Introduction
The economic gains of a dairy farm increase as cattle reproductive efficiency improves. However, the historical decline in fertility of Holstein dairy cows hampers profitability, but at the same time offers a challenge to develop strategies to enhance reproductive performance. The cause of low fertility in modern Holstein dairy cattle is multifactorial. The main associated are the improvement in genetic merit to milk production, the inability to meet nutritional requirements, the adverse environmental conditions and the susceptibility to diseases that compromise oocyte and embryo viability1.
The exact cause of low fertility is unknown, but oxidative stress could be implicated. Oxidative stress results when free radicals exceed the organism’s antioxidant capacity2. Free radicals are molecules with an unpaired electron, highly reactive and normally produced in living aerobic organisms3. At a controlled production rate, they serve as molecular signals, but over production may result in a pathological process4. Sources of free radicals that may surpass the cow’s antioxidant capacity include milk production yield and heat stress. High milk producers have higher blood concentrations of oxidative stress markers than those that produce less milk5, and are also more susceptible to heat stress6. This is relevant because heat stress produces oxidative stress in dairy cattle7. Oxidative stress creates unfavorable intraoviductal conditions8 that result in embryo death9.
Oxidative stress is counteracted by antioxidants, which suppress the deleterious effect of free radicals by giving them one electron. One antioxidant that is relevant to mammalian reproduction is water soluble ascorbic acid (vitamin C, here after referred to as VC)10. The chemistry and biological functions of VC in cattle have been reviewed by others11, and will therefore be not further addressed here. However, the impact of VC supplementation on dairy cattle fertility has been poorly studied, probably because bovines can synthetize their own VC in the liver from glucose11, and thus have no need for external supplementation12. Nevertheless, the same factors that are blamed for disrupting fertility (high milk yield and heat stress) decreased blood VC concentration in dairy cattle13,14. It might be suspected that if VC is necessary for reproduction, a diminished supply could affect fertility. Previous research has shown that supplementation of VC is advantageous to improving reproductive performance of repeat breeder cows15 and dairy cattle under heat stress conditions16. It is important to consider that VC supplementation and impacts on dairy cattle fertility deserve more attention.
The objective of this review is to contribute to the current knowledge regarding the relationship between VC and fertility, and to share the experiences on the relevance of VC supplementation to improve dairy cattle reproductive performance.
Ovarian follicle and corpus luteum development
Vitamin C deficiency increases the number of atretic follicles17. However, supplementation attenuates follicular cell apoptosis18, promotes primordial follicle activation19, increases the population of growing follicles20 and reduces those in atretic state21. These findings suggest that VC supports the development of healthy ovarian follicles.
The ovarian follicle is under constant structural remodeling. Its diameter increases up to 475 times from the primordial to the ovulatory size22,23. This increase in size implies a constant remodeling of the follicular basal lamina24 and changing intrafollicular concentrations of VC, which are higher in smaller follicles25. The follicular basal lamina gives the follicle stability and serves as a molecule filter24, but it needs increasing amounts of collagen as it increases in size26. Since VC is a cofactor in collagen synthesis27, it is logical to assume that VC would be required in higher quantities in developing follicles. In fact, supplementation of VC improves follicle survival and increases the odds of a follicle reaching preovulatory size28. This could be explained by VC preventing follicular cell death and maintaining base membrane integrity as the follicle grows18,29.
Under an environment with a regressing corpus luteum, the dominant follicle will reach the preovulatory state. At this stage, VC is needed for normal follicular steroidogenesis30, which is accomplished by promoting the expression of key enzymes involved in steroidogenesis such as aromatase and P450 cholesterol side-chain cleavage31. However, as the follicle grows there is a reduction in the concentration of VC. Preovulatory follicle has lower intrafollicular concentrations of VC than large follicles from other stages of the estrus cycle32. This reduction may result from a higher intrafollicular concentration of IGF-I, which induces the uptake of VC by granulosa cells33. The LH surge also causes a reduction in VC concentrations34, probably by increasing intrafollicular reactive oxygen species (ROS) concentrations35.
The reduced intrafollicular concentrations of VC at the preovulatory stage may be part of the mechanism controlling ovulation. The collagen in the follicular basal lamina is reduced as the follicle grows, which makes it more expandable and easier to remodel36. The reduced intrafollicular concentrations of VC, together with degradation of collagen in preovulatory follicles, results in the weakening and rupture of the basal lamina, which are crucial events that can lead to preovulatory follicle rupture37,38.
