepatic lobe were evaluated as follows, zone 1, located at a further distance from the centrilobular vein, specifically in the periphery of the lobule. Zone 2 corresponded to the middle of the periphery and centrilobular vein, and the zone 3, it was considered as closest to the centrilobular vein.
In aforementioned areas, the following variables were evaluated:
Alterations in architecture: vacuolation, lobular inversion, canalicular hyperplasia and parenchymal fibrosis.
Microcirculatory alterations: in this case, centrilobular congestion, generalized congestion, focal hemorrhage, and edema were considered.
Inflammatory alterations: presence of polymorphonuclear, mononuclear and mixed cells. An evaluation of the lobular areas was done either.
Changes similar to cell death: apoptosis and necrosis were considered.
For each of the variables, the degree of the lesion was classified as follows: (0) apparently normal, (1) mild, (2) moderate and (3) severe14.
Statistical analysis
This study was observational with simple random sampling. The data was recorded in Microsoft Office Excel 2016 spreadsheet and analyzed with STATA MP14 statistical package. A descriptive analysis of the origin, breed, gender and age variables was carried out. To analyze the effect of gender, age and histopathological variables with ivermectin concentration, a Chi2 (P<0.05) association test was performed.
Results
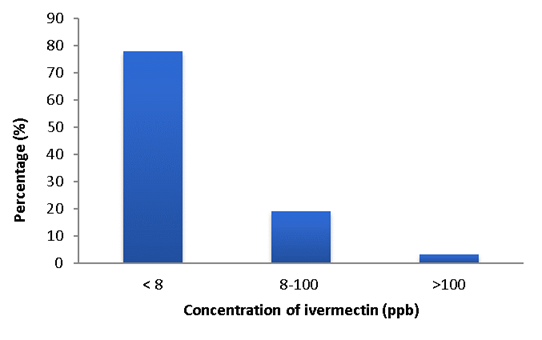
The coefficients of determination obtained to calculate residue concentration were 97 % on average. Most individuals presented levels lower than 8 ppb (78 %), 19 % between 8 and 100 ppb and only 3 % presented residues over 100 ppb, exceeding the MRL (Figure 1).
The population without residues (LOD <8 ppb) was mostly originated from Zipaquira 44 % (28/70), followed by Cogua 26 % (18/70), and to Sopo and Chiquinquira respectively corresponded the remaining 10 % (7/70) of the evaluated animals. For the breed variable, the highest proportion of animals were Normande 34 % (24/70), followed by Holstein 28 % (20/70) and Half-Blood 23 % (16/70). In the case of the age variable, only 13 % (9/70) were animals under 1.5 yr old and 87 % were older than 1.5 yr old. For the gender variable, it was observed that 59 % (41/70) of the sampled animals were females and 41 % (21/70) were males.
The individuals with residues prescense were mostly originated from Cogua 35 % (7/20), Zipaquira 30 % (6/20), Sopo 20 % (4/20). For La Vega, Bogota and Guasca, only one individual was positive respectively. The breeds in which residues were found corresponded to Half-Blood 35 % (7/20), Zebu 25 % (5/20), Normande 20 % (4/20) and Jersey x Holstein 15 % (3/20); for Holstein there was only one individual. 85 % (17/20) of the animals were older than 1.5 yr old and the remaining 15 % (3/20) were less than 1.5 yr old. In terms of the gender variable, they were molstly males 65 % (13/20) and 35 % (7/20) were females.
Only 3 % (3/90) of samples exceeded the MRL. All of the positive animals were male, two samples came from Sopo and one from Cogua and two of them were older than 1.5 yr old. These animals were Half-Blooded, Holstein and Jersey x Holstein breeds (Table 2). When performing the Chi2 association test between variables age (P=0.84), gender (P=0.06) and ivermectin concentration, no association was found.
