Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.10 no.2 Mérida abr./jun. 2019
https://doi.org/10.22319/rmcp.v10i2.4529
Articles
Quantifying ruminal fermentation and methane production using the in vitro gas technique in the forages of a sheep silvopastoral system in Chiapas, Mexico
a ECOSUR (El Colegio de la Frontera Sur, Unidad SCLC), Departamento de Agricultura, Sociedad y Ambiente. Carr. Panamericana s/n, San Cristóbal de las Casas, Chiapas, México.
b ECOSUR ((El Colegio de la Frontera Sur Unidad Campeche). Campeche, México.
c UNACH (Universidad Autónoma de Chiapas), Facultad de Agronomía. Chiapas, México.
d Tecnológico Nacional de México. IT Conkal. Yucatán, México.
e ECOSUR ((Universidad Autónoma de Chiapas Unidad Tapachula). Chiapas, México.
Ruminal fermentation and methane production in a sheep silvopastoral system were quantified with the in vitro gas production technique. Evaluations were done of local energy sources (molasses, Zea mays L. and Musa paradisiaca L.), of the base forage (Panicum maximum cv. Tanzania), of forage tree foliage (Gliricidia sepium (Jacq.) and Leucaena leucocephala cv. Cunningham), and diets combining these elements. Ruminal fluid was collected from five sheep (Pelibuey x Katahdin; 40 ± 3 kg). Five treatments (diets) containing different mixtures of forage tree foliage, energy sources and the base forage were analyzed in a completely random experimental design. Maximum gas volume production (V) was observed in M. paradisiaca (544 ml/g-1 DM) and Z. mays (467 ml/g-1 DM) (P≤0.05). The lowest V values were for the foliage of G. sepium (253 ml/g-1 DM) and L. leucocephala (180 ml/g-1 DM) (P≤0.05). Of the diets, D4GMP (48% P. maximum, 30% G. sepium, 7% Z. mays, 15% M. paradisiaca) had the highest V value. Methane production ranged from 6.31 to 9.60 L/Kg digested DM, and did not differ between treatments (P>0.05). Data were used to generate a potential fermentable gases emission index, which suggested that the diets containing slow fermenting carbohydrates resulted in higher gas emission rates. Inclusion of forage trees and local energy sources in sheep silvopastoral management systems can improve diet quality and contribute to reducing CH4 emissions.
Key words: Mitigation; Climate Change; Energy; Agroforestry
Se evaluaron mediante la técnica de producción de gas in vitro, fuentes energéticas locales (melaza, Zea mays L. y Musa paradisiaca L.) sobre la fermentación ruminal y producción de metano de diversos forrajes usados en un sistema silvopastoril con Panicum maximum cv. Tanzania, Gliricidia sepium (Jacq.) y Leucaena leucocephala cv. Cunningham, con ovinos. Se usaron cinco borregos Pelibuey x Katahdin 40 ± 3 (µ±DE) kg como donantes de líquido ruminal. Se analizaron cinco tratamientos (dietas) con diferentes mezclas de follaje de arbóreas y fuentes energéticas en un diseño experimental completamente al azar. M. paradisiaca y Z. mays presentaron los mayores registros de volumen (V) máximo en producción de gas (544 y 467 ml/g-1 MS, respectivamente) (P≤0.05). El follaje de G. sepium y L. leucocephala tuvieron los menores valores de V (253 y 180 ml/g-1 MS, respectivamente) (P≤0.05). La dieta D4 GMP (48 % P. maximum, 30 % G. sepium, 7 % Zea mays, 15 % M. paradisiaca) registro el mayor valor de V. No hubo diferencia (P>0.05) en la producción de metano en las dietas usadas, teniendo un rango de 6.31 a 9.60 de LCH4/kg MSDIG. Se generó un índice de emisión potencial de gases fermentables (IPEGF), el cual sugirió que dietas con carbohidratos de lenta fermentación, contribuyen a un índice más alto de emisión de gases. Por su mejoramiento en la calidad de las dietas y en contribuir en una baja de emisiones de CH4, se sugiere el manejo de arbóreas forrajeras como G. sepium y L. leucocephala, incorporando fuentes energéticas locales.
Palabras clave: Mitigación; Cambio climático; Energía; Agroforesteria
Introduction
Livestock are key to the survival of more than 800 million of the world’s poor1. However, animal production also contributes to natural resource degradation, environmental pollution and climate change2, mainly through greenhouse gas (GHG) emissions3. Tropical livestock farming in Latin America is primarily based on grazing native and introduced grasses in extensive systems4, with little or no supplementation, minimal infrastructure and low capital investment5. In this context, silvopastoral systems and use of local forage trees and shrubs have been shown to improve livestock production systems, reduce their environmental impact and contribute to GHG mitigation6-9.
In silvopastoral systems, the protein in the foliage of multiple-use trees (e.g. the genera Leucaena, Gliricidia and Erythrina, among others) degrades rapidly in the rumen. Addition of ingredients providing energy to the diet are therefore required to improve rumen fermentation efficiency, synchrony and nutrient balance10,11. High-quality commercial energy byproducts for use in livestock meat and dairy systems can be costly12, highlighting the need to search for energy supplements among local resources that are both easily accessible and provide adequate nutritional value13. The foliage of many forage trees contains secondary metabolites14, and many of these can mitigate enteric methane emissions in ruminants15,16. Indeed, the foliage from some forage trees is known to reduce rumen populations of protozoa and methanogenic archaea17,18,19, leading to lower enteric CH4 synthesis and production20.