The number of pregnant women with luteal phase defects increases after supplementing VC, which likely worked by increasing corpus luteum progesterone39. Corpus luteum diameter32 and concentration of progesterone40 has been related to VC concentration. In addition, the content of VC is higher during the early stages of corpus luteum development41, reaching the highest concentration, at least in bovines, on d 12 of the estrous cycle42. Furthermore, one key element in the relationship between VC and the corpus luteum is that this vitamin is required, as mentioned previously, for the synthesis of collagen, which is essential for corpus luteum development43.
Vitamin C and fertility
Vitamin C improved fertility44. The enhancement in oocyte and embryo development could explain these results45,46. Unfortunately, the limited information available on this topic has been obtained mostly under in vitro conditions. In contrary, high doses of VC might harm both the oocyte (750 µM mL-1) and embryo development (˃200 󠅾µM in culture medium)47,48, possibly resulting from a pro-oxidant effect of VC. The VC at low concentrations can act as an antioxidant while the opposite occurs at high concentrations, which may depend on the concentration of metal ions (iron)49. A pro-oxidant effect of VC could be expected as the concentration of metal ions increases50. The latter may be true under in vitro conditions, but it is unlikely to occur in living organisms51.
Relationship between vitamin C and vitamin E
Vitamin C may control follicular development by interacting with other elements known to affect fertility. It is well accepted that after vitamin E fulfills its antioxidant activity, it can be reactivated by VC52, which increases its availability53. Vitamin E deficiency disrupt follicle development, produces estrous cycle abnormalities and pregnancies loss54. It is not known exactly the blood concentration at which vitamin E can be considered as adequate or deficient in cattle. Vitamin E blood concentrations ˃1 µg mL-1 can considered as adequate, but there is not agreement on this topic55. In addition, it is unaware of any vitamin E recommendation for optimal reproduction performance in cattle. However, previous work (see next section of this manuscript and reference 16), have shown that supplementation of 3,000 IU of vitamin E during a synchronized estrus is advantageous to improve fertility in dairy cattle.
The relationship between vitamins C and E in reproductive issues has received little attention. An antioxidant system, that includes vitamins C and E, is activated during ovarian steroidogenesis42. The supplementation with vitamin C (125 mg kg-1 d-1) and E (75 mg kg-1 d-1) to rats increases blood concentrations of testosterone, FSH and LH56. These higher concentrations of gonadotropins are in agreement with the fact that VC stimulates its secretion from pituitary57. Studies in vitro have shown a positive effect of vitamin C and E on oocyte quality and embryo development when supplemented separately, but not together53,58,59. Addition of vitamin C and E to the maturation medium impairs blastocyst occurrence rate by preventing the formation of the amount of ROS necessary for oocyte developmental competence53. This is acceptable because a tonic supply of ROS has proved to interrupt oocyte meiotic arrest60. However, it is unlikely that the situation described by Dalvit et al53 also occur in vivo because supplementation of both vitamins has resulted in more pregnancies in dairy cattle (see next section of this manuscript and reference 16). In addition, an improvement in embryo quality after injecting superovulated cattle donors before estrus with two antioxidants, β-carotene and vitamin E, has been reported61.
In vitro studies resemble conditions found under physiological conditions. However, contrary to in vivo conditions, in vitro systems are static, where metabolic activity, nutrient adsorption and storage, as well as waste disposal are limited by time and medium culture conditions. In addition, adaptation to changing conditions is faster in in vivo systems. Therefore, when supplementing vitamin C concomitant with vitamin E, the living organism choose between to storage, to excrete or to distribute them to where they are needed. This avoid possible harmful effects on cell biological process such as those affecting oocyte quality and embryo development.
Experiences supplementing vitamin C to dairy cattle
The evidence presented here supports a prominent role of VC on fertility. The first approach to evaluating the effect of VC on dairy cattle fertility was carried out on cows under heat stress conditions16. The results of this study revealed that injecting both vitamin C and E results in more pregnant cows than administering one or the other separately. In addition, no effect of vitamins supplementation was found on preovulatory follicle and corpus luteum size. These findings led to assume that the increased number of pregnant cows, obtained after supplementing both vitamins, was the result of cows carrying a healthier follicle, which eventually becomes a corpus luteum that produces more progesterone than that carried by non-supplemented cows. To prove this assumption a second trial was carried out (T2).