Table 2 Descriptive statistics of the population with residues presence
| Variable | Samples with residue \ Total population | % Samples with residues | Concentration (≥ 8 ppb) | Samples >MRL* | ||
|---|---|---|---|---|---|---|
| Range | Average | SE | ||||
| Gender: | ||||||
| Males | 13\42 | 31 | 8.40-154.72 | 58.50 | 14.71 | 3 |
| Females | 7\48 | 15 | 8.32-26.92 | 16.30 | 2.38 | 0 |
| Origin: | ||||||
| Cogua | 7\25 | 28 | 8.86-154.72 | 37.86 | 19.58 | 1 |
| Sopo | 4\11 | 36 | 8.32-140.25 | 86.05 | 31.92 | 2 |
| Zipaquira | 6\34 | 18 | 8.40-87.65 | 32.75 | 10.50 | 0 |
| Age: | ||||||
| < 1.5 yr old | 3\12 | 25 | 8.86-154.72 | 60.98 | 46.96 | 1 |
| ≥ 1.5 yr old | 17\78 | 22 | 8.32-140.25 | 40.68 | 10.11 | 2 |
| Breed ** | ||||||
| Half-Blood | 7\23 | 30 | 8.32-136.40 | 33.74 | 17.45 | 1 |
| Zebu | 5\12 | 42 | 18.28-87.65 | 34.67 | 13.32 | 0 |
| Normande | 6\28 | 14 | 8.40-59.22 | 31.43 | 8.87 | 0 |
| Jersey x Holstein | 3\4 | 75 | 15.12-140.25 | 61.53 | 39.56 | 1 |
*MRL= Maximum Residue Limit.
**Only one individual of Holstein breed exceeded the MRL with 154.72 ppb.
For the histopathological analysis, 86 samples were evaluated, and as it was mentioned before, 20 livers presented ivermectin residues and 66 livers where considered as negative. According to lesion severity and the different variables analyzed, the data was recorded in Table 3. It is worth mentioning that no lobular inversion and lesions compatible with necrosis were observed in the analyzed samples. The majority of changes observed were mild in both cases. For the samples without residues, the main changes observed corresponded to the inflammatory level, with a mild presence of monocytes stood out (28/66), moderate (6/66) and severe (3/66). Mild changes in vacuolization (41/66), hyperplasia (15/66), moderate hyperplasia (22/66), mild fibrosis (27/66) and moderate fibrosis (21/66) were also described. We found association between variables microcirculatory alteration, inflammatory alteration and similar to the cell death (P<0.05) (Table 3).
Table 3 Histopathological findings in the studied population
| Severity Degree | LOD < 8ppb | LOD ≥ 8ppb | P value | ||
|---|---|---|---|---|---|
| Architecture alteration | Vacuolization | Normal | 21 | 10 | 0.318 |
| Mild | 41 | 7 | |||
| Moderate | 4 | 3 | |||
| Hyperplasia | Normal | 29 | 11 | 0.447 | |
| Mild | 15 | 8 | |||
| Moderate | 22 | 1 | |||
| Fibrosis | Normal | 18 | 6 | 0.708 | |
| Mild | 27 | 11 | |||
| Moderate | 21 | 2 | |||
| Severe | 0 | 1 | |||
| Microcirculatory alteration | Lobulillar congestion | Normal | 49 | 2 | 0.000* |
| Mild | 17 | 11 | |||
| Moderate | 0 | 7 | |||
| Generalized congestion | Normal | 64 | 7 | 0.000* | |
| Mild | 1 | 9 | |||
| Moderate | 0 | 4 | |||
| Severe | 1 | 0 | |||
| Focal hemorrhage | Normal | 66 | 16 | 0.006* | |
| Mild | 0 | 1 | |||
| Moderate | 0 | 3 | |||
| Edema | Normal | 66 | 15 | 0.000* | |
| Mild | 0 | 6 | |||
| Moderate | 0 | 1 | |||
| Inflamma tory alteration |
Polymorphonuclear | Normal | 66 | 5 | 0.000* |
| Mild | 0 | 13 | |||
| Moderate | 0 | 2 | |||
| Monocytes | Normal | 29 | 3 | 0.027* | |
| Mild | 28 | 15 | |||
| Moderate | 6 | 2 | |||
| Severe | 3 | 0 | |||
| Mixed | Normal | 66 | 7 | 0.000* | |
| Mild | 0 | 12 | |||
| Moderate | 0 | 1 | |||
| Zone 3 | Normal | 33 | 17 | 0.003* | |
| Mild | 33 | 3 | |||
| Similar to the cell death |
Apoptosis | Normal | 66 | 12 | 0.000* |
| Mild | 0 | 7 | |||
| Moderate | 0 | 1 | |||
P<0.05.
Animals with residues evidence (20) presented alterations on architectural variables as follows: 10 samples had vacuolar changes (7 mild and 3 moderate); 9 had canalicular hyperplasia (8 mild, 1 moderate) and 14 had portal fibrosis (11 mild, 2 moderate and 1 severe) (Figure 2). Eighteen (18) samples that contained ivermectin residues presented centrilobular congestion (11 mild, 7 moderate); 13 samples presented generalized congestion and sinusoidal dilatation (10 mild, 4 moderate) and 4 hemorrhagic foci (1 mild, 3 moderate) (Figure 3).

