Campylobacter fetus subspecies venerealis is the causal agent of bovine genital campylobacteriosis1. Bulls can carry the bacterium asymptomatically in the prepuce for indefinite time and transmit the agent to females at mating. Infected females can develop infertility, embryonic death or abortion. Abortion can occur at any gestational age, but it is more commonly diagnosed in the fourth to sixth month of gestation2. Lesions caused by C. fetus venerealis include endometritis, placentitis, fetal serositis, hepatitis and pneumonia1.
The protozoan Neospora caninum is an important cause of abortion in beef and dairy cattle in South America3. Members of the Canidae family are definitive hosts and shed oocysts in feces4. Cattle are intermediate hosts and get infected with N. caninum by ingestion of oocysts or by transplacental transmission. The definitive hosts acquire the infection by ingesting bradyzoites that are encysted in the tissues of the intermediate hosts. Depending on the gestational age at the time of infection, fetal death with either abortion or mummification can occur. If the infection takes place within the first 100 d of gestation, the chances of fetal survival are low because there is incomplete development of the fetal immune system4. Abortion due to N. caninum frequently occurs during the second or third trimester. If the fetus develops an immune reaction against N. caninum, it is born as seropositive calf. However, the birth of seronegative calves from seropositive dams can occasionally occur4. Necropsy findings in aborted fetuses are scant, fetuses can be severely autolytic or mummified. Grossly, the placenta can show necrosis of the cotyledons with no changes of the intercotyledonary region. The fetal heart and skeletal muscles can have gray to whitish foci, that microscopically are characterized by necrosis and inflammation. The main microscopic lesions in the fetus are multifocal non-suppurative necrotizing encephalitis with gliosis, myocarditis and myositis, which are highly specific of this protozoon4.
This report describes a bovine abortion outbreak in a commercial dairy farm caused by the concurrent action of two different pathogens. The importance of performing multiple diagnostic tests in various fetuses and serological studies in the cows is highlighted.
The outbreak occurred in a brucellosis free dairy herd with 650 milking Holstein cows in a semi-extensive system with periods of confinement of variable lengths depending on pasture availability. The farm was located in Florida department, Uruguay. The average daily milk production was approximately 20 L/cow. Calving was scheduled in autumn-winter, and artificial insemination was performed from May to October, followed by natural breeding with bulls. The affected farm worked with a second dairy farm where they received cows for insemination that have calved at least two months before. After being inseminated, these cows remained in the farm during lactation. A total of 45 cows aborted during a period of 3 wk in November 2015. Five fetuses (cases 1-5) were necropsied, the gestational age was estimated based on the crown-to-rump length and other gross characteristics of the fetuses5. There was no placenta submitted for examination in any of the cases. For histology, fetal tissues were fixed in 10 % neutral buffered formalin, embedded in paraffin, sectioned at 4 to 6 µm, and stained with hematoxylin and eosin. Immunohistochemistry (IHC) was performed in sections of brain for N. caninum, sections of kidney and liver for Leptospira spp., and in liver, heart, and lung for bovine viral diarrhea virus (BVDV)6-8.
Titers of antibodies against Leptospira spp. were determined by microagglutination test (MAT) on samples of pericardial/thoracic fluid from the five fetuses, with a cutoff point >1/10. Samples of abomasal fluid, and liver from the five aborted fetuses were spiked in blood Agar in microaerophilic conditions.
The molecular identification of N. caninum was done from formalin-fixed paraffin-embedded sections of brain in those fetuses with microscopic brain lesions typical of neosporosis. The DNA was isolated using a commercially available kit (DNeasy Tissue Kit, QIAGEN Group, Germany) according to the manufacturer’s recommendations, and DNA concentration was measured using an Epoch micro-volume spectrophotometer system (Epoc, Bioteck® Instruments, Inc., Vermont, USA). Neospora caninum DNA was assessed by a nested-PCR targeting the internal transcribed spacer one (ITS1) region with four oligonucleotides, as previously described9.
The diagnosis of Campylobacter infection was done by bacterial culture, inoculating samples of fetal abomasal fluid, lung, and liver in Skirrow agar. Incubation was performed for 48 h at 37 ºC in an AnaeroJar™ (Oxoid) with a microaerobic environment (approximately 5 to 10% O2, 5 to 10% CO2) generated with CampyGen™ sachets (Oxoid)10. Direct immunofluorescence was done on smears of fetal abomasal fluid (20 µL) fixed in acetone at 20 0C for 30 min, using a commercially available fluorescein-isothiocyanate conjugated antiserum (FITC) against Campylobacter fetus (Biotandil, Argentina), with appropriate positive and negative controls provided with the kit. Incubation was performed inside a humid chamber at 37 ºC for 30 min, slides were then visualized under an AXI0 Lab A.1 microscope with a FITC filter and 470 nm UV light.