The general procedure, as well as justification of the doses and time of vitamins injections used in T2 is explained in detail elsewhere16. Briefly, the follicular wave of the cows was synchronized with a device containing 1.0 g of progesterone (Sincrogest®, Ourofino Agronegocio), inserted intravaginally for 8 d, and an intramuscular (i.m.) injection of 250 µg of GnRH analogue (GnRH, Sanfer). Estrus behavior was induced by an i.m. injection of 500 µg of cloprostenol (Celosil, MSD, Animal Health) at intravaginal device removal. Once the intravaginal device was withdrawn, the animals were constantly monitored by direct observation for signs of standing estrus. The cows were artificially inseminated 12 h after standing estrus with a single dose (approximately 20 x 106 spermatozoa) of semen from a single bull of proven fertility. Cows that received vitamins (n=32. Control group, n=28) were injected with a single i.m. injection of 3,000 IU of vitamin E ((±) α-tocopherol, Sigma-Aldrich)) on d-5 (day 0 is the day of intravaginal device removal) and subcutaneous (s.c.) injections with a total dose of 3,000 mg of VC (ascorbic acid, Q.P., Reasol) on d-5, immediately after estrus detection and 2 d after artificial insemination.
As depicted in Table 1, vitamin supplementation did not affect preovulatory follicle or corpus luteum size. In addition, no effect was noted on blood estradiol and progesterone production. However, in agreement with previous findings16, pregnancy rate was higher (P=0.06) in cows injected with vitamins 45 d after artificial insemination (Figure 1).
Table 1 Least square means (±SE) for the effect of injecting vitamin C and E on ovarian structures size, estrus presentation and hormone concentrations in Holstein dairy cows
| Treatment | |||
|---|---|---|---|
| Variable | Control (n=28) |
Vitamin Cand E (n=32) |
P-value |
| Time to estrus, h | 57.1±4.89 | 58.4±4.57 | 0.67 |
| Diameter of the preovulatory follicle, mm | 18.3±0.57 | 17.2±0.60 | 0.21 |
| Estradiol concentration, pg mL-1 | 45.1±3.12 | 46.8±3.26 | 0.71 |
| Area of the corpus luteum, cm2 | 6.9±0.39 | 6.7±0.37 | 0.74 |
| Progesterone concentration, ng mL-1 | 10.8±1.60 | 12.5±1.60 | 0.26 |
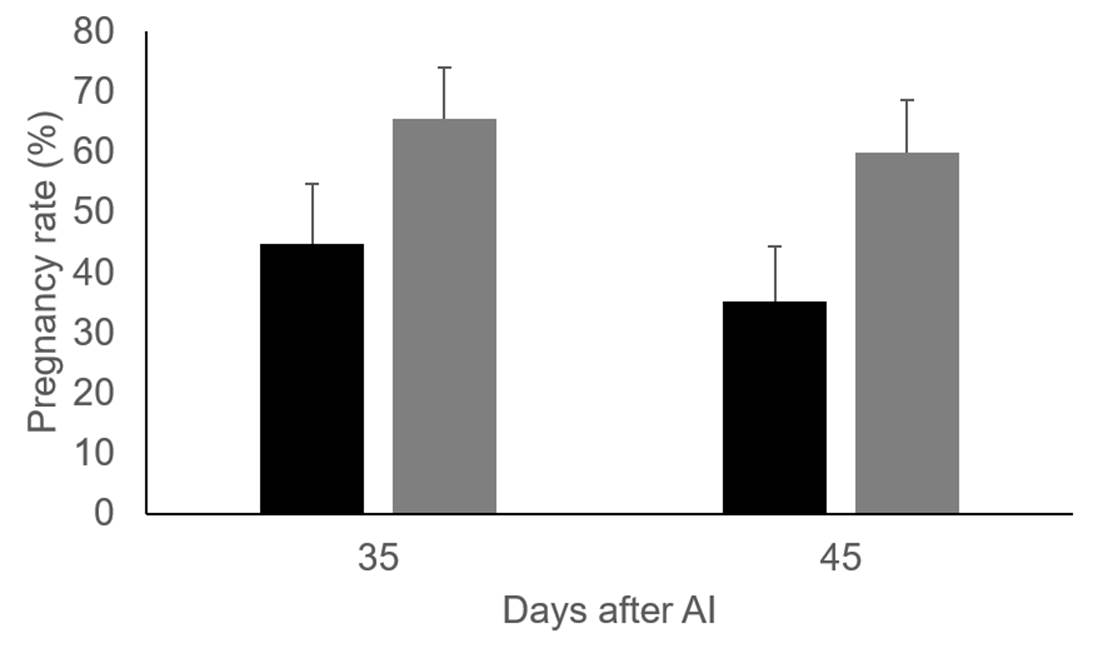
Figure 1: Pregnancy rate 35 and 45 days after AI in control group (black bars, n=28) and Holstein dairy cows injected with vitamins C and E (grey bars, n=32)
Estrus synchronization is a reproductive tool used in dairy cattle to improve fertility because it makes possible to control the onset of estrus. However, most technicians prefer to use fixed-time artificial insemination because it avoids the need for estrus detection. In addition, it is very convenient because all the cows are scheduled to be inseminated at the same time. Based on previous findings, it was decided to incorporate vitamin C and E injections to a fixed-time artificial insemination protocol (T3) to increase the number of pregnant cows. Briefly, cows were injected i.m. with 250 µg of GnRH analogue on d 0, 7 days after administering an i.m. injection of 500 µg of cloprostenol. A second dose of GnRH was given to cows 48 h after injecting cloprostenol. Insemination was performed 14 to 16 h after the second injection of GnRH. Injections of vitamins C and E were carried out as mentioned in T2, but the first injection of vitamin C and E was given 3 d after the first injection of GnRH. The second and third injections of VC were administered just after the second injection of GnRH and 2 d after artificial insemination.