The present study objective was to evaluate the effect of addition of local energy sources on ruminal fermentation and methane emissions parameters when combined with forages in a sheep silvopastoral system involving P. maximum supplemented with Gliricidia sepium and Leucaena leucocephala foliage.
Materials and methods
Study area
Materials were obtained from a sheep ranch managed with silvopastoral techniques and located in the municipality of Chiapa de Corzo, in the state of Chiapas, Mexico (16°42’ N; 93°00' W). Altitude at the ranch ranges from 400 to 450 m asl, average annual precipitation in the region is 900 mm, and average annual temperature is 26.0 °C. Soils in the area are mainly clay loam, with 2.4 % organic matter content, 7.0 pH, and slightly poor nitrogen content (0.15 %)21. Ranch surface area is 12 ha and average herd size is 55 Pelibuey x Katahadin sheep. Of the total area 10 ha is covered with Tanzania grass (P. maximum) with living fences consisting of the trees L. leucocephala, G. sepium and Cordia dentata (Vahl). Several paddocks (3 ha) contain L. leucocephala in alleys, and trees such as Enterolobium cyclocarpum (Jacq) and Ceiba pentandra L. are scattered across 7 ha of grazing areas. A nature reserve of dry tropical forest covers 2 ha. No chemical fertilization of pastures is done. Paddocks are managed in a rotation controlled by electric fences, and pastures are irrigated in the dry season. Animal production is focused on lamb meat for sale in regional and national markets.
Feed chemical analysis
Dry matter (DM) content of the forages and supplements was determined by drying in a forced air stove at 55 °C for 48 h (constant weight) and processing following the regulation NOM-116-SSA1-1994. Crude protein content was measured by an internal method (ECOSUR-ET-BR04) based on the standard NMX-F-608-NORMEX-2002. Organic matter (OM) content was measured by incineration in a muffle oven at 550 °C for 3 h according to the standard NMX-F-607-NORMEX-2002. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were quantified following Van Soest22, using the sequential procedure, with alpha-amylase and no ash correction in all samples (AOAC)23. Condensed tannins were measured with the acidified vanillin method (1% w/v vanillin in methanol)24.
In vitro gas production
An in vitro gas assay was done following the cumulative gas technique suggested by Theodorou25 and Williams26. Five diets (treatments) were designed using six raw materials, (Table 1): P. maximum as base forage (control); G. sepium and L. leucocephala foliage as protein sources; and molasses, Zea mays and M. paradisiaca as energy sources. Diets were isoenergetic and isoproteic, and formulated to meet the demands of adult sheep in the evaluated silvopastoral unit: 2,200 kcal/kg, 14% crude protein (CP).
Table 1 Treatments and percent ingredients used in in vitro gas experiment
| Feed | P. maximum | G. sepium | L. leucocephala | M. paradisiaca | Z. mays | Molasses |
|---|---|---|---|---|---|---|
| P100 (control) | 100 | 0 | 0 | 0 | 0 | 0 |
| G100 | 0 | 100 | 0 | 0 | 0 | 0 |
| L100 | 0 | 0 | 100 | 0 | 0 | 0 |
| MP100 | 0 | 0 | 0 | 100 | 0 | 0 |
| Z100 | 0 | 0 | 0 | 0 | 100 | 0 |
| M100 | 0 | 0 | 0 | 0 | 0 | 100 |
| Treatments | ||||||
| D1LM | 47 | 0 | 30 | 0 | 8 | 15 |
| D2LMP | 47 | 0 | 30 | 15 | 8 | 0 |
| D3GM | 47 | 30 | 0 | 0 | 8 | 15 |
| D4GMP | 48 | 30 | 0 | 15 | 7 | 0 |
| D5GLMPM | 47 | 16 | 17 | 5 | 5 | 10 |
P100 (control) = P. maximum; G100= G. sepium; L100= L. leucocephala; MP100 = M. paradisiaca; Z100= Z. mays; M100 = molasses; D1LM= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% molasses; D2LMP= 47% P. maximum, 30% L. leucocephala 8% Z. mays, 15% M. paradisiaca; D3GM= 47% P. maximum, 30% G. sepium, 8% Z. mays, 15% molasses ; D4GMP= 48% P. maximum, 30% G. sepium, 7% Zea mays, 15% M. paradisiaca; D5GLMPM= 47% P. maximum, 16% G. sepium 17% L. leucocephala, 5% M. paradisiaca, 5% Z. mays, 10% molasses.
Sheep were managed and ruminal fluid extracted from them according to Alexander and McGowan27 and Blummel and Orskov28, and following the animal welfare norms of the ECOSUR Sustainable Livestock Production Research Group. Ruminal fluid was extracted from five ewes in the experimental area; all had a live weight of 40.0 ± 3.0 kg, were of similar ages and good body condition. An esophageal probe was used to extract 300 ml ruminal fluid from each animal, for a total of 1.5 L ruminal fluid. All ruminal fluid samples were stored at 39 °C and protected from sunlight.
