Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias pecuarias
On-line version ISSN 2448-6698Print version ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.10 n.1 Mérida Jan./Mar. 2019
https://doi.org/10.22319/rmcp.v10i1.4241
Articles
Potential distribution of Musca domestica in Jesús María Municipality, Aguascalientes, Mexico, based on climate change scenarios
a Universidad Autónoma de Aguascalientes. Centro de Ciencias Agropecuarias, Jesús María, Aguascalientes, México.
b Universidad Autónoma Chapingo. Unidad Regional Universitaria de Zonas Áridas. Bermejillo, Durango, México.
c Universidad de Guanajuato. Departamento de Veterinaria y Zootecnia, DICIVA. Irapuato, Guanajuato, México.
The housefly M. domestica is a primary domestic pest responsible for food decomposition, and is a vector for more than 100 pathogens in humans and animals. Climate conditions including temperature and relative humidity influence M. domestica development and prevalence. As climate change advances control programs for this species will need to adapt to evolving conditions. A development assay was done of M. domestica at different temperatures and relative humidities to estimate its current potential incidence in Jesús María Municipality, Aguascalientes, Mexico. Local climate is temperate semi-dry (BS1k) with 16 to 18 °C annual average temperature and 500 to 600 mm annual average rainfall. In a completely randomized design, six treatments involving different temperatures and relative humidities during the entire fly lifecycle were analyzed. Development conditions were ideal between 20 and 30 °C, conditions present in the study area between June and August. The CNRMCM5 (RCP 4.5) climate change model was used to predict extreme minimum temperatures in three time horizons: Short (2015-2039); Medium (2040-2060); and Long (2075-2090). Under the Medium and Long scenarios ideal development conditions could last as long as five months, representing a potential increase in the time M. domestica is present in the region, and in the duration of the public and animal health challenges it generates. The present results are important for planning future prevention, monitoring and control programs and strategies.
Key words: Housefly; Vector; Temperature; Relative humidity; Precipitation
M. domestica es el mayor parásito doméstico que causa descomposición de alimentos y actúa como vector de más de 100 patógenos para la medicina humana y veterinaria, tiene una cercana asociación con el hombre y su ambiente. El objetivo del presente estudio fue evaluar el desarrollo de M. domestica a diferentes niveles de temperatura y humedad relativa y determinar su potencial incidencia en el municipio de Jesús María, Aguascalientes. El estudio se desarrolló en el Centro de Ciencias Agropecuarias de la Universidad Autónoma de Aguascalientes (CCA-UAA), que presenta un clima semiseco templado (BS1k) con promedios anuales de temperaturas y precipitación de 16 a 18 °C y 500 a 600 mm, respectivamente; se evaluaron seis tratamientos con diferentes temperaturas y humedad relativa. Los resultados se analizaron en un diseño completamente al azar y mediante comparación de medias de Tukey (α=0.005). Los resultados muestran que la mosca doméstica se puede desarrollar a temperaturas ambientales de 20 a 30 °C; y con base en el modelo CNRMCM5 (RCP4.5) en diferentes horizontes de tiempo cercano (2015-2039), medio (2040-2060) y lejano (2090) de escenarios de cambio climático, la mosca tendrá una duración de alrededor de tres meses para el horizonte cercano y de cinco meses para el medio y lejano. Con estos datos se provee información importante que permite planear el desarrollo de estrategias para su prevención, monitoreo y control.
Palabras clave: Parásito; Vector; Temperatura; Humedad relativa; Precipitación pluvial
Introduction
The housefly Musca domestica is a synanthropic insect in close association with humans and their environment. It is present wherever humans live, but is also associated with livestock such as poultry, cattle, horses and swine1. It is therefore a potential disease vector for various diseases among humans and for zoonoses2. Prevention, monitoring and controlling M. domestica is vital because it is the principal domestic parasite responsible for food decomposition and a vector for more than 100 pathogens3. It can also propagate approximately thirty bacterial and protozoan diseases, although M. domestica can thrive without causing infection4. Controlling M. domestica is costly and ongoing; for example, an estimated 1.6 million dollars annually is spent in the United States of America on insecticides for control of this parasite on poultry farms5.
