Introduction
For many years, the in vitro production of porcine embryos has been the object of study of multiple investigations, and although there are currently several research groups working in this technique, the success rate remains low, especially compared with other species1. This makes it difficult to gain any progress implementing this technology in the production units, limiting its use only for research purposes.
The low rate of development of in vitro produced porcine embryos, could be due to inadequate conditions of culture and the high incidence of polyspermy1,2,3. Currently, the standard medium for the in vitro embryo production is the North Carolina State University (NCSU) 23 or NCSU-37, supplemented with follicular fluid for maturation (medium chemically undefined)4.
The defined or semi-defined culture media removes unknown factors present in biological materials such as the follicular fluid, fetal bovine serum or serum albumin, and the use of these media for in vitro embryo production have had major progress. In addition, the use of defined and semi-defined media, facilitates the physiological evaluation of substances on maturation, fertilization and embryo development, enhances the reliability of the media and allows a high repeatability and reproducibility of the results5. There are substrates that can replace the biological materials in the culture media as the polyvinyl alcohol (PVA), hyaluronic acid, bovine serum albumin (BSA) fatty acids free, recombinant albumin and serum synthetic.
The objective of this study was to determine the effect of a semi-defined culture media system developed at the National Genetic Resources Center (CNRG), named Pigs Media System (PMS) on the in vitro production of porcine embryos.
Material and methods
The study was conducted at the CNRG, of the Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias (INIFAP), located in Tepatitlán of Morelos, Jalisco, Mexico. It was developed a semi-defined system culture media, named PMS, for in vitro production of porcine embryos that consists of the following solutions: oocytes washing pre-maturation (H-PMS-M), maturation (PMS-M), oocytes washing pre-fertilization (H-PMS-F), fertilization (PMS-F), zygotes washing pre-embryo culture 1 (H-PMS-E1), embryo culture 1 (PMS-E1), embryos washing pre-embryo culture 2 (H-PMS-E2) and embryo culture 2 (PMS-E2) (Table 1). The PMS was supplemented with 0.4% BSA fatty acids free. All chemicals used in this study were acquired in the company Sigma-Aldrich (St. Louis, MO, USA). The preparation of the media was performed with ultra-pure water. The osmolarity of the media was of 270 to 290 milliosmoles (mOsm) and the pH was adjusted to 7.4.
Table 1 Composition of cultured media for in vitro production of porcine embryos PMS
| Component (mM) |
H-PMS- M |
PMS-M | H-PMS-F | PMS-F | H-PMS- E1 |
PMS-E1 | H-PMS-E2 | PMS- E2 |
|---|---|---|---|---|---|---|---|---|
| NaCl | 100 | 94 | 97 | 94 | 100 | 94 | 100 | 94 |
| KCl | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| KH2PO4 | 1.2 | 1.2 | - | - | 1.2 | 1.2 | 1.2 | 1.2 |
| MgSO4 7H2O | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Glucose | 5.6 | 5.6 | 5.6 | 5.6 | - | - | 5.6 | 5.6 |
| Alanine-Glutamine | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 | 1.9 |
| Arginine | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 |
| NaHCO3 | 5 | 25 | 5 | 25 | 5 | 25 | 5 | 25 |
| Hepes | 20 | - | 20 | - | 20 | - | 20 | - |
| Na-pyruvate | - | - | 2 | 2 | 0.33 | 0.33 | - | - |
| Na-Lactate | - | - | - | - | 4.5 | 4.5 | - | - |
| Melatonin (µM) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| CaCl2 2H2O | 1.7 | 1.7 | 7 | 7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Caffeine | - | - | 2 | 2 | - | - | - | - |
| Taurine | 7 | 7 | - | - | 7 | 7 | 7 | 7 |
| Hypotaurine | 5 | 5 | - | - | 5 | 5 | 5 | 5 |
| NEAA´S* ml/L | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| EAA'S* ml/L | - | 20 | - | - | - | - | 20 | 20 |
| β-mercaptoetanol | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Cysteine | - | 0.6 | - | - | - | - | - | - |
| EGF* (ng/ml) | - | 10 | - | - | - | - | - | - |
| Osmolarity | 287 | 286 | 283 | 287 | 291 | 289 | 288 | 286 |
* NEAA´S= non-essential amino acids; EAA'S= essential amino acids; BSA= bovine serum albumin; EGF= Epidermal growth factor.