In vitro fermentation of each treatment was done by introducing 0.5 ± 0.001 g substrate in 90 ml amber glass vials and evaluating fermentation as represented by gas production at different times (0, 2, 4, 6, 8, 10, 12, 16, 20, 24, 30, 36, 48, 60, 72 h). Three replicates were done per treatment. The pressure generated in each vial was monitored with an analog manometer (Metron Mod. 63100, Range: 0-1 kg/cm2), and the resulting data used to calculate six response variables: maximum gas volume (V); gas production rate (S); lag phase (L); rapid fermentation fractional gas volume generated in first eight hours (V8); intermediate fermentation volume generated from eight to 24 h (V24); and slow fermentation volume generated from 24 to 72 h (V72). Two batches were incubated simultaneously, each comprised of three replicates (vials) per feed and treatment. In the first batch total accumulated gas production at 72 h was evaluated in each fermentable fraction: rapid, intermediate and slow. For each fraction three groups of fermentable carbohydrates were estimated (monosaccharides, starch and cellulose) based on the gas volumes recorded in three time intervals: 0 to 8 h incubation (Vf0-8); 8 to 24 h (Vf8-24); and 24 to 72 h (Vf24-72). These volumes were used to estimate the rapid (FR), intermediate (FI) and slow (FS) fermentable fractions using the linear regression equations proposed by Miranda et al29: FR = Vf0-8/0.4266, FI = Vf8-24/0.6152, and FL = Vf24-72/0.3453). Values for accumulated gas production were fit to the model of Menke and Steingas30:
Where:
Y = Total volume of gas produced;
v = Maximum production volume;
s = Constant gas production rate;
t = Time;
L = Lag or delay phase.
In vitro dry matter digestibility (IVDMD) was measured by gravimetric analysis, considering initial dry matter weight, and final weights at 24 and 72 h fermentation. Dry matter (DM) weight was measured by recovering the matter with a 200 μm filter and drying at 65 °C to constant weight. Calculation of IVDMD was done with the formula:
Where:
% IVDMD = percentage in vitro dry matter digestibility;
IW = initial weight incubated dry matter in grams;
FW = final weight incubated dry matter in grams.
Using the data for IVDMD24/72 and emitted gas volumes a potential fermentable gas emission index (PFGEI) was generated. This refers to the amount of gas that can be produced by a substrate per gram of fermented DM or OM in the rumen31.
Methane and carbon dioxide production
Production of CO2, CH4 and minor gases was analyzed during the first 24 hours of fermentation in samples from the second incubation batch. Following Bartha and Pramer32 as modified by Miranda29, CO2 separation was done using a trap (hermetically sealed glass jar with rubber stopper and aluminum ring) containing 90 ml 1 M potassium hydroxide (KOH) and a dilution of 56.10 g KOH in 1 L deionized water. Samples were taken and placed in sterile vials under a vacuum for later analysis with gas chromatography and quantification of CH4 for each substrate. Analysis of CH4 production was done in a gas chromatographer (Clarus 500, Perkin Elmer; Software version 6.3.2.0646; 0.530 mm column diameter; 50 m length; 35 °C injection temperature). Analysis was done of a total of 36 samples collected during the 24 h in vitro fermentation, in the second incubation run; 20 µl of sample were used in each assay. Correction of CH4 concentrations was done for each treatment by subtracting average methane production from the three blanks. For the purposes of calculating CH4 concentration and the effect of the treatments on CH4 production, it was expressed as L CH4/kg DMDIG.
Statistical analysis
Gas production parameters, IVDMD and methane production were analyzed with an ANOVA in a completely random design. The mathematical model was:
Where:
Yij = Response variable in j-th replicate (flasks) of i-th treatment;
μ= overall mean of all experimental data;
Ti= Effect of treatment I;
ε ij = experimental error associated with j under treatment i.
Data from all the response variables were processed with an ANOVA33, and differences between treatment means compared with a Tukey test (P≤0.05) using the PROC GLM procedure in the SAS statistics package34.
Results and discussion
Analysis of forage, energy source and treatment (diet) chemical composition showed crude protein (CP) content to be high in the G. sepium and L. leucocephala foliage (Table 2); indeed, it was higher than reported elsewhere32,33. As expected, the energy sources had low CP and NDF contents. The grass P. maximum (control) had a CP higher than the 7 to 9 % average in many tropical grasses. This relatively high grass CP may be linked to natural fertilization via sheep feces in the studied controlled grazing management system. The P. maximum also had high NDF and ADF contents. Compared with previous reports35,36, the L. leucocephala leaves analyzed in the present study contained very little tannins (CT). This discrepancy could be due to variability in the nutritional value of foliage from the same fodder tree species in response to site conditions, management, phenological stage and specific characteristics of the study area37. Lignin content in L. leucocephala was high but within the range suggested by the FAO. This lignin content very probably affected the digestibility of L. leucocephala, and ration components, reducing energy use38,39.
Table 2 Chemical composition (g/Kg DM) of forages, energy sources, and treatments used in in vitro gas experiment
| DM | OM | CP | Lignin | NDF | ADF | CT | CHO | |
|---|---|---|---|---|---|---|---|---|
| P. maximum (control) | 933 | 853 | 124 | 103 | 712 | 490 | NA | 231 |
| G. sepium | 930 | 889 | 367 | 133 | 353 | 250 | 0 | 269 |
| L. leucocephala | 932 | 883 | 261 | 207 | 462 | 308 | 56 | 352 |
| M. paradisiaca | 925 | 953 | 52 | NA | 137 | 37 | NA | 763 |
| Z. mays | 866 | 984 | 59 | 6 | 86 | 16 | NA | 795 |
| Molasses | 788 | 866 | 53 | 3* | 8* | 5* | NA | 600 |
| D1LM | 906 | 874 | 149 | 111 | 481 | 324 | 16 | 368 |
| D2LMP | 926 | 887 | 149 | 111 | 501 | 329 | 16 | 392 |
| D3GM | 905 | 876 | 181 | 89 | 448 | 307 | NA | 343 |
| D4GMP | 926 | 888 | 182 | 90 | 474 | 317 | NA | 361 |
| D5GLMPM | 914 | 877 | 172 | 105 | 482 | 326 | 9 | 349 |
DM= dry matter; OM = organic matter; CP= crude protein; NDF= neutral detergent fiber; ADF = acid detergent fiber; CT= condensed tannins; CHO= carbohydrates; NA = not analyzed. * https://www.feedipedia.org/01/05/2018. ; D1LM= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% molasses; D2LMP= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% M. paradisiaca, D3GM= 47% P. maximum, 30% G. sepium, 8% Z. mays, 15% molasses; D4GMP= 48% P. maximum, 30% G. sepium, 7% Z. mays, 15% M. paradisiaca; D5GLMPM= 47% P. maximum, 16% G. sepium, 17% L. leucocephala. 5% M. paradisiaca, 5%, Z. mays, 10% molasses.