Survival in ectotherms such as flies is limited by temperature. They often remain exposed to extreme thermal variations in their natural habitat, especially during the summer months6. Optimum development of M. domestica occurs at about 25 °C7. Environmental factors such as temperature, precipitation, relative humidity and soil type and use directly affect M. domestica population dynamics8.
Livestock can exacerbate M. domestica presence and persistence. In 2015 the state of Aguascalientes, Mexico, had a dairy cattle population of approximately twenty thousand head9. Public health problems can emerge when dairy cattle production is in proximity to densely populated urban areas; for example, in livestock regions such as the Comarca Lagunera, in the states of Coahuila and Durango, and Jesús María Municipality in the state of Aguascalientes. Musca domestica can then effectively transmit myriad diseases from livestock to human populations, and extreme measures are required to control its propagation.
The present study objective was to evaluate the effects of variations in temperature and relative humidity on M. domestica population development in Jesús María Municipality, Aguascalientes, and estimate its potential distribution under climate change scenarios based on the CNRMCM5 (RCP 4.5) model for three periods: Near (2015-2039), Middle (2045-2069) and Long (2075-2099).
Material and methods
Development of M. domestica was evaluated under temperature and humidity conditions similar to the study area. Jesús María Municipality, Aguascalientes, has a temperate semi-dry (BS1k) climate with 16 to 18 °C annual average temperature and 500 to 600 mm annual average rainfall. Fly pupae for use as progenitors were collected from the livestock area of the Agricultural Sciences Center (Centro de Ciencias Agriculturales - CCA) of the Autonomous University of Aguascalientes (Universidad Autónoma de Aguascalientes- UAA). These were placed in stainless steel wire cages (30 x 30 x 30 cm), covered with 100% nylon fabric and incubated at 24 ± 2 °C in the Veterinary Clinic and Greenhouse Research Laboratory of the UAA. Once emerged the flies were fed a 10% sugar water solution in which pieces of cotton were saturated. The substrate for oviposition and larvae feeding was a mixture of wheat bran and water (70 %) or wheat bran, powdered milk and water (30 %). The resulting pupae were used in the evaluation of development under different temperatures and relative humidities (RH) done at the Laboratory of Natural Resources and Agrarian Systems Analysis of the CCA at UAA. Each pupa was placed in a one-ounce polypropylene container with lid to prevent copulation between females and males. After emergence they were sexed and placed in different cages (30 x 30 x 30 cm). Females and males were removed from these cages, three of each sex placed in stainless steel cages (size 10 x 10 x 10 cm), and these covered with nylon fabric. Each cage was subjected to one of six temperature/RH treatments (Table 1). Experimental design was completely random, with three replicates per treatment. A Tukey test was used to compare the means for the larvae count, pupae count, and fly emergence per cage variables (α= 0.005). Statistical analyses were run using the STATISTICA® ver. 13 program.
Table 1: Temperature and relative humidity in M. domestica development treatments
| Treatments | Temperature (°C) | Relative humidity (%) |
|---|---|---|
| T1 | 27.5 ± 2.5 | 90 - 95 |
| T2 | 22.5 ± 2.5 | 60 - 65 |
| T3 | 32.5 ± 2.5 | 20 - 25 |
| T4 | 32.5 ± 2.5 | 35 - 40 |
| T5 | 35 | 90 |
| T6 | 32.5 ± 2.5 | 15 - 20 |
Data for maximum, minimum and mean temperature, as well as HR, for Jesús María municipality were acquired from the National Network of State Agroclimatological Stations (a.k.a., Agroclima)10 of the National Institute of Forestry, Agriculture and Livestock Research (Instituto Nacional de Investigación Forestal, Agrícola y Pecuaria - INIFAP). These were analyzed considering the M. domestica development variables and the species’ possible infestation in the study area. Climate data were interpolated by the method based on inverse distance weighting (IDW), which has been described and applied previously11,12.