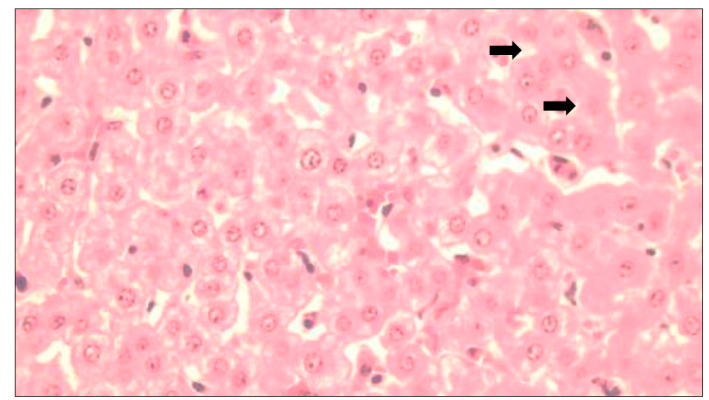
A. Normal architecture in the hepatocytes (400X) and B. the portal area (100X). Architectural changes in the hepatic parenchyma of cattle with residues. C. Vacuolar changes, note the presence of intracytoplasmic vacuoles (arrow tip) (400x). D. Mild fibrosis of the portal area (arrow) (100X).
Figure 2 Bovine liver without ivermectin residues
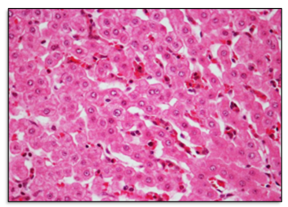
Figure 3 Microcirculatory changes in the hepatic parenchyma of cattle positive for ivermectin residues. Mild congestion and sinusoidal dilatation were evident
In relation to the inflammatory infiltrate, 13 of the 20 animals presented mixed infiltration, predominantly by mononuclear cells (15 mild, 1 moderate) in centrilobular distribution. Comparatively, in both groups, a wide range of changes were found associated to microcirculatory, architectural, inflammatory and cell death (apoptosis) processes (Figure 4). In relation to the last alteration, only eight of the analyzed samples showed changes associated with similar to cell death, characterized by a multifocal distribution in the positive tissues with ivermectin residues (Table 3).
Discussion
Ivermectin is an active compound that has been used for almost 20 yr in numerous countries and different animal production systems. As a result of its usage, control testing on food products derived from livestock is carried out in order to harmonize the commercialization processes. Several studies have been done reporting different methodologies for this purpose, such as the competitive ELISA technique. This method was developed before4, using rabbit produced polyclonal antiserum and the limit of detection (LOD) was 1.6 μg/kg and the antibodies presented a cross-reaction with doramectin but not with moxidectin. In that study, negative liver (1.6 ng/g), 100 ng/g ivermectin fortified liver and 0.5 mg/kg topically treated liver samples from animals slaughtered on day 7, 14, 21 and 28 were analyzed. In this case, ivermectin levels on d 7 post-treatment were 52.7 ng/g decreasing to 4.1 ng/g by d 28. The data was confirmed by another complementary technique such as high-performance liquid chromatography (HPLC). The range of both tests was 1 to 58 ng/g with a close correlation (r = 0.99).
In a study carried out by other authors12 where an ELISA was developed for the detection of multiple avermectins (abamectin, eprinomectin, and ivermectin) the modified avermectin 4C-O-succinoylavermectin was conjugated with bovine albumin and ovoalbumin as immunogens for polyclonal antibodies the preparation. The LOD for this test corresponded to 1.06 ng/ml for all three avermectins. The percentage of recovery ranged from 53.8 % to 80.6 % and it was similar to previous reports using HPLC, converting this technique as a rapid method for screening avermectins in bovine liver. In the present study the LOD of 8 ppb (reported by the manufacturer), 70 samples were under, 17 between 8 and 100 ppb and only 3 exceeded 100 ppb. It is important to bear in mind that in this case no additional confirmatory tests were performed, however considering the obtained data it would be relevant to expand this kind of approach in other production systems with a greater usage of this active compound, situation that has been reported in other country areas.
Turning to the MRL, only 3 % of the samples exceeded the maximum value reported by Colombian authorities (>100 ppb), this could be considered as a low level. In Colombia, a study determining residuality of organophosphorus, carbamates and ivermectin insecticides in raw milk from farms in tropical areas. Of 609 samples analyzed, 37.44 % contained organophosphate levels exceeding the MRL, with Magdalena Region containing almost all the positive samples. Although, 180 milk samples were analyzed for ivermectin detection and quantification but any sample exceeded the MRL15. Data obtained in this study were similar to the observed in other countries as Mexico, where evaluated the presence of ivermectin residues due to the increased use of this molecule on the farms. In this case, 234 liver samples were taken during the course of a month and analyzed by HPLC. Only one sample was not within parameters (149 μg/kg), situation that was similar to another previously reported by a control scheme for this area16.