Collection of oocytes and in vitro maturation
Oocytes were obtained from ovaries collected from gilts at the local slaughterhouse and were transported in saline solution 0.9% at 35°C. Ovarian follicles of 3-6 mm were pick up and aspirated with a needle caliber 18-gauge and 10 ml syringe. The cummulus-oocytes complexes (COCs) with a compact cumulus mass and a dark evenly granulated cytoplasm were selected and washed in H-PMS-M. The COCs were cultured in 1 ml of medium of maturation in four-well multidish (Nunc, Roskilde, Denmark) during 22 h with 0.5 μg/ml of LH, 0.5 μg/ml of FSH and 1 millimolar (mM) dibutiryl cAMP, and subsequently for 22 h in the same medium, but without hormones and dibutiryl cAMP, at 38.5 °C with 5% CO2 in air.
In vitro fertilization
After maturation, thirty COCs were placed in 90 μl of fertilization medium and covered with mineral oil, until fertilization. Frozen semen was thawed for 30 sec in water at 37 °C and centrifuged (700 ×g for 20 min) in gradients of Percoll 45:90 %. The sperm pellet was resuspended and washed by centrifugation (400 ×g for 5 min) with fertilization medium. The COCs were cultured during 3 hours with sperm at a final concentration of 5 × 105 cells/ml at 38.5 °C with 5% CO2 in air.
Embryo culture
The presumptive zygotes were removed from the fertilization media, washed and ten zygotes were placed in 90 μl of early culture medium, and cultured 48 h at 38.5 °C with 5% CO2, 5% O2 and 90 % N2. Afterwards, embryos were transferred to late culture medium, where they remained for 120 h under the previous conditions.
Evaluation of nuclear maturation, activation and fertilization
After maturation, some oocytes were removed from the cumulus cells, fixed with 25% (v/v) acetic acid and ethanol, for 48 to 72 h. The samples were stained with aceto-orcein 1% (w/v) and the percentage of mature oocytes (oocytes in metaphase II and first polar body formed) was evaluated with a clear field microscope. After 10 h post fertilization, some presumptive zygotes were removed from the cumulus cells, fixed, stained and evaluated as previously mentioned. The variables measured were: percentage of oocytes penetrated (the proportion of whole oocytes having a single female pronucleus and a single or multiple penetrated sperm nuclei or male pronuclei; the pronuclei with sperm tail were regarded as male pronuclei); monospermy (the proportion of monospermic penetration oocytes having female and male pronuclei); polyspermy (the proportion of oocytes having a single female pronucleus and multiple penetrated sperm nuclei or male pronucleus); and formation of male pronuclei (the proportion of whole oocytes with male pronuclei).
Evaluation of embryo development
The following variables were evaluated for embryo development: percentage of embryos divided at 48 h and blastocyst development at 120 h per observation, under inverted microscope (200X); total cell number of the blastocysts were counted with staining Hoechst-33342 in an Eclipse 200 microscope equipped with fluorescence at a wavelength of 330 to 380 nm (Nikon Corp., Tokyo, Japan).
Lipid quantification
The lipid quantification in the blastocyst cytoplasm was made through the Sudan-Black B. The embryos were fixed in 70% (v/v) formalin by at least 2 h. Afterwards, the embryos were washed in ultra-pure water for 1 min and subsequently in a 50% (v/v) ethanol solution for 2 min. The embryos were stained with 1% (w/v) Sudan-Black B in 70% ethanol for 30 sec to 1 min and were washed three times in a 50% (v/v) ethanol solution, for 5 min each time. Then, the embryos were washed in a 0.05% (w/v) PVA solution for 5 min and were mounted on a slide with glycerol. Pictures of each embryo were taken with an inverted microscope (Leica, DM IL-LED; camera Leica, DFC295; the equatorial part of the embryo was set in focus). The images were digitized and analyzed with the Software ImageJ™. The percentage of lipids was expressed as relative values with respect to the area occupied by the stained lipids of the embryo total area. The experimental unit was each dyed embryo and the space occupied by the lipids was expressed in percentage.
Experimental design
Assay 1. Effect of PMS on the in vitro production of porcine embryos. The COCs were washed with H-PMS-M or NCSU-23 media (added with follicular fluid)4 and matured in PMS-M or NCSU-23 media. The mature oocytes were washed in H-PMS-F or Tris-buffer medium (TBMm)6 and cultured with sperm in PMS-F or TBMm. The presumptive zygotes were washed in H-PMS-E1 or NCSU-23 media and cultured in PMS-E1 or NCSU-23 media, during 48 h and then, the embryos were washed in H-PMS-E2 or NCSU-23 media and cultured in PMS-E2 or NCSU-23 media, during 120 h as previously described. Two groups were defined: group 1, PMS; group 2, NCSU-23.