Geospatial processing first produced a raster image using the maximum and minimum temperatures as extreme values. The second product was an image classified based on changes in its properties in five classes associated with its distribution at the municipal level. The minimum temperature raster images were built based on the model created by the National Center for Meteorological Research (Centre National de Recherche Météorologique - CNRM), under the nomenclature CNRMCM5 (RCO 4.5)13, which formed part of the IPCC’s 5th Climate Change Assessment Report14. After testing and rescaling from 0.5° x 0.5° (55 x 55 km, approximately) to 30" x 30" (926 x 926 m) for application in the Mexico, southern United States and Caribbean region, this and other models (GDFL-CM3, HADGEM2-ES, etc.) were downloaded from the webpage of the Atmospheric and Environmental Sciences Data Unit (Unidad de Informática para las Ciencias Atmosféricas y Ambientales - UNIATMOS) of the Atmospheric Sciences Center of the National Autonomous University of Mexico (Universidad Nacional Autónoma de México - UNAM)15. Using different modules of the ARCMAP 10.2.2® program (ESRI, Redlands, CA), the CNRMCM5 model was processed for three time horizons: Short (2015-2039), Medium (2045-2069) and Long (2075-2099).
Results and discussion
Larvae development
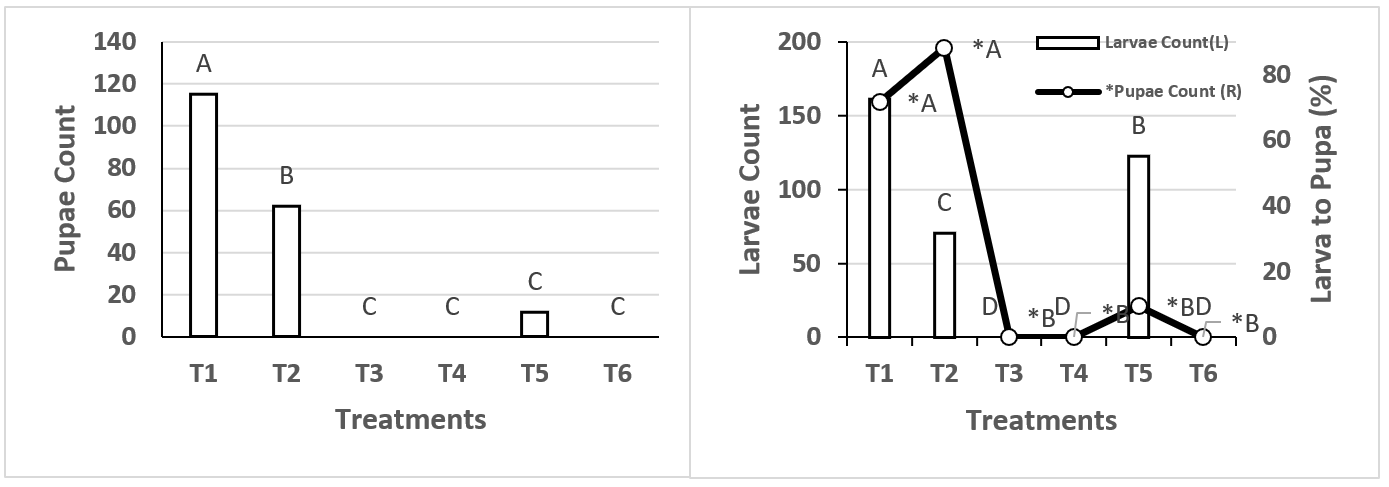
Comparison of median number of larvae per treatment showed treatment one (T1) to have the highest number (161 larvae per cage) (Figure 1). Treatments T3, T4 and T6 produced no larvae due to temperature variability, since larval development depends on there being at least 8 °C16,17. Musca domestica larvae prefer to develop at 35 °C and high humidity, but when fully developed prefer 15 to 20 °C with low HR, and do not withstand temperatures above 45 °C18. In another study fly larva distribution was higher in manure at temperatures from 17 to 35 °C19.