The effect of vitamin C and E injections on preovulatory follicle diameter (16.8 ± 0.70 vs 16.2 ± 0.77 mm, for control group and cows injected with vitamins) and area of the corpus luteum (5.4 ± 0.48 vs 6.1 ± 0.50 cm2, for control group and cows injected with vitamins) were not significant. Similar to previous results20 and with T2, a greater percentage of cows were found to be pregnant 30 and 45 d after artificial insemination in the group of cows supplemented with vitamins than those in the control. However, the differences are not significant, most likely because of the small sample size used in T3 (cows injected with vitamins, n=16. Control group, n=17), Figure 2.
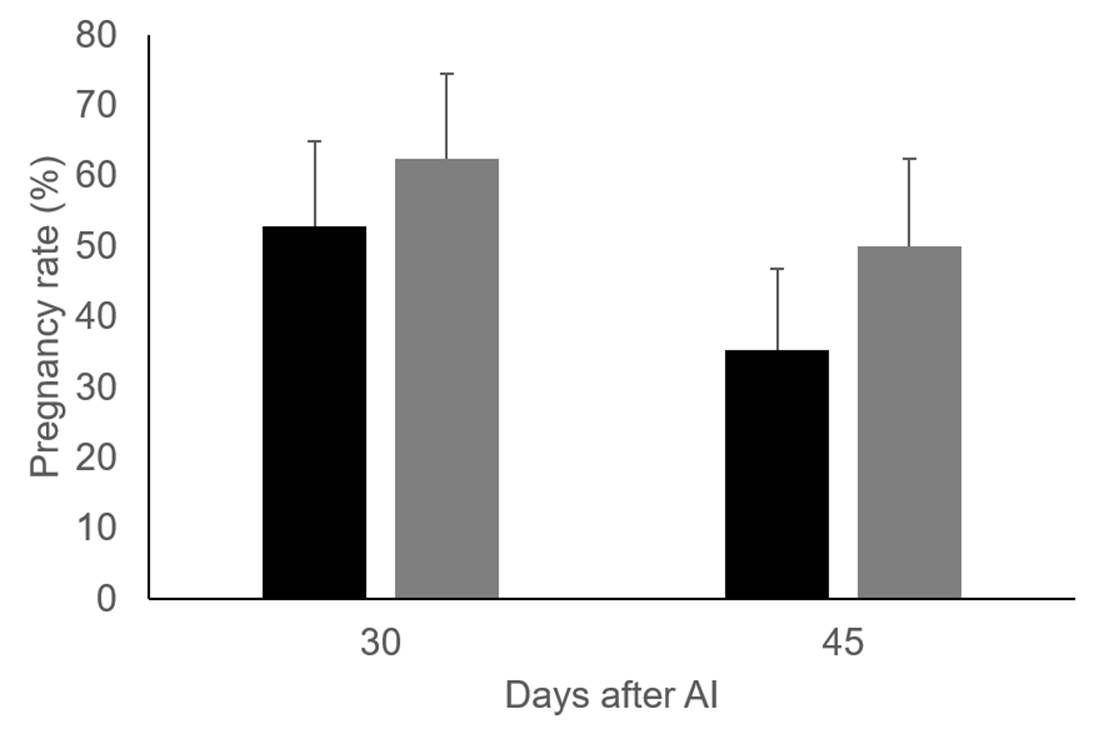
Figure 2: Pregnancy rate 30 and 45 days after AI in control group (black bars, n=17) and Holstein dairy cows injected with vitamins C and E (grey bars, n=16)
The results obtained show that VC injections in combination with vitamin E are a feasible way to improve dairy cattle fertility. This effect is not mediated by changes in preovulatory or corpus luteum size, nor by affecting estradiol or progesterone production. A likely explanation for the increased pregnancy rate in dairy cattle injected with vitamins C and E is that cows supplemented with vitamins produce better quality oocytes and embryos than those not supplemented.











 texto en
texto en 


