Introduction
Ticks are globally widespread ectoparasites and their eco-epidemiology is dependent on the regional environmental conditions1. Ticks are arthropods with great capacity for transmission of human and animal pathogens and are considered the most important vectors of disease-causing pathogens in domestic and wild animals second to mosquitoes1,2. There are many species of ticks present in Mexico3 but the most economically important in the cattle industry is Rhipicephalus microplus both in Mexico and Latin America. R. microplus is responsible for direct damage and transmission of bovine babesiosis and bovine anaplasmosis3,4.
While transovarian transmission of bovine babesiosis has been clearly established5, transovarian transmission of bovine anaplasmosis is still controversial.
Bovine anaplasmosis a rickettsial tick-borne disease of worldwide distribution6. Anaplasma marginale, the causal agent, infects mature erythrocytes of several ruminant species but it is of greater impact in adult beef cattle; the clinical syndrome includes fever, anemia, losses in weight and production and, death if timely treatment is not provided7,8.
In cattle, A. marginale infects mature erythrocytes and endothelial cells9,10 and, despite specific treatment, cattle may remain as asymptomatic carriers for the rest of their lives11,12. The emergence of antigenic variants of membrane proteins of the rickettsia in the mammalian host has also been shown to be fundamental for its permanence and transmission to naïve hosts13.
Transmission of A. marginale between cattle occurs both mechanically and biologically. Mechanically, by blood sucking arthropods and veterinary procedures that transfer infected blood from carriers to naïve hosts14,15. Biological transmission is carried mainly by Rhipicephalus and Dermacentor ticks8 but in Latin America, the cattle tick Rhipicephalus microplus is the main biological vector4. Ticks can transmit biologically the rickettsia within the same stage (intrastadial) and from one stage to another (transstadial). Tick larvae, nymphs and adults acquire the rickettsia by feeding on cattle and these replicate within R. microplus midgut cells16,17,18. After an initial cycle of replication, tick transmissible Anaplasma strains migrate from the midgut through the hemolymph to other tissues, including the salivary gland acinar cells where the rickettsia undergoes several cycles of final logarithmic replication19. Upon a second round of feeding, and usually, after molting (transstadial transmission), ticks secrete the infective forms of the rickettsia in their saliva while feeding, thus transmitting the rickettsia18. Previous evidence has shown that hand-transferred adult R. microplus males transmit A. marginale4,20. A. marginale infected R. microplus nymphs and young adults (larvae and nymphs incubated in the laboratory and allowed to molt to the next stage) and hand-transferred to susceptible animals were capable to transmit Mexican Aguascalientes and Yucatan A. marginale strains of high and low virulence respectively in the laboratory21.
R. microplus is a one-host tick which spends its entire parasitic life on the same animal until engorged females drop to lay their eggs. Adult male ticks can migrate through physical contact from one host to another22,23. Their importance as A. marginale vector between different animals though has not been fully evaluated. The apparent inefficiency of male ticks as transmitters, and the presence of anaplasmosis outbreaks at the beginning of the tick season has led to propose that, larvae of infected ticks may acquire the infection through the ovary (transovarian transmission). Larvae are then hatched infected, becoming potential vectors for A. marginale24. Further experimental efforts to prove transovarian transmission in Dermacentor ticks have been inconclusive25. In an effort to clarify transovarian transmission in R. microplus, Shimada and coworkers26 collected R. microplus larvae from an infected pasture during the first five months of the year in Brazil, and found that 7/50 samples were positive when tested for msp5 by nested PCR (nPCR). In this same study, female ticks engorged on an A. marginale infected carrier were incubated at 18 ºC or 28º C for oviposition; eleven percent of larvae from engorged ticks incubated at 18 ºC and none from those incubated at 28 ºC were positive when tested for msp5 by nPCR. These authors however, make no reference of msp5-positive larvae from any of these groups to be infested onto susceptible cattle to confirm feed-transmission26.
In light of these findings it was hypothesized that R. microplus larvae can acquire A. marginale from their A. marginale-engorged progenitor (transovarian transmission) and when incubated at 18 ºC and, through feeding, transmit the infection to uninfected cattle under laboratory conditions
Material and methods
Ethics statement
This study was approved by the CENID-PAVET branch of the INIFAP Animal Experimentation and Ethics Committee and conducted considering ethic and methodological aspects in agreement with the Mexican regulations related to use, housing and transport of experimental animals NOM-062-ZOO-1999.