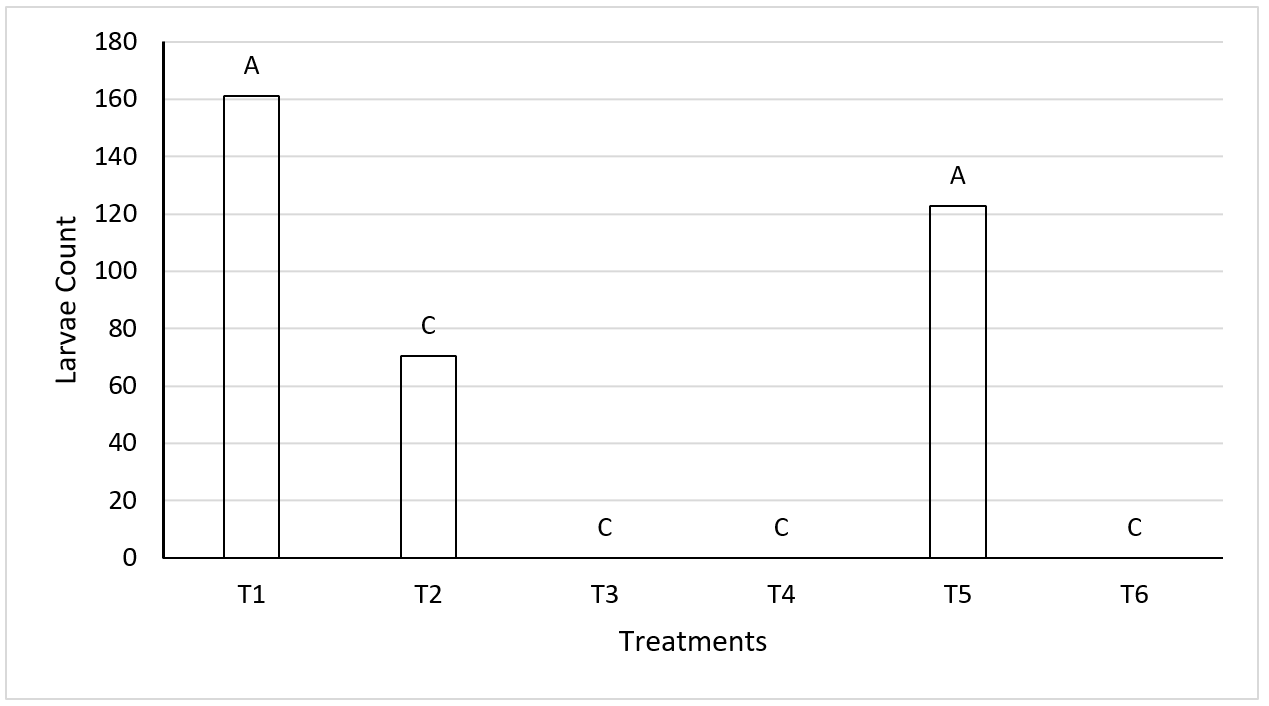
*Different letters on different bars indicate significant difference (P≤0.01).
Figure 1: Comparison of median M. domestica larvae count per treatment
Treatment T5 had the highest larvae count with 122 larvae per cage. The discrepancy between these treatments was a lower initial temperature in T1 (25 to 30 °C for T1 vs 35 °C for T5) and constant RH in T5. Relative humidity (RH) determines larvae survival, since insufficient RH will dry them out, while excess moisture can cause them to drown20.
Larvae to pupae development
Larvae were not present in treatments T3, T4 and T6 because initial conditions (>30 °C and low RH) did not allow for egg development. Maximum temperature for M. domestica oviposition is 25 to 30 °C, and eggs must remain moist or they will not hatch21.