Assay 2: Effect of PMS with different macromolecules on the in-vitro oocytes maturation, fertilization and production of porcine embryos. The COCs were washed with H-PMS-M and maturated in PMS-M supplemented with 0.4% BSA or 0.01% of PVA; after maturation, the oocytes were washed using H-PMS-F and cultured with sperm in PMS-F. Then, the zygotes were washed in H-PMS-E1 and cultured in PMS-E1 supplemented with 0.4% BSA or 0.01% of PVA, 48 h. After this step, the embryos were washed in H-PMS-E2 and cultured in PMS-E2 supplemented with 0.4% BSA or 0.01% of PVA, for 120 h as previously described. Maturation and culture were performed in PMS using BSA or PVA in a 2 × 2 factorial arrangement. It was defined four groups: PMS-BSA/BSA. PMS-BSA/PVA, PMS-PVA/PVA, PMS-PVA/BSA.
Statistical analysis
Data was submitted to an analysis of variance, for a completely randomized design (on experiments 2, a 2 × 2 factorial arrangement, being the factors: BSA or PVA in the maturation, and BSA or PVA in the culture), the GLM procedure (SAS Version 9.3, 2012; SAS Institute, Cary, NC, USA) was used. Prior to the analysis, the data expressed as proportions (p) were subjected to a variance homogeneity test, if necessary, they were transformed to its arcsine (√p), to be analyzed using GLM and subsequently transformed back to real values and are expressed as percentages in the results.
Results
Assay 1
There were no significant differences for the oocytes maturation and fertilization, between the group PMS (88.6 ± 0.8 %) and the group NCSU-23 (87.5 ± 3.4 %) (Figure 1; Table 2). The use of PMS significantly increased the total cell number in blastocyst and reduced the percentage of total lipids (58.04 ± 1.8 and 49.4 ± 5.6 %; P<0.05) compared with the group NCSU-23 (37.9 ± 1 and 59.2 ± 2.2 %) (Table 3). However, there were no significant differences in the percentages of embryo division and blastocyst development between groups.
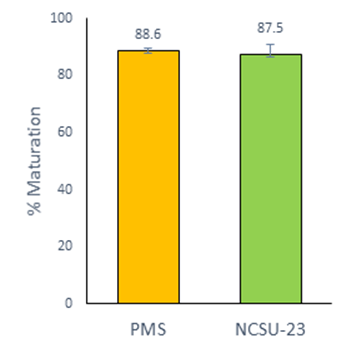
Figure 1 In vitro maturation of porcine oocytes with different media. The graphic shows the Least Square Means ± SEM. Data from five replicates
Table 2 In vitro fertilization of porcine oocytes matured in vitro with different media
| Medium | Oocytes examined |
Percentage of oocytes | |||
|---|---|---|---|---|---|
| Penetrated | Monospermy* | Polyspermy* | MPN-formed* | ||
| PMS | 109 | 82.6 ± 1.2 | 45.4 ± 1.6 | 46.6 ± 0.6 | 92.1 ± 1.3 |
| NCSU-23 | 98 | 84.9 ± 1.4 | 41.5 ± 2.7 | 43.7 ± 4.5 | 85.1 ± 6.0 |
Data from five replicates. Percentages are expressed as Least Square Means ± SEM. *Calculated as a percentage of penetrated oocytes.
Table 3 In vitro development of porcine oocytes matured in vitro with different media after in vitro fertilization
| Medium | Presumptive zygotes cultured | Cleaved at day 2 (%) | Blastocyst at day 7 (%) | Total No. of cells in blastocysts | Total lipids (%) |
|---|---|---|---|---|---|
| PMS | 236 | 72.0 ± 3.3 | 25.4 ± 7.1 | 58.0a ± 1.8 | 49.4a ± 5.6 |
| NCSU-23 | 253 | 77.6 ± 5.7 | 23.7 ± 2.6 | 37.9b ± 1.0 | 59.2b ± 2.2 |
Data from five replicates. Percentages are expressed as least square means ± SEM.
ab Values with different superscripts within each column are significantly different (P<0.05).
Assay 2
There were no significant differences in the oocyte maturation between the group PMS-BSA (92.8 ± 0.9 %) and the group PMS-PVA (91.5 ± 1.3 %) (Figure 2). However, the percentage of monospermic fertilization was significantly lower (42.3 ± 3.1 %; P<0.05) when the oocytes were matured with PMS-BSA in comparison with those matured with PMS-PVA (52.6 ± 3.3 %) (Table 4).