Pathogen and vector strains
The Anaplasma marginale Tlapacoyan-2 strain used in this study was originally collected in the Municipality of Tlapacoyan, Veracruz state, Mexico, from a natural outbreak and has been characterized with regards to the msp1( variable region and msp4 genes27. This strain has been shown to be transmissible by R. microplus adult males in CENID-PAVET laboratory.
The “Media Joya” colony of R. microplus was originally collected from the Tapalpa Municipality, Jalisco State, Mexico28,29, and is routinely maintained through passages in tick-borne disease-free steers at the CENID-PAVET. The colony efficiently transmits multiple A. marginale strains, including the Tlapacoyan-2 strain21. To ascertain that the strain is free of A. marginale, ticks from every generation are routinely tested for the absence of A. marginale by nPCR for the msp5 gene (see below).
Five 12-month old Bos taurus-cross steers were purchased from a local breeder in the Municipality of Cuauhtémoc, west-central Chihuahua state, Mexico which is classified as tick-free by The Mexican National Service of Health, Safety and Agro-Food Quality (http://www.gob.mx/senasica/documentos/34495). These steers were certified free of tuberculosis and brucellosis by Federally certified laboratories. The animals were tick-free, and were also free of A. marginale as certified by endpoint nPCR for msp5 gene. Steer 027 was purchased first and the remaining four steers (1756, 1776, 6963 and 6964) were purchased later from the same breeder to assure they all met sanitary standards as required by Mexican authorities and our own, with regards to age and absence of ticks and, tick-borne and other infectious diseases.
The CENID-PAVET laboratories and stalls are located on the outskirts, within the city limits of the small urban area of Progreso, in the Jiutepec Municipality of the state of Morelos, central Mexico. The quarters are free of ticks. Tick treatment is required for animals entering these quarters and all animals housed in outdoor stalls are periodically sprayed for fly control. All animals used in these experiments were housed at the Cattle Isolation Unit of the CENID-PAVET, a tick and fly-proof confinement stall.
Infection of carrier animal
Steer 027 was intravenously inoculated with a dose of 8.2 x 109 infected erythrocytes of A. marginale Tlapacoyan-2 strain preserved under liquid nitrogen. The infection was monitored and the animal required no treatment. Steer 027 remained as an asymptomatic carrier as tested both by microscopic examination of Giemsa-stained blood slides and amplification of msp5 gene by nPCR, for the 15 mo prior to infestation with R. microplus for this study. Steer 027 was infested with (approximately 10,000) Media-Joya R. microplus mature larvae hatched from 0.5 g of eggs to feed-acquire the infection. Twenty-one (21) days later, mature engorged females were collected directly from steer 027, rinsed in distilled water to eliminate debris and groups of 10 females were set into petri dishes and incubated at 80 % humidity.
Incubation for oviposition
Engorged females were set in two different lots to complete oviposition as follows. The first lot was incubated at 18 ºC for oviposition in a climatized room which is set to have maximal variations of 2 ºC. In order to avoid temperature variations due to regular use of the room, ticks were kept within a small portable cooler and 80 % humidity was provided by use of damp wicks and controlled by use of a Traceable( hygrometer (Fisher Scientific). An equal number of dishes were set at 28 ºC for oviposition into a Nor-Lake Scientific incubator (Nor-Lake LRF201WWW-0). Humidity was maintained at 80 % saturation as described. Once oviposition was complete, egg masses from each temperature were pooled, weighted and divided into 0.25 g lots and kept in 5 ml glass vials capped with cotton plugs. Both egg-lots were incubated at 28 ºC and 80 % humidity as described, for another two weeks until hatching. Mature larvae (approximately 5,000) from 0.25 g egg-lots were used for feed-transmission on intact steers. Additional mature larvae from 0.25 g lots from females kept at 18 ºC and 28 ºC were frozen for further determination and identification of A. marginale DNA by amplification of msp5 and msp1( variable region.
Feed-transmission infection of naïve steers, clinical monitoring, and sample collection
Four non-splenectomized steers were each infested with mature larvae from 0.25 g egg lots; steers 1756 and 1776 were infested with larvae from engorged females incubated at 18 ºC while steers 6963 and 6964 were infested with larvae from engorged females incubated at 28 ºC.