Larvae did hatch in treatments T1, T2 and T5. Transition from larva to pupa was better in T1 than in the other treatments: an average of 115 pupae survived of the 160 larvae per cage. Treatment T2 had 62 pupae develop from 72 larvae per cage (Figure 2), and in T5 12 pupae survived from 122 larvae. The very low development rate in T5 and slightly lower rate in T1 may be due to the 90 % RH in both. In a previous study maximum larva mortality occurred at 100 % RH and temperatures higher than 30 °C22. Pupae tolerate less moisture than larvae and can tolerate temperatures from 35 to 40 °C, but only for a minimum period of 3 to 4 d18. In the present study the pupae remained for sixteen days at temperatures as high as 35 °C. Temperature clearly affects survival in different stages of M. domestica. In one study it was found that at 48 °C for 15 min all lifecycle stages died, at 37 °C all stages survived for just over 4 h, and at 42 °C adults died after approximately one hour; pupae were found to resist temperatures of 44 to 46 °C23. Exposure periods in this study were relatively short (a few hours at most), in contrast the periods used in the present study for the different treatments were until completion of the fly lifecycle, that is, from 19 to 22 d. An analysis of variance between treatments comparing larva to pupa transformation rates found T1 (74 %) and T2 (86 %) to have the best rates.
Pupa to adult transition
Treatment 1 (T1) had the highest adult emergence per cage (n= 85), followed by T2 (n= 54) (Figure 3). This suggests that the ideal temperature for M. domestica development in the present study was 20 to 30 °C. This agrees with a previous study in which fly density was highest at an average temperature of 20 to 25 ºC and no flies were present at temperatures higher than 45 °C or lower than 10 °C17. In another similar study the highest adult fly counts were observed at temperatures between 25 and 35 ºC24.
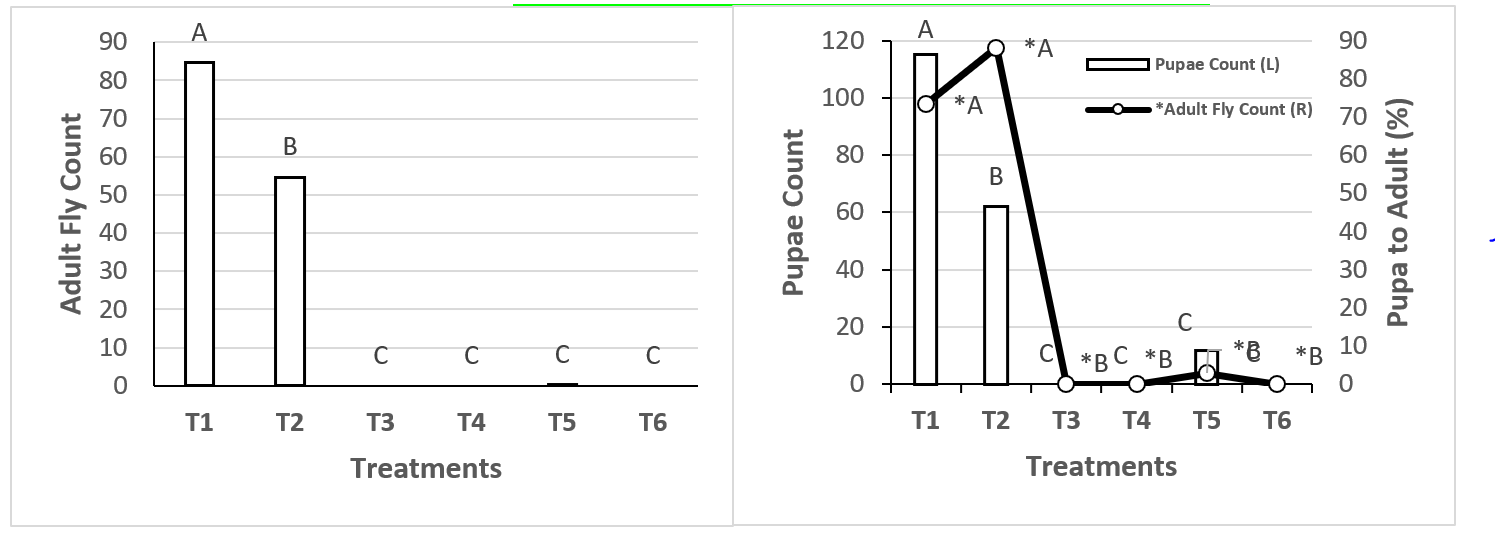
*Different letters on different bars indicate significant difference (P≤0.01).