For the molecular identification of the isolates, DNA was extracted using the Gene bacterial genomic DNA extraction kit (Sigma-Aldrich, USA), following by two separate multiplex PCR protocols that amplify specific regions of the C. fetus genome that discriminate between C. fetus subspecies11,12. Additionally, the almost complete gen that codifies the 16S rRNA was amplified using the universal primers 27F and 1492R13. The PCR products were purified and sequenced at Macrogen Inc., Seoul, South Korea. The obtained sequences were compared with sequences from public databases using the "Classifier” tool from the Ribosomal Database Project and BLASTn from the National Center for Biotechnology Information14,15.
Indirect fluorescent antibody test for the detection of anti-Neospora caninum antibodies was done in serum of 27 cows from the affected herd at the Division of Veterinary Laboratory of the Uruguayan Ministry of Agriculture, Livestock and Fishery, following their standard protocols.
All five necropsied fetuses (cases 1-5) had approximate gestational ages of 180 d. Grossly, case 1 had diffuse fibrinous epicarditis (Figure 1) and peritonitis. Histologically in this fetus, there was neutrophilic bronchopneumonia and epicarditis. Campylobacter fetus was detected by direct immunofluorescence and isolated from abomasal fluid and lung. Identification of the isolate was further confirmed by PCR, which yielded amplification products of sizes corresponding to those described for C. fetus subsp. venerealis11,12. Additionally, the 16S rDNA gene sequence of the isolates was compatible with C. fetus. No Campylobacter spp. were isolated from samples of cases 2-5.
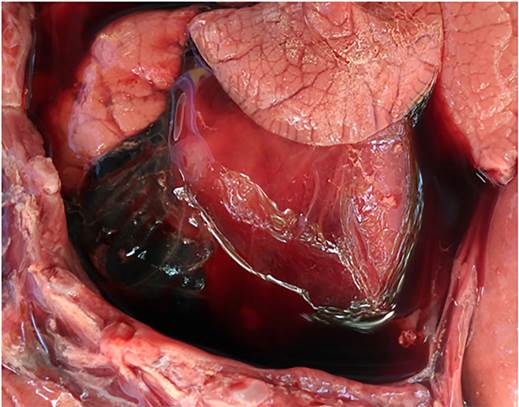
Figure 1 Case 1, aborted by Campylobacter fetus subsp. venerealis. The epicardium is covered by moderate to large amount of fibrinous material along with serosanguinous fluid
No significant gross lesions were observed in cases 2 to 5, and no microscopic lesions were observed in cases 2 to 3. However, in cases 4 and 5, histology revealed multifocal necrotizing non-suppurative encephalitis (Figure 2), lymphocytic and histiocytic myocarditis and myositis, and lymphocytic interstitial nephritis. Neospora caninum antigen was detected intralesionally in the brain of these two fetuses by IHC (Figure 2 inset) antibody titers to N. caninum ranged from 1/200 to 1/3200 in 10 of the 27 examined cows. Additionally, PCR for N. caninum DNA was positive in both cases. BVDV IHC was negative in liver, heart, and lung in cases 2, 4 and 5. Lastly, the IHC for Leptospira spp. was negative in kidney and liver from all fetuses. No antibody titers against Leptospira spp. were detected in any of the five fetuses. Other abortigenic pathogens were ruled out, such as Brucella spp. Their negativity was based on negative isolation of the pathogen and the absence of the pathogen in association with the compatible lesions.
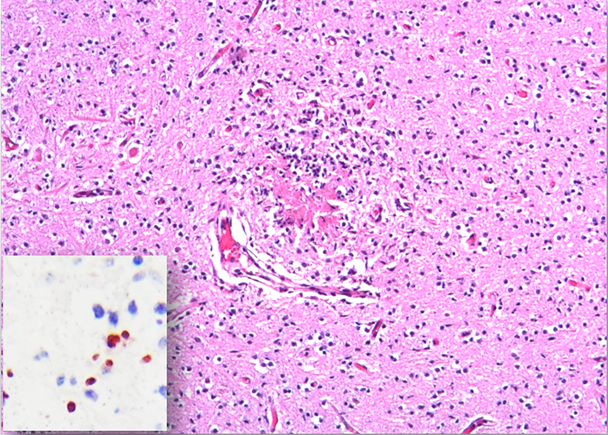
The neuropil is disrupted by necrotizing non-suppurative multifocal encephalitis. Hematoxylin and eosin stain.