Clinical monitoring
Clinical monitoring of experimental animals included daily registration of rectal temperature (between 8 and 9 in the morning), daily collection of blood with anticoagulant by venipuncture of the caudal vein for examination of Giemsa-stained blood smears and evaluation of packed cell volume by the microhematocrit method and weekly amplification of msp5 gene by nPCR and, msp1( gene variable region by PCR and sequencing27.
DNA extraction, PCR, cloning and sequencing
Larvae from engorged females incubated at 18 ºC and at 28 ºC were used for extraction of genomic DNA (gDNA). Larvae from 100 mg eggs masses were extracted as follows: frozen (-70 ºC) larvae were pulverized using a -70 ºC frozen mortar. The pulverized larvae were then solubilized in 1M Tris-HCl, 0.5 M EDTA, proteinase K (1 mg/7 ml) solution and centrifuged 10,000 xg; the supernatant was separated from DNA with two cycles of phenol-chloroform-isoamyl alcohol and chloroform. Supernatants were washed stepwise first with absolute ethanol and then with 70% ethanol. The DNA was hydrated in double distilled-deionized sterile water and kept frozen at -20 ºC until use.
Anticoagulated blood samples from transmission-infected steers were centrifuged at 2,250 xg for 15 min at 4 °C: plasma and buffy coat were discarded. gDNA was extracted by means of a commercial kit (UltraClean® BloodSpin® DNA Isolation Kit, MO-BIO Laboratories Inc.), following manufacturer’s instructions. gDNA was kept at -20 ºC until use.
DNA samples from blood and larvae were assayed for msp5 gene as a universal marker for A. marginale by nested PCR using forward: 5’-GCATAGCCTCCGCGTCTTTC -3’ and reverse 5’-TCCTCGCCTTGCCCTCAGA-3’ primers in the first round of amplification and forward 5’-TACACGTGCCCTACCGACTTA-3’ and reverse 5’-TCCTCGCCTTGCCCTCAGA-3’ primers in the second round as described30. msp5 nPCR was run in two rounds in a 25 µl final volume with a commercial kit (PCR master mix, system, Promega, Madison, WI, USA) in a T-Professional Thermocycler (Biometra, Germany), 0.1 - 1 ng DNA and 10 pM primers. Cycling conditions for msp5 were a preheating step at 95 °C for 3 min and 35 cycles of 95 °C for 30 s, 65 °C for 58 s, and 72 °C for 30 s with final extension step at 72 °C for 10 min.
msp5 nPCR positive samples were assayed for the msp1( variable region by PCR (forward: 5’ -GTGCTTATGGCAGACATTTCC-3’ and reverse 5’-CTCAACACTCGCAACCTTGG-3’ primers)27,31 for strain verification. For the msp1α variable region cycling conditions were preheating step 95 ºC for 3 min and 35 cycles 60 s, 58 ºC 60 s, 72 ºC 60 s, and final extension at 72 ºC for 10 min. nPCR and PCR products were separated in 2% agarose gels following electrophoresis in 1x TAE buffer and staining with 0.015% ethidium bromide at 100 volts. msp1α variable region PCR products were cloned in pJet1.2 plasmid system using CloneJet PCR cloning kit (Thermo Fisher Scientific), and E. coli TOP10 competent cells were transformed with the constructions following manufacturer instructions. Positive colonies were grown in LB+ampicillin (100 mg/ml) and plasmid DNA was isolated by use of Wizard® Plus SV Minipreps (Promega, Madison WI, USA). Plasmid DNA from at least three isolated colonies was sequenced for determination of the consensus sequence. DNA sequences derived from Sanger sequencing were analyzed with ApE Plasmid Editor v2.0.47. Consensus sequences were aligned with ClustalW (http://www.clustal.org/).
Results
Initial infection and feed-acquisition of Anaplasma marginale
Intravenous inoculation of steer 027 with A. marginale Tlapacoyan-2 strain resulted in positive blood-smears and mild clinical signs of anaplasmosis (39 ºC rectal temperature, depression, loss of appetite and anemia), reaching a 3.2 % maximal rickettsemia, and a loss of 50 % packed cell volume by d 25. Chemotherapy was no required and steer 027 returned to normal clinical values within 2 wk after onset of infection. Steer 027 remained as a subclinical carrier for the next 15 mo, as corroborated by periodic nPCR for msp5 gene amplification. Mature R. microplus larvae negative to A. marginale as determined by msp5 nPCR, were infested on steer 027 for feed-acquisition infection. Engorged females collected 21 d later and incubated at 28 ºC, completed oviposition over 15 d, a period considered normal. In contrast, engorged females collected at the same time but incubated at 18 ºC took over 30 d to complete oviposition. Regardless of the temperature at which the mothers were incubated, larvae from both lots completed hatching within 15 d after oviposition.