Figure 3: Comparison of median M. domestica adult fly count per treatment
Treatment 2 (T2) had the highest pupa to adult transformation rate (84 %), followed by T1 (74 %). The 62.5 ± 2.5% RH used in T2 is near the 65 to 75 % RH reported as optimal for fly development22, which is why this treatment exhibited a more consistent development rate among the different stages. In T5, counts for both pupae (n= 11) and adults (n= 1) were minimal (Figure 3).
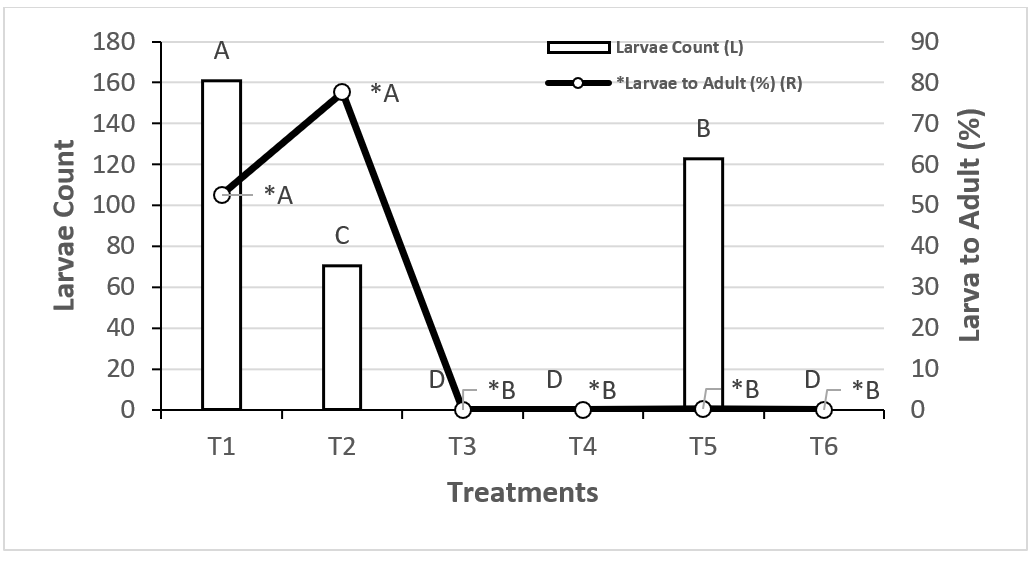
Larva to adult transition
Larva to adult development was highest in T2 (73 %), followed by T1 (55 %)(Figure 4).
Role of temperature and relative humidity in M. domestica development
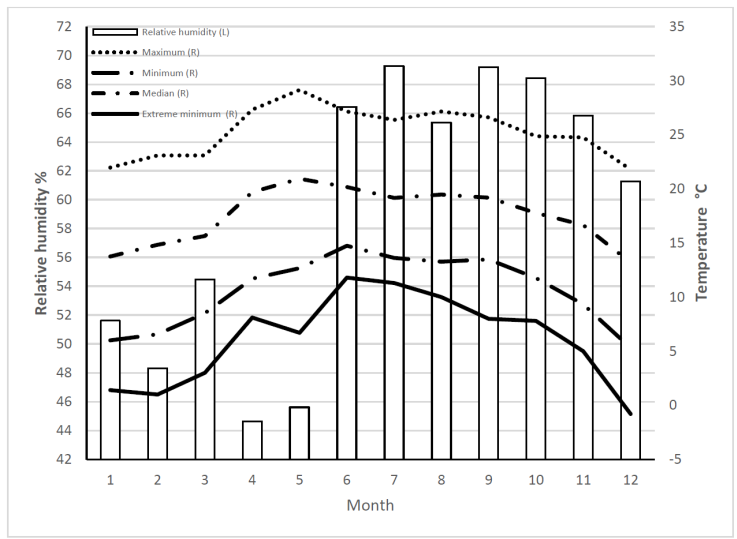
An analysis was done incorporating temperature and RH data for Jesús María municipality in 2015, M. domestica frequency, and the experimental results. Population increase was highest in June (extreme minimum= 11.8 °C) to August (extreme minimum= 10 °C)(Figure 5). The latter temperature is the lower limit for egg hatching and at which adults can barely fly17; it can be inferred that little copulation occurs. Oviposition generally does not happen below 15 °C18, which is probably why M. domestica populations are lower in April and May than in warmer months. This data agrees with reports of higher activity in this species at milder temperatures (30 to 33 °C) and low RH, and lower activity at very high temperatures and high RH17,25. In another study in which temperature remained nearly constant year round fly abundance was only influenced by changes in rainfall and RH24. A study done in horse farms also found that temperature and RH affected fly populations, causing them to grow in the spring when temperatures rise and decrease in autumn when temperatures fall26. Temperature is known to influence daytime M. domestica physical activity. As temperature increases (10 to 30-35 °C) during the day so does activity, and consequently pathogen dispersion and transmission, but when it surpasses 35 °C activity decreases notably27. This species therefore has a wide temperature range within which to function, which is important information when designing disease outbreak prevention measures.