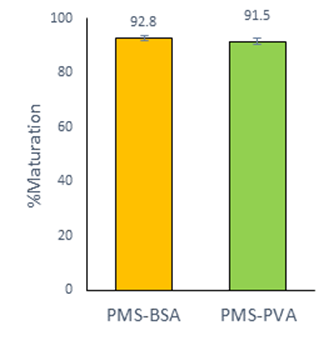
Figure 2 In vitro maturation of porcine oocytes in medium PMS-M with different macromolecule. The graphic shows the Least Square Means ± SEM. Data from five replicates
Table 4 In vitro fertilization of porcine oocytes matured in vitro with different media
| Medium | Oocytes examined | Percentage of oocytes | |||
|---|---|---|---|---|---|
| Penetrated | Monospermy* | Polyspermy* | MPN-formed* | ||
| PMS-BSA | 100 | 94.9 ± 2.5 | 42.3a ± 3.1 | 43.3a ± 2.0 | 85.6 ± 4.0 |
| PMS-PVA | 103 | 88.7 ± 2.1 | 52.6b ± 3.3 | 34.2b ± 2.8 | 86.8 ± 4.0 |
Data from five replicates. Percentages are expressed as Least Square Means ± SEM.
abc Values with different superscripts within each column are significantly different (P<0.05). *Calculated as a percentage of penetrated oocytes
The interaction of using either BSA or PVA in maturation and culture PMS was not significant, for any of the variables evaluated. Nevertheless, the percentage of blastocyst development was significantly higher in the group PMS-BSA/BSA (36.8 ± 6.0 %; P<0.05), compared to the PMS-BSA/PVA and PMS-PVA/PVA (23.5 ± 3.2 and 23.9 ± 4.1 %, respectively) groups, whereas the group PMS-PVA/BSA (30.5 ± 5.1 %) was intermediate. The cell number in the blastocyst was significantly higher for the group PMS-PVA/BSA (46.9 ± 2.5; P<0.05), compared to the PMS-BSA/PVA and PMS-PVA/PVA (38.0 ± 2.3 and 32.8 ± 1.3 respectively) groups, while the group PMS-BSA/BSA (44.9 ± 3.0 was intermediate. The group PMS-BSA/BSA (49.6 ± 3.0 %; P<0.05) was significantly lower for the content of total lipids, compared to the PMS-BSA/PVA and PMS-PVA/PVA (62.4 ± 3.2 % and 61.3 ± 2.0 %) groups, the group PMS-PVA/BSA (51.2 ± 5.6 %) was intermediate (Table 5).
Table 5 In vitro development of porcine oocytes matured in vitro with different media after in vitro fertilization
| Medium | Presumptive zygotes cultured |
Cleaved
at day 2 (%) |
Blastocyst
at day 7 (%) |
Total No.
of cells in blastocysts |
Total
lipids (%) |
|---|---|---|---|---|---|
| PMS-BSA/BSA | 160 | 80.2 ± 2.3 | 36.8a ± 6.0 | 44.9ab ± 3.0 | 49.6a ± 2.9 |
| PMS-BSA/PVA | 155 | 76.8 ± 5.4 | 23.5b ± 3.2 | 38.0bc ± 2.3 | 62.4b ± 3.2 |
| PMS-PVA/PVA | 147 | 79.3 ± 3.9 | 23.9b ± 4.1 | 32.8c ± 1.3 | 61.3b ± 2.0 |
| PMS-PVA/BSA | 148 | 79.4 ± 3.2 | 30.5ab ± 5.1 | 46.9a ± 2.5 | 51.2ab ± 5.6 |
Discussion
Results obtained in the first experiment showed that the porcine oocytes can be matured, fertilized and developed in vitro until the blastocyst stage using the semi-defined PMS. There were no significant differences for the oocytes maturation using PMS (88.6 %) and NCSU-23 medium (87.5 %) supplemented with follicular fluid during this study. These results are similar to other studies that used the NCSU-32 medium supplemented with follicular fluid and a defined medium supplemented with PVA5,7. Similar data for the rate of penetration and monospermic fertilization were obtained (87.6 % and 44.8 %) in oocytes matured in media supplemented with follicular fluid and subsequently fertilized5, compared to this study (82.6 % and 45.4 %).