Inset. There is immunoreaction against Neospora caninum antigen within the affected sections of brain.
Figure 2 Fetal brain from case 4 aborted by Neospora caninum
The etiologic diagnosis of bovine abortion is complex because multiple potential causes can be involved, and fetal autolysis can preclude the identification of the etiologic agent. In this outbreak, the identification of two abortigenic pathogens in 3/5 fetuses suggests that the examination of various fetuses is recommended. While coinfection by multiple abortifacients has been reported16,17, little has been discussed about the concurrent detection of different pathogens in different aborted fetuses and in outbreaks of abortion in dairy farms. Abortion outbreaks can be caused by different infectious agents contemporaneously.
The main identified causes of bovine abortion in dairy and beef cattle in South America are infectious18-22. In one study from Uruguay, the most frequent cause of bovine abortion identified in laboratory submissions was leptospirosis (41 % of 241 cases with diagnosis), followed by neosporosis (36 %) and campylobacteriosis (12 %)20. In Argentina, leptospirosis was the third most frequently detected cause (7.3 %) 21 while in Brazil it was diagnosed in 0.6 % of the abortions22. Such differences may be due to the different frequencies of leptospirosis in these countries, but also to the different laboratory techniques used for the diagnosis and interpretation of the results. The diagnosis in the Uruguayan study was based on the presence of high titers of antibodies in aborted cows (>1/800) and/or fetuses (>1/10), whereas in Argentina and Brazil the etiologic diagnosis was based on detection of Leptospira spp. by PCR, immunofluorescence, immunohistochemistry, and/or Warthin Starry stain in fetal samples19,22. Tests that aim at detecting the agent in the aborted fetuses are more suitable for the confirmatory diagnosis of abortion by Leptospira spp. than serologic tests performed on the dam´s serum of fetal fluids.
The microbiologic and pathologic evaluation of the placenta in cases of abortion is key to increase the chances of reaching a diagnosis. For some diseases, such as coxiellosis or chlamydiosis, it is difficult to arrive to an etiologic diagnosis if the placenta of aborted cows is not evaluated. In this outbreak, no placentas were examined, and this could have been a limitation for the determination of the diagnosis in two of the five fetuses. Previous reports in the USA show that placentitis can result in abortion, in the absence of fetal lesions23.
In this report, the diagnosis of C. fetus subsp. venerealis was confirmed in one of the fetuses. The affected herd practiced artificial insemination followed by natural breeding. A national survey including 340 farmers indicated that only 21 % of the dairy farms used artificial insemination and 29 % used natural breeding after artificial insemination24. Half of the farms used only natural breeding22. These data suggest that bovine campylobacteriosis, diagnosed for the first time in Uruguay in 1970 in dairy cows25, is still a health problem in dairy farms in the country. Nevertheless, natural breeding maintains the risk of bovine genital campylobacteriosis and should be avoided when possible. Neosporosis was diagnosed in two fetuses and about 37 % of the examined cows had titers against N. caninum. A serologic survey done in beef cattle in Uruguay showed that in 2006, neosporosis was present in 69.2 % of 229 farms, and that 14.3 % of the cows and 12.9 % of the heifers were seropositive26 proving that N. caninum is endemic in the Uruguayan bovine population, including dairy bovines27.
In this outbreak, antibody titers against L. interrogans serovars were detected in serum of 15/18 cows examined by MAT (data not shown). Unfortunately, the vaccination status of the herd unknown, and whether these dams had aborted or not was not recorded. The antibodies detected were against serovars Pomona (13 cows), Hardjo-prajitno (9 cows), Wolfii (9 cows) and Hadjo-bovis (seven cows), with titers ranging from 1/200 to 1/3200. In the absence of fetal lesions compatible with leptospirosis coupled with negative IHC results, and the negative MAT results in the thoracic/cavitary fetal fluids, it is possible to conclude that none of the five examined fetuses were infected with Leptospira spp.
Abortion outbreaks can be caused by different infectious agents contemporaneously in the same herd. In such cases, it is necessary to perform necropsies in many fetuses using the specific techniques for each agent and, if possible, to evaluate the placenta, along with the serum of the dams.
The authors thank Cecilia Monesiglio, Anderson Saravia, Yisell Perdomo and all graduate students of the animal health platform at INIA. This work was funded by grant FSSA_X_2014_1_105696 of the Uruguayan “Agencia Nacional de Investigación e Innovación” (ANII).











 texto en
texto en 