Minimum temperature projection model
Prediction of future extreme minimum temperatures in Jesús María Municipality can indicate when M. domestica could have ideal development conditions. The experimental results demonstrated that temperature is the most significant parameter influencing mortality in this species22, while RH was important in regulating the viability of different stages within a moderate temperature range.
In the Short time horizon model, current conditions are predicted to remain relatively unchanged and M. domestica development will peak from June to August. Under the Medium and Long horizon models favorable development conditions will exist for a total of five months, from May to September (Figure 6). These projections will also depend on other factors such as wind, food availability, light and RH in the region. If these scenarios hold true, M. domestica populations will remain stable until 2039, but could then increase due to the longer periods apt for development. A similar prediction has been made for the United Kingdom, for which climate change scenarios predict increases of up to 244 % in the fly population in the summer months by 208028.
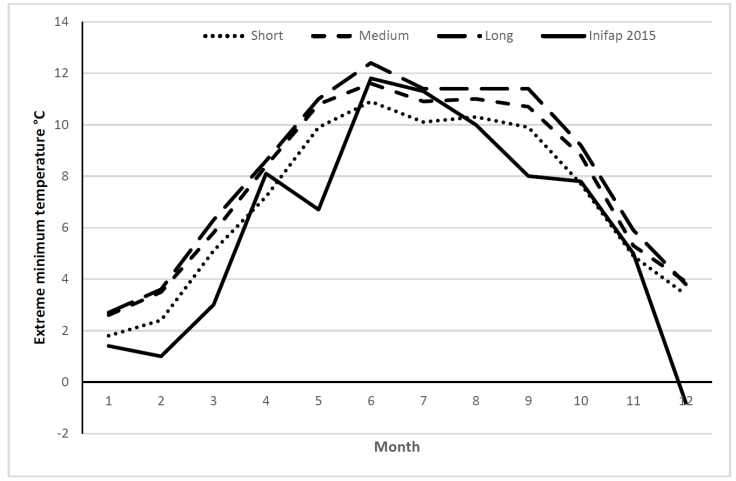
Figure 6: Extreme minimum temperatures in Jesús María Municipality in 2015, and its projection over three time horizons: Short, 2015-2039; Medium, 2045-2069; and Long, 2075-2099
This predictive information can help in planning control programs during more favorable fly development periods. Reducing M. domestica proliferation can help to avoid negative effects on livestock farms, reduce food contamination in the food industry and lower disease occurrence in human populations. The species’ high reproductive potential requires effective and timely control practices to protect human and animal health29. Control measures in developed countries have been established, especially for agriculture and the dairy industry, control agents have been formulated, and they are applied in both urban and rural areas1. If insecticide is to be used, the present data can help to program application schedules and efficacy. It must be considered that these projections do not take into account biotic factors such as predators and parasitoids that could arise in coming years28.