Gas production data at 8, 24 and 72 h fermentation showed the highest gas volumes (V) to be 544.0 ml/g-1 DM in MP100 (M. paradisiaca), 467.3 ml/g-1 DM in Z100 (Z. mays) and 325.7 ml/g-1 DM in M100 (molasses)(Table 3). These levels differed (P<0.05) between each other and from the diets. This behavior is typical of foods containing carbohydrates such as monosaccharides and starches40. Both G. sepium (G100) and L. leucocephala (L100) had relatively low gas production volumes (V), which differed from each other (P<0.05) (Figure 1). These low production levels may be due to the presence of secondary metabolites (tannins) in L. leucocephala40, and/or the high lignin and fiber contents in both species’ leaves (111 g/kg DM), all of which can result in lower gas production compared to higher carbohydrate content diets41. Treatments with energy-protein mixtures had higher gas production (V) (P<0.05) due to the additive effect of the carbohydrates to L. leucocephala and G. sepium leaves (Figure 2). Overall, gas production rate (S) was similar among the treatments although slight differences were present (P<0.05).
Table 3 Total gas production parameters and fractional volumes in feed ingredients and treatments in in vitro gas production experiment
| Parameters | Fractional volumes (ml g-1 DM) | |||||
|---|---|---|---|---|---|---|
| Feed ingredients | V (ml / g-1 DM) | S (ml h-1) | L (h) | V8 | V24 | V72 |
| P100 (control) | 266.3de | 0.03ab | 11.2a | 15.1e | 100.5d | 159.7b |
| G100 | 253.0e | 0.03ab | 9.0b | 28.7ed | 85.9de | 144.8bcd |
| L100 | 180.8f | 0.03ab | 2.7f | 40.6cd | 63.2e | 81.9e |
| MP100 | 544.9a | 0.03ab | 3.7ef | 117.7a | 250.0a | 206.4a |
| Z100 | 467.3b | 0.04a | 6.2c | 44.1cd | 271.2a | 194.7a |
| M100 | 325.7c | 0.04a | 2.6f | 71.6b | 166.9b | 119.5d |
| Treatments | ||||||
| D1LM | 299.8cd | 0.03b | 4.7cde | 51.7c | 105.0c | 149.9bc |
| D2LMP | 308.9cd | 0.03ab | 5.7cd | 46.8c | 119.7cd | 152.4bc |
| D3GM | 293.6cde | 0.03ab | 4.5de | 54.0c | 115.6cd | 134.3bcd |
| D4GMP | 337.4c | 0.03ab | 5.6cd | 52.6c | 147.1bc | 151.5cb |
| D5GLMPM | 292.3cde | 0.03ab | 3.6fe | 57.5cb | 122.2cd | 128.7cd |
V = maximum gas production volume; S = constant gas production rate; L = Lag phase (h); V8 = fractional volume generated in rapid fermentation fraction (0-8 h); V24 = fractional volume generated in intermediate fermentation fraction (8-24 h); V72 = fractional volume generated in slow fermentation fraction (24-72 h).
P100 (control)= P. maximum; G100= G. sepium; L100= L. leucocephala; MP100= M. paradisiaca; Z100= Z. mays; M100 = molasses; D1LM= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% molasses; D2LMP= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% M. paradisiaca; D3GM= 47% P. maximum, 30% G. sepium, 8% Z. mays, 15% molasses; D4GMP= 48% P. maximum, 30% G. sepium, 7% Z. mays, 15% M. paradisiaca; D5GLMPM= 47% P. maximum, 16% G. sepium, 17% L. leucocephala, 5% M. paradisiaca, 5% Z. mays, 10% molasses.
abcdef Different letter superscripts in the same column indicate significant differences between treatments (α= 0.05).
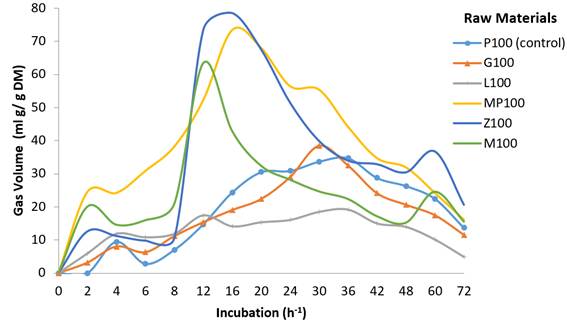
P100 (control)= Panicum maximum; G100= Gliricidia sepium; L100= Leucaena leucocephala; MP100= Musa paradisiaca; Z100= Zea mays; M100= molasses.