Percentages of embryos divided and blastocyst production were reported (79.4 % and 28.8 %) in a defined medium8. In this work, was obtained 25.4 % and 23.7 % of blastocysts for PMS and NCSU-23 medium respectively. Other studies reported similar data for production of blastocysts with defined medium and the NCSU-23 medium supplemented with follicular fluid5,7,9. In pigs, the supplementation of follicular fluid in maturation medium has a beneficial effect on the nuclear maturation of oocytes, fertilization and embryo development in vitro10,11,12. However, follicular fluid can be replaced by other macromolecules such as the PVA or BSA fatty acids free5,13. The PMS contains BSA, a total of 22 amino acids, supplement of arginine, melatonin and β-mercaptoethanol, whereas the NCSU-23 medium does not, this perhaps could be favoring the maturation, fertilization, development and embryo quality in this study. It has been demonstrated that the amino acids in a culture medium play an important role, as osmoregulator14, intracellular buffers15, heavy metals chelators16 and energy substrates17. The beneficial effects of the essential and non-essential amino acids in a chemically defined NCSU-23 medium on the porcine oocytes maturation have been reported13.
The melatonin and its metabolites are effective antioxidants, which scavenger the reactive oxygen species (ROS) and regulate several antioxidant enzymes18,19. Melatonin has been found to regulate the oocyte maturation in carp20 and improves the in vitro embryo development and total cell number in porcine blastocyst21. The PMS showed a lower percentage of lipids (49.4 %), compared to the NCSU-23 medium (59.2 %). It also has been demonstrated that melatonin has large lipolytic properties22. Melatonin promotes lipid metabolism have been found in porcine oocytes, providing a source of essential energy for oocyte maturation and subsequent embryo development23. It has been reported that the decrease of intracitoplasmatic lipids in embryos, increases their cryotolerance due to a lower lipid peroxidation and therefore a lower cell damage24,25.
Arginine is a vital amino acid for many metabolic processes in the cell, such as protein synthesis, creatine production, polyamines synthesis and nitric oxide generation26. Arginine has a positive effect on the oocytes maturation27 and improves the in vitro embryo development in pig28. The addition of arginine in the embryo culture medium increases the percentage of embryos that develop up to the stage of blastocyst and the total cell number of blastocyst29. The PMS showed a significant greater total cell number (58.04) compared to the NCSU-23 medium (37.9). An indicator of the embryo quality is the total cell number of blastocyst29.
The implementation of a semi-defined or defined cultured media system for in vitro embryo production is considered important to observe the effects of some supplements of interest in the medium on the maturation, fertilization and embryo development by eliminating these unknown factors that the follicular fluid provides to the medium30.
In the second assay, the results showed a percentage of monospermic fertilization significantly lower (42.3 ± 3.1 %; P<0.05) when the oocytes were matured with PMS-BSA compared with PMS-PVA (52.6 ± 3.3 %). It has been demonstrated that the addition of PVA to maturation medium improves the percentage of monospermic embryos during the in vitro fertilization of porcine oocytes5,8. When the maturation medium is supplemented with PVA, monospermic fertilization can reach between up to 70-80 % of success5,7. However, a decline has been observed in the penetration rate of matured oocytes when replacing the follicular fluid with PVA in the NSCU-37 medium, perhaps due to that, the number of oocyte that reach the metaphase II stage during fertilization, decreases5. During this study, was observed that the sperm penetration does not decrease when replacing BSA with PVA in the maturation medium, probably due to the fact that the PMS has a higher content of amino acids and factors, that could be contributing conditions than the BSA provides. Future histological and biochemical analysis of matured and fertilized oocytes are required to understand the mechanism of polyspermic fertilization in the system of in vitro production of porcine embryos with the addition of different macromolecules.
Addition of BSA during the embryo culture, improves the embryo development, the cell number in the blastocyst and decreases the total lipids, probably due to the nutrients that BSA brings. BSA plays a role in embryo development31, formation and hatching of blastocyst32. However, it did not find differences in the percentage of bovine embryos that reached the stage of blastocyst in a medium supplemented with PVA, BSA or fetal bovine serum33. However, the blastocysts produced in PVA had a lower amount of cell number. Future studies are required to determine if there is a relationship between the total cell number and lipids in the porcine blastocyst.
Conclusions and implications
Results suggest that the semi-defined PMS, has favorable effects on the total cell number and decrease of total lipids of blastocyst in pigs; so they can be used for the in vitro production of porcine embryos. The use of PVA instead of BSA during the maturation, has a positive effect on the rate of in vitro monospermic fertilization of porcine oocytes. Future histological and biochemical analysis on the oocytes maturation and fertilization, as well as the embryo development, are required to understand the mechanisms of action of macromolecules during in vitro production of porcine embryos. The PMS developed by the CNRG could be used to favor the cryopreservation of in vitro produced porcine embryos.











 texto em
texto em 