Feed-Transmission
Steers were infested with R. microplus larvae from engorged females incubated at 18 ºC (steers 1756 and 1776) and 28 ºC (steers 6963 and 6964), respectively. Mature unfed larvae were allowed to feed and reach adult stage on designated steers. None of the four steers developed clinical signs of anaplasmosis or showed infected erythrocytes at microscopic evaluation of blood smears during this period. Eight weeks after infestation, all four animals were subjected to experimental splenectomy in order to immunosuppress and induce rickettsemia. Steer 6964 (larvae oviposited at 28 ºC) developed a 7.5 % rickettsemia detectable by microscopy recorded 2 d after splenectomy moment when the animal received specific treatment (oxytetracyclin 20 mg/kg for three consecutive days). Despite splenectomy, none of the other three steers developed microscopically detectable rickettsemia. msp5 specific nPCR amplification corroborated the presence of A. marginale DNA in steers 6964 and 6963 but did not amplify in blood from steers 1756 and 1776 (Figure 1).
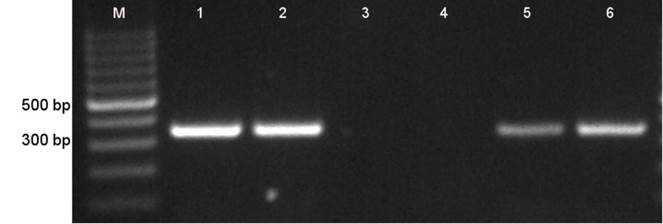
M. Molecular weight marker; 1, Tlapacoyan-2 original frozen stabilate; 2, steer 027; 3, steer 1756; 4, steer 1776; 5, steer 6963; 6, steer 6964
Figure 1 Anaplasma marginale msp5 nPCR detection. nPCR products were separated by electrophoresis in 1% agarose gel
In order to corroborate that Tlapacoyan-2 strain was the same pathogen infecting steers 027, 6964 and 6963, blood samples from these steers, a sample of the original cryopreserved stabilate and, larvae hatched from ticks incubated a 28 °C, were assayed for the msp1( variable region. Figure 2 shows a 750 bp PCR product (lane 2) for Tlapacoyan-2 cryopreserved stabilate, in agreement with the reported sequence27; (GenBank accession number JN564641.1). A band with the same apparent molecular weight was present in all other samples tested for the variable region of msp1( in blood samples from steers 027 (Lane 3), 6964 (Lane 5) and 6963 (lane 6) and R. microplus larvae from engorged ticks incubated at 28º C (Lane 4).

Panel A; M, molecular weight marker; lane 1, (-) control; lane 2(+) control Mex-31 infected blood; lane 3, Tlapacoyan-1 infected blood; lane 4, Tlapacoyan-2 infected blood. Panel B: lane 1, Tlapacoyan-2 cryostabilate; lane 2, steer 027 blood sample; lane 3, steer 6963 week 9 blood sample; lane 4, R. microplus larvae from engorged ticks incubated at 28º C and lane 5, steer 6964 week 9 blood sample.
Figure 2 msp1a variable region specific PCR
All sequences derived from either blood or larvae were identical for the msp1( variable region of Tlapacoyan-2 strain as reported27. This finding is consistent with the hypothesis that R. microplus larvae from engorged ticks acquire the infection from their mothers and are capable of transmitting the infection to naïve hosts. It was not demonstrated presence (PCR or blood smear) of A. marginale in either larvae from engorged ticks incubated at 18 ºC nor steers infested with them.
Discussion
Intrastadial and transstadial transmission of A. marginale during the vector parasitic stages have been well documented in Dermacentor and Rhipicephalus ticks4,32,33. The role of R. microplus ticks as the most important biological vector of Anaplasma marginale in areas where Dermacentor ticks are not present has also been documented4. Efforts to document transovarian transmission however, have been carried without success or have been inconclusive.