Figure 1 Gas volume over time in control treatment and raw material ingredients in in vitro gas production trial
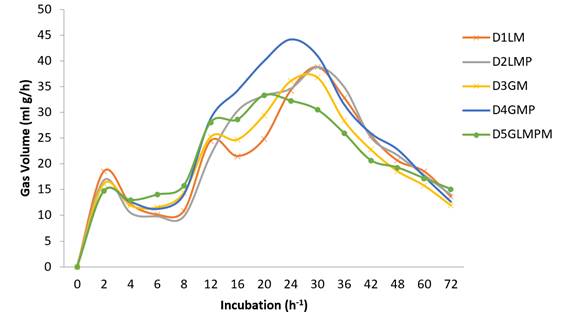
P100 (control)= P. maximum; D1LM= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% molasses; D2LM= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% M. paradisiaca; D3GM= 47% P. maximum, 30% G. sepium, 8% Z. mays, 15% molasses; D4GMP= 48% P. maximum, 30% G. sepium, 7% Zea mays, 15% M. paradisiaca; D5GLMPM= 47% P. maximum, 16% G. sepium, 17% L. leucocephala, 5% M. paradisiaca, 5% Z. mays, 10% molasses.
Figure 2 In vitro gas production (ml gas/h) of five diets used in sheep in a silvopastoral system in Chiapas, Mexico.
The fermentation profiles clearly varied between the energy sources, forages and treatments. Energy sources such as bananas (MP100) and molasses (M100) began to ferment quickly, increased gas production during the intermediate incubation phase and then declined rapidly. In the treatments containing mixtures of forages with energy sources gas production and fermentation rate were initially slow but increased notably in the intermediate phase and remained higher for longer (Figure 2). During fermentation the substrate is hydrated and colonized by ruminal microorganisms. The quantity and type of carbohydrates present in the substrate influence gas volume and its effect on DM digestibility42,43.
In vitro dry matter digestibility (IVDMD) was lowest at 72 h with P. maximum (50.9 %) and L. leucocephala (29.9 %), which differed from G. sepium and the diets (P≤0.05) (Table 4). The IVDMD values for L. leucocephala were lower than reported in other in vitro and in vivo studies34,42,43, probably due to the maturity of the forage tree foliage used in the present study and its consequently high lignin content. At both 24 and 72 h IVDMD was highest (P≤0.05) in M100 (Z. mays), Z100 (molasses) and MP100 (M. paradisiaca). The treatments (D1LM, D2LMP, D3GM, D4GMP and D5GLMPM) exhibited a range of values between these highs and lows (P<0.05). The linear increases observed in the treatments resulted from the contributions of G. sepium and L. leucocephala foliage to fermentation and digestibility (Figure 1). Inclusion of energy sources (D3GM and D4GMP) improved digestibility (P≤0.05) compared to D2LMP, and P100 and L100 (P≤0.05). The digestibility observed for G. sepium was similar to that reported elsewhere43. The energy sources’ (MP100, Z100 and M100) high digestibility was due to their high soluble sugars contents. When diets are balanced with high G. sepium and molasses contents, digestibility and utilization are higher due to the synchrony between protein and energy contents44.
Table 4 CH4, CO2, IVDMD, PFGEI and Total CH4 produced by fermentation of treatments in in vitro gas production experiment
| Treatments | CH4 (%) | CO2 (%) | IVDMD 24 h (%) | IVDMD 72 h (%) | PFGEI/DM 24 h | PFGEI/DM 72 h | CH4 (L CH4/kg DMDIG) |
|---|---|---|---|---|---|---|---|
| P100 (control) | 22.5bcd | 77.5abc | 33.7f | 50.9e | 791.0a | 523.5cd | 1.55d |
| G100 | 23.2bcd | 76.8abc | 51.0cd | 60.1cd | 496.8e | 420.9e | 1.68d |
| L100 | 30.8a,b | 69.2cd | 28.8f | 29.9f | 628.1bcd | 606.6ab | 1.94d |
| MP100 | 18.1d | 81.9a | 77.0b | 83.6b | 708.1ab | 652.1a | 15.75b |
| Z100 | 16.4d | 83.6a | 80.1b | 87.0b | 583.6cde | 537.2cd | 28.59a |
| M100 | 17.9d | 82.1a | 92.7a | 92.4a | 351.4f | 352.5f | 9.03c |
| D1LM | 31.9a | 68.1d | 44.4e | 56.6d | 678.6bc | 529.7cd | 8.82c |
| D2LMP | 27.0abc | 73.0bcd | 44.9de | 50.9e | 690.0b | 606.5ab | 8.83c |
| D3GM | 24.2abcd | 75.8abcd | 55.1c | 61.9c | 533.0de | 474.2de | 6.32cd |
| D4GMP | 21.9cd | 78.1ab | 54.5c | 61.1cd | 619.4bcd | 552.1bc | 9.60c |
| D5GLMPM | 22.3bcd | 77.7abc | 51.7c | 56.6d | 565.5de | 516.9cd | 6.31cd |
CH4= in vitro methane + minor gases; CO2= in vitro carbon dioxide; IVDMD 24 h= in vitro dry matter digestibility at 24 h; IVDMD 72 h= in vitro dry matter digestibility at 72 h; PFGEI/DM 24 h= potential fermentable gas emission index at 24 h; PFGEI/DM 72 h= potential fermentable gas emission index at 72 h; CH4= methane concentration at 24 h.