The aforementioned data differ from another reported in Brazil. Due to residuality problems during 2010 several detection techniques were developed in muscle matrices, allowing the establishment of the MRL and pertinent measures were taken to exercise drug control within the production systems17. Some of the strategies used corresponded to education campaigns for producers in order to prevent parasitic disease by management and medication use, considering several factors that can lead to exceed the residues maximum permitted levels, such as product formulation, active ingredient physicochemical properties, overdose, administration method, product use in non-recommended species, heterogeneous animal lots, and finally non-compliance with withdrawal times2,11.
In terms of the epidemiological characteristics, no association was observed between gender and age variables with the residues presence. Despite that fact, there was a greater presence of residues in males than females. Considering this, reported pharmacokinetic data show that there are differences related to gender and plasma disposition of the active ingredient. These characteristics have been studied in various animal species and specifically for cattle, found differences related to gender in terms of plasma concentration when they evaluated ivermectin and doramectin, bioavailability was 10% higher in heifers than in steers for both molecules18. This difference was associated with the amount of adipose tissue in males and females, considering the characteristics of the active ingredient such as the high liposolubility of the endectocide agent that promotes its storage in this tissue and favors its persistence9.
Despite that residues were observed in females, the percentage found was very low (15%), possibly the evaluated animals were in nonlactating (dry) period, driving to their discard and slaughter, but any case exceeded the limits and most relevant data was found in males. Even so, it is important to bear in mind that the only avermectin approved for dairy farming is eprinomectin and that other factors, such as hormonal influence can affect drug metabolism in terms of its transformation and elimination15,19.
The histopathological evaluation of this study allowed a wide range of hepatic parenchymal alteration findings, even in animals with and without residues. When the microcirculatory changes were evaluated, some animals with residues presented a greater degree of the congestive changes and hemorrhagic foci severity, suggesting a greater susceptibility to develop disorders associated with alterations of the vascular network of the liver parenchyma; however, the number of animals that developed these kind of disorders does not turn out to be significant.
Numerous chemical substances suffer part of their biotransformation in the hepatic tissue, this also means that a major part of these compounds could generate cellular alterations of the parenchyma, leading to possible disorders associated to liver function alterations such as protein synthesis, carbohydrate transformation and lipid metabolism. Included amongst these are pharmaceuticals, bacterial toxins, and toxic plants. In this sense, the unique or concomitant effect of this type of compounds on the analyzed samples cannot be ruled out.
A study conducted in rats for the evaluation of the effect of massive and long-lasting treatments with ivermectin among other molecules against various parasites, showed some clinical alterations compatible with hepatotoxicity and anatomopathological changes revealed architecture distortion, hepatocellular necrosis, and Kupffer cell hyperplasia20. In hyperacute cases of intoxication in goats, sudden death is reported without systemic pathological alterations21. In humans, a case report showed the hepatotoxic potential of ivermectin after administration in a patient with filarial parasitism, finding intralobular inflammatory infiltrate and accumulation of ceroid pigment, perivenular necrosis, necrosis and apoptosis22. According to the international guidelines, the acceptable daily intake established for humans is 10 μg/kg (600 μg/person/d)5, levels that were not observed in the analyzed samples. It is worth noting that comparatively with these reports, the present study also evidenced changes similar to apoptosis only in animals with residues presence, however further studies like imnunohistochemistry should be conducted in order to clarify the association between residues and hepatic lesion development.
The ivermectin poisoning has been associated with overdosing or with the presence of a P-glycoprotein mutation that promotes the development of neurological symptoms in several animal species. The human intoxication has been associated with mild to moderate effects of a Mazzotti reaction and encephalopathy linked to microfilaricidal therapy or macrocyclic lactones exposure23.
Conclusions and implications
In conclusion, the competitive ELISA test used in this study served as a screening method to analyze ivermectin residues in bovine livers. Only 3 % of the samples exceeded the MRL and no correlation was found between the presence of residues with gender and age variables (P>0.05). The majority of the histopathological changes were mild or moderate, with alterations in architecture and inflammatory changes standing out. Exist statistical association between the presence of residue of ivermectin and variables microcirculatory alteration, inflammatory alteration and changes similar to the cell death (P<0.05).











 texto en
texto en