Step-wise infestations on splenectomized calves with unfed R. microplus larvae from engorged ticks fed on cattle infected with Babesia bovis, B. bigemina, A. marginale and other blood-borne pathogens were carried in Madagascar34. Patent infection was demonstrated with B. bovis and B. bigemina but not with A. marginale, in these splenectomized calves. In two different studies carried in Australia, unfed larvae hatched from acquisition-fed engorged ticks were used only four days or, four and 15 d after hatching respectively23,35; in both cases, engorged females were incubated at 28 ºC to complete oviposition. Infection with A. marginale was not corroborated in either of these works. A study carried in Colombia36, reported transmission of anaplasmosis to naïve 6 mo-old calves by larvae hatched from experimentally acquisition-fed ticks. These authors reported that transmission also occurred when the second-generation larvae were infested on a non-splenectomized steer. The cattle housing conditions for this study however were not fully described. Recently, authors in Brazil26 documented the presence of A. marginale DNA in larvae progeny of engorged ticks collected from anaplasmosis endemic paddocks and unfed larvae from experimentally acquisition-fed females engorged on Anaplasma infected calves. These authors however showed no evidence of infestation of susceptible cattle with msp5 PCR positive larvae.
In order to determine transovarial transmission of A. marginale, a range of conditions have been reported for oviposition, length of maturation of “infected larvae”, variable values of observable rickettsemia both in naturally or experimentally infected donors, use of intact and splenectomized recipients of unfed larvae and even locations where the studies were conducted.
In order to guaranty that the animals were free of A. marginale, they were purchased from a tick-free area and were PCR and serology tested at purchase and right before infection or infestation. Housing conditions for the experimental steers in this study precluded transmission of the rickettsia amongst themselves or from the carrier steer (027) by flies or ticks. In an attempt to replicate natural conditions, a bovine carrier with no detectable rickettsemia fed ticks at the moment of feed-acquisition of progenitor ticks. Conventional 28 ºC and low 18 ºC temperatures for oviposition were chosen, as there were reports, that transmission could occur at any of these temperatures26.
The original hypothesis postulated that incubation of engorged ticks at 18 ºC lengthened the oviposition period in such a manner that allowed A. marginale to reach and infect the ovary and therefore the progeny as well. Consistent with previous results, incubation at 18 ºC lengthened the oviposition period twofold compared to conventional 28 ºC incubation37. It was also confirmed the presence of A. marginale DNA in the progeny from acquisition-fed ticks incubated at 28 ºC, but not in larvae from ticks incubated at 18 ºC however. Consistent with the presence of A. marginale DNA in these larvae, it was found that one of the two steers infested with larvae from ticks incubated at 28 ºC developed observable rickettsemia on blood smears (6964) 2 d after splenectomy. Steer 6963 also infested with the same larvae, was msp5 nPCR positive.
It was hypothesized that infestation with larvae from ticks incubated at 18 ºC would transmit the rickettsia yet the present results show that these larvae failed to induce patent rickettsemia while infestation with larvae from incubation at 28 ºC did. The results however are consistent, with others who used larvae from females engorged on infected cattle and achieved infection to susceptible 6-mo-old cattle36.
It is unknown why transmission was achieved only from larvae hatched from females incubated at 28 ºC and not from those from mothers incubated at 18 ºC, yet the evidence from these and other studies where transmission has failed or achieved infection seem to point towards a phenomenon that may occur under the influence of many variables, including the tick strain, the rickettsial strain and very likely, the genetic makeup of the host and not only from the temperature at which the engorged ticks oviposit their eggs.
This is the first study in which the presence of A. marginale DNA in larvae and infested naïve cattle was characterized as the same, indicating that transmission from unfed larvae to susceptible cattle occurred. The possibility of using a 73 β β β γ msp1α strain facilitated the follow up of the infection along the experiment. Our results confirmed that Tlapacoyan-2 was the same organism in the carrier, larvae and steers in these experiments.
In light of previous evidence, these results provide additional support for the contention that Anaplasma marginale is transovarially transmitted through R. microplus ticks and that these larvae were capable of transmitting the rickettsia to the mammalian host. There are still many questions to answer; more studies will have to be carried to respond them. In the meantime, and based on these results it is important that transovarian transmission of Anaplasma marginale be considered within the A. marginale life cycle.











 texto em
texto em 