P100 (control)= P. maximum; G100= G. sepium; L100= L. leucocephala; MP100= M. paradisiaca; Z100= Z. mays; M100= molasses; D1LM= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% molasses; D2LMP= 47% P. maximum, 30% L. leucocephala, 8% Z. mays, 15% M. paradisiaca; D3GM= 47% P. maximum, 30% G. sepium, 8% Z. mays, 15% molasses; D4GMP= 48% P. maximum, 30% G. sepium, 7% Z. mays, 15% M. paradisiaca; D5GLMPM= 47% P. maximum, 16% G. sepium, 17% L. leucocephala, 5% M. paradisiaca, 5% Z. mays, 10% molasses.
abcdef Different letter superscripts in the same column indicate significant differences between treatments (α= 0.05).
Total CH4 production (L/Kg DMDG) was highest in the Z. mays (Z100) and M. paradisiaca (MP100) energy sources (P≤0.05) (Table 4). The lowest production values were in the control (P100), G. sepium (G100) and L. leucocephala (L100), which did not differ (P>0.05). The diet treatments (D5GLMPM, D3GM, D1LM, D2LMP and D4GMP) exhibited intermediate values (P>0.05). Of the treatments containing mixed energy source and protein, D5GLMPM had the lowest CH4 production, highlighting the importance of associating forages with carbohydrates45,46. These authors emphasize that carbohydrate type determines transit time, thus affecting CH4 production per gram of digested substrate. Carbohydrate type appears to be a determining factor in CH4 production47, since it can be mediated by lower availability of digestible carbohydrates48. Concentrations of 550 g kg-1 DM surpass the concentration which negatively affects voluntary consumption of feed and nutrient digestibility in animals49. In addition, tree and shrub foliage contains low concentrations of structural fractions44, making them more susceptible to degradation and bacterial action, resulting in increased transit time, which decreases total gas production and therefore results in lower enteric CH4 production36,50.
Both research and development agencies have been focusing on quantification of GHG from ruminal fermentation, creation of indices to evaluate the potential for environmental pollution from ruminal GHG, and design of sustainable animal management strategies51,52. In the present results wide variation (P<0.001) was apparent in the PFGEI/DM, both at 24 and 72 h, and in the evaluated energy sources and treatments (Table 4). Of note is that the lowest PFGEI rates at 24 and 72 h correspond to M100 (496.8 ml.g-1/IVDMD) and G100 (420.9 ml.g-1/IVDMD), whereas the highest rates occurred with MP100 at 24 h (708.1 ml.g-1/IVDMD) and 72 h (652.1 ml.g-1/IVDMD). Of the treatments including tree foliage and energy sources, the lowest index corresponded to the D3GM mixture. The present data suggest that the type of foliage from forage trees, in association with carbohydrate type, can affect ruminal GHG production, especially if the carbohydrate exhibits slow fermentation, as is the case with starches53.
Conclusions and implications
The present results suggest that in silvopastoral systems the combination of foliage from forage trees with local energy sources, especially molasses and bananas, can improve diet nutritional value and animal production parameters while mitigating generation of greenhouse gases such as methane. The combination of 30% DM foliage from trees such as G. sepium and L. leucocephala with local energy sources such as molasses and bananas contributed to lowering CH4 emission in sheep. Management of forage trees (e.g. G. sepium and L. leucocephala) is recommended in silvopastoral systems because they improve diet quality, particularly when combined with local energy sources, and contribute to lowering CH4 emissions. Future research will need to address animal response (e.g. weight gain) and bio-economic balance in these systems to understand how to make them economically and socially viable, and to develop adaptation strategies that will improve animal production, contribute to producers’ social welfare and mitigate greenhouse gas emission.
Acknowledgements
The research reported here was financed by the SEP-CONACYT through the project “Cuantificación de emisiones de metano entérico y óxido nitroso en ganadería bovina en pastoreo y diseño de estrategias para la mitigación en el sureste de México” (SEP-CONACYT CB 2014-1 No. 242541)
REFERENCES
1. FAO. Food and Agriculture: Key to Achieving in the 2030. Agenda for Sustainable Development. FAO, Rome. 2016. [ Links ]
2. IPCC. Climate Change 2014: Impacts, adaptation, and vulnerability report of the Intergovernmental Panel on Climate Change. Field C, et al. editors. Cambridge, UK 2014. [ Links ]
3. O´Mara FP. The significance of livestock in global greenhouse gas emissions today and in the near future. Anim Feed Sci Technol 2011;(166-167):7-15. [ Links ]
4. Ibrahim M, Chacón M, Cuartas C, Naranjo J, Ponce G, Vega P, Casasola F, Rojas J. Almacenamiento de carbono en el suelo y la biomasa arbórea en sistemas de usos de la tierra en paisajes ganaderos de Colombia, Costa Rica y Nicaragua. Agroforestería las Américas 2007;(45):27-36. [ Links ]
5. Avila-Foucalt V, Revollo FD. Análisis financiero y percepción de los servicios ambientales de un sistema silvopastoril: un estudio de caso en los Tuxtlas, México. Rev Iber Econ Ecol 2014;(21):17-33. [ Links ]
6. Solorio S, Wright M, Franco M, Basu S, Sarabia S, Ramirez L, et al. Silvopastoral systems: Best agroecological practice for resilient production systems under dryland and drought conditons. Ahmed M, Stockle C. editors. Quantificaction of climate variability, adaptation and mitigation for agricultural sutainability. Australis: Springer; 2002:233-250. [ Links ]
7. Murgueitio E, Chará J, Barahona R, Cuartas C, Naranjo J. Los Sistemas Silvopastoriles Intensivos (SSPI), herramienta de mitigación y adaptación al cambio climático. Trop Subtrop Agroecosystems 2014;(17):501-507. [ Links ]
8. Palmer L. A new climate for grazing livestock. Nat Clim Chang 2014;(4):321-323. [ Links ]
9. Ibrahim M, Villanueva C, Casasola F. Sistemas silvopastoriles como una herramienta para el mejoramiento de la productividad y rehabilitación ecológica de paisajes ganaderos en Centro América. Agroforesteria las Américas 2007;(15):1-34. [ Links ]
10. Kass ML. Determinación del nitrógeno en los alimentos. Ruiz, M, Ruiz A. editores. Nutrición de rumiantes, Guía Metodológica de Investigación. Costa Rica, ALPA-RISPAL 1990:49-58. [ Links ]
11. Sirohi SK. Mitigation options for enteric methane emissions from dairy animals: an evaluation for potential projects in India. Mitig Adapt Strateg Glob Chang 2007;(12):259-274. [ Links ]
12. Piñeiro-Vázquez A, Ayala-Burgos A, Chay-Canul A, Ku-Vera J. Dry matter intake and digestibility of rations replacing concentrates with graded levels of Enterolobium cyclocarpum in Pelibuey lambs. Trop Anim Health Prod 2013;(45):577-583. [ Links ]
13. Jiménez-Ferrer G, Mendoza-Martinez G, Soto-Pinto L, Alayón-Gamboa A. Evaluation of local energy sources in milk production in a tropical silvopastoral system with Erythrina poeppigiana. Trop Anim Health Prod 2015;(47):903-908. [ Links ]
14. Norton B. Anti-nutritive and toxic factors in forage tree legumes. Gutteridge R, Shelton H editors. Forage tree legumes in tropical agriculture. UK: Cab International; 1993:202-215. [ Links ]
15. Piñeiro-Vázquez A, Canul-Solís J, Alayón-Gamboa J, Chay-Canul A, Ayala-Burgos A, Aguilar-Pérez C, et al. Potential of condensed tannins for the reduction of emissions of enteric methane and their effect on ruminant productivity. Arch Med Vet 2015;(47):263-272. [ Links ]
16. Yáñez-Ruiz D, Hart K, Martin-Garcia A, Ramos S, Newbold C. Diet composition at weaning affects the rumen microbial population and methane emissions by lambs. Aust J Exp Agric 2008;(48):186-188. [ Links ]
17. Min B, Solaiman S, Shange R, Eun J. Gastrointestinal bacterial and methanogenic Archaea diversity dynamics associated with condensed tannin-containing pine bark diet in goats using 16S rDNA amplicon pyrosequencing. Int J Microbiol 2014; Article ID 141909, 11 pages. doi.org/10.1155/2014/141909 [ Links ]
18. Herrera-López M, Rivera-Lorca J, Ortega-Reyes L, Escobedo-Mex J, Magaña M, Sanginés-García J, Sierra-Vázquez Á, Contenido nutritivo y factores antinutricionales de plantas nativas forrajeras del norte de Quintana Roo. Tec Pecu Mex 2008;(46):205-215. [ Links ]
19. Puchala R, Animut G, Patra A K, Detweiler G D, Wells J E, Varel V H, Sahlu T, Goetsch A. Methane emissions by goats consuming Sericea lespedeza at different feeding frequencies. Anim Feed Sci Technol 2012;(175):76-84. [ Links ]
20. Soltan YA, Morsy AS, Lucas RC, Abdalla AL. Potential of mimosine of Leucaena leucocephala for modulating ruminal nutrient degradability and methanogenesis. Anim Feed Sci Technol 2017; (223):30-41. [ Links ]
21. Pérez Esaú, Soca Mildrey, Díaz L, Corzo M. Comportamiento etológico de bovinos en sistemas silvopastoriles en Chiapas, Pastos y Forrajes 2008;(31):161-172. [ Links ]
22. Van-Soest, P, Robertson J, Lewis B. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition, J Dairy Sci 1991;74:3583-3597. [ Links ]
23. AOAC. Official Methods for Analysis. Association of Official Analysis Chemist, Gaitersburgh, MD, USA. 2006. [ Links ]
24. Price ML, Van SC, Butler L. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain, J Agric Food Chem, 1978;26(5):1214-1218. doi: 10.1021/jf60219a031 [ Links ]
25. Theodorou M K, Williams BA, Dhanoa M S, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol 1994;(48):185-197. [ Links ]
26. Williams, BA. Cumulative gas-production techniques for forage evaluation. In: Forage evaluation in ruminant nutrition. Givens, D et al., editors. UK : CABI, Pub; 2000:189-213. [ Links ]
27. Alexander RH, McGown M. The routine determination of in vitro digestibility of organic matter in forages-an investigation of the problems associated with continuos large-scale operation. J Brit Grass Soc 1966;(21):140-147. [ Links ]
28. Blummel M, Orskov ER. Comparison of in vitro gas production and nylon bag degradability of roughages in predicting food intake in cattle. Anim Feed Sci Technol 1993;(40):109-119. [ Links ]
29. Miranda LA, Vázquez MP, Améndola MR, Sandoval GL, González OR. Cuantificación de las fracciones fermentables de alfalfa y tuna por la técnica de producción de gas [resumen]. Congreso de la Asociación Latinoamericana de Producción Animal. Puerto Varas, Chile. 2015:575. [ Links ]
30. Menke K, Steingass H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 1988;(28):7-55. [ Links ]
31. Cáceres LM. Índice de contaminación atmosférica y productividad de vacas Jersey y sus cruzas con Holstein en pastoreo, [tesis maestría]. Texcoco, México. Universidad Autónoma Chapingo; 2016. [ Links ]
32. Bartha R, Pramer D. Features of flask and method for measuring the persistence and biological effects of pesticides in soil. Soil Sci. 1965; 100; (1): 68-70 [ Links ]
33. Cochran WG, Cox GM. Diseños experimentales. 2ª ed. México: Trillas; 1991. [ Links ]
34. SAS. User´s Guide SAS/STAT. Sas Institute, Cary, NC, USA. 2015. [ Links ]
35. Abdulrazak S, Muinga R, Thorpe W, Ørskov ER. Supplementation with Gliricidia sepium and Leucaena leucocephala on voluntary food intake, digestibility, rumen fermentation and live weight of crossbred steers offered Zea mays stover. Livest Prod Sci 1997;(49):53-62. [ Links ]
36. Morgavi D P, Archimède H, Marie-Magdeleine C, Popova M, Bousseboua H, Doreau M. Potential of tannin-rich plants for modulating ruminal microbes and ruminal fermentation in sheep. J Anim Sci 2015;(93):334-347. [ Links ]
37. Borel, R. Nutrición de rumiantes: Aspectos críticos de las metodologías de evaluación nutritiva de árboles y arbustos forrajeros. Guía metodológica de investigación. ALPA-IICA, Costa Rica 1990:21-31. [ Links ]
38. Piñeiro-Vázquez AT, Jiménez-Ferrer G, Chay-Canul AJ, Casanova-Lugo F, Díaz-Echeverría VF, Ayala-Burgos A, et al. Intake, digestibility, nitrogen balance and energy utilization in heifers fed low-quality forage and Leucaena leucocephala. Anim Feed Sci Technol 2017. Vol. 228, pag. 194-201. doi.org/10.1016/j.anifeedsci.2017.04.009 [ Links ]
39. Van Soest PJ. Development of a comprehensive system of feed analyses and its application to forages. J Anim Sci 1967;(26):119-128. [ Links ]
40. Van Soest PJ. Nutritional ecology of the ruminant. Corvallis, Oregon: O&B Books Inc; 1982. [ Links ]
41. Cárdenas J, Sandoval C, Solorio F. Composición química de ensilajes mixtos de gramíneas y especies arbóreas de Yucatán. Tec Pecu Méx 2003;(41):283-294. [ Links ]
42. Juárez F, Contreras J, Montero M. Tasa de cambios con relación a edad en rendimiento, composición química y digestibilidad de cinco pastos tropicales. Decima Cuarta Reun Cient Tecnol Forest Agropecu. Veracruz, Mex. 2001. [ Links ]
43. Williams BA. Cumulative gas-production technique for forage evaluation. Eds: Givens D, et al. editors. Forage Evaluation in Ruminant Nutrition, UK 2000; 189-213. [ Links ]
44. Reed J. Nutritional toxicology of tannins and related polyphenols in forage legumes. J Anim Sci 1995;(73):1516-1528. [ Links ]
45. Molina-Botero C, Cantet J, Montoya S, Correa-Londoño G, Barahona-Rosales R. In vitro methane production from two tropical grasses alone or in combination with Leucaena leucocephala or Gliricidia sepium. CES Med Vet y Zootec 2013;(8):15-31. [ Links ]
46. Archiméde H, Rira M, Barde D, Labirin F, Marie-Magdeleine C, et al. Potential of tannin-rich plants, Leucaena leucocephala, Glyricidia sepium and Manihot esculenta, to reduce enteric methane emissions in sheep. J Anim Physiol Anim Nutr 2016;(100):1149-1158. [ Links ]
47. Fernández-Mayer A. Efecto de la sincronización de energía-proteína sobre la performance animal. EEA INTA Bordenave 2001:7-13. [ Links ]
48. Eugene M, Archimèede H, Sauvant, D. Quantitative metanalysis on the effects of defaunation of the rumen on growth, intake and digestion in ruminants, Livestock Prod Sci 2004;85:81-97. [ Links ]
49. Van Soest PJ. Symposium on factors influencing the voluntary intake of herbage by ruminants: voluntary intake in relation to chemical composition and digestibility. J Anim Sci 1965: 4(3):834-843. [ Links ]
50. Animut G, Puchala R, Goetsch AL, Patra AK, Sahlu T, Varel VH, Wells J. Methane emission by goats consuming diets with different levels of condensed tannins from lespedeza. Anim Feed Sci Technol 2008;(144):212-227. [ Links ]
51. Johnson KA, Johnson DE. Methane emissions from cattle. J Anim Sci 1995;73 (8):2483-2492. [ Links ]
52. McAllister TA, Cheng KJ, Okine EK y Mathison GW. Dietary, environmental and microbiological aspects of methane production in ruminants. Can J Anim Sci 1996;76(2):231-243. [ Links ]
53. Nunes L, Salomon S, Castro L, Robson E, Abdalla A. Chemical composition, degradability and methane emission potential of banana crop residues for ruminants. Trop Subtrop Agroecosyst 2014;(17):197-206. [ Links ]
Received: June 13, 2017; Accepted: May 29, 2018











 texto en
texto en 