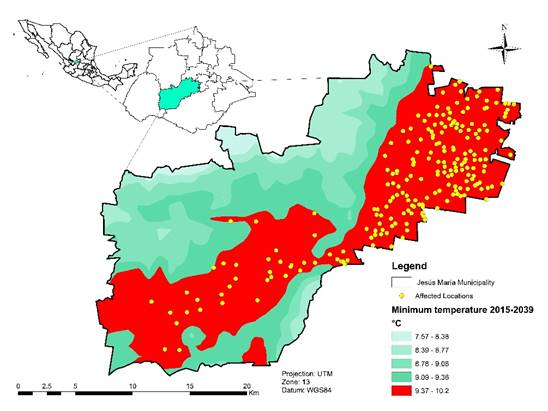
Prediction of temperature ranges in Jesús María Municipality over the Short horizon shows that approximately 100,000 inhabitants in the municipality’s 235 settlements30 could be affected by increased M. domestica populations (Figure 7). The problem could be most acute in the map’s red-toned areas where most of the population is concentrated (219 villages; 99,046 inhabitants). Musca domestica is often associated with livestock and household waste disposal facilities, since accumulation of organic matter provides appropriate breeding conditions28. It also prefers to feed on decomposing vegetal and animal matter, which puts it in contact with the pathogenic organisms present in human garbage and animal waste, making it a disease vector for humans and animals31.
Conclusions and implications
Under the experimental conditions M. domestica development was ideal between 20 and 30 °C, a temperature range that currently occurs in the study area for three months a year (June to August). Predictive analysis of regional climate suggested that this species will be favored by longer periods of ideal temperatures in the Medium and Long horizons; these will probably stretch over the five months from May to September. Longer periods with apt conditions for M. domestica development could exacerbate contamination problems in livestock and agricultural production and the food industry, and promote the spread of diseases among animal and human populations. Almost all the populated areas in Jesús María Municipality will experience this climate change, highlighting the need to consider it when designing monitoring and control programs.
Literatura citada:
1. Malik A, Singh N, Satya S. House fly (Musca domestica): A review of control strategies for a challenging pest. J Env Sci & Health, Part B 2007;42(4):453-469. doi: http://dx.doi.org/10.1080/03601230701316481. [ Links ]
2. Mehrabi MR, Zoghimofrad I, Mazinani M, Akbarzadeh, A, Rahimi A. A study of the effect of Bacillus thuringiensis serotype H14 (subspecies israelensis) delta endotoxin on Musca larva. Turk J Med Sci 2015;45(4):794-799. doi: http://dx.doi.org/10.3906/sag-1406-91. [ Links ]
3. Burgess ER, King BH. Compatibility of the parasitoid Wasp Spalangia endius (Hymenoptera: Pteromalidae) and insecticides against Musca domestica (Diptera: Muscidae) as evaluated by a new index. J Econ Entomol 2015;108(3):986-992. doi: http://dx.doi.org/10.1093/jee/tov104. [ Links ]
4. Kumar P, Mishra S, Malik A, Satya S. Insecticidal evaluation of essential oils of Citrus sinensis L. (Myrtales: Myrtaceae) against housefly Musca domestica L. (Diptera: Muscidae). Parasitol Res 2012;(110):1929-1936. [ Links ]
5. Lu X, Shen J, Jin X, Ma Y, Huang Y, Mei H, Zhu J. Bactericidal activity of Musca domestica cecropin (Mdc) on multidrug-resistant clinical isolate of Escherichia coli. Appli Microbiol & Biotechnol 2012;95(4):939-945. doi: http://dx.doi.org/10.1007/ s00253-011-3793-2. [ Links ]
6. Sharma S, Rohilla MS, Tiwari PK. Developmental and hyperthermia-induced expression of the heat shock proteins HSP60 and HSP70 in tissues of the housefly Musca domestica: An in vitro study. Genet Mol Bio 2007;30(1):159-168. [ Links ]
7. Chapman JW, Goulson D. Environmental versus genetic influences on fluctuating asymmetry in the housefly, Musca domestica. Biol J Linn Soc 2000;(70):403-413. doi: http://dx.doi.org/10.1006/bijl.1999.0408. [ Links ]
8. Kassem HA, El-Sayed YA, Baz MM, Kenawy MA, El Sawaf BM. Climatic factors influencing the abundance of Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae) in the Nile Delta. J Egypt Soc Parasitol 2009;39(1):305-316. [ Links ]
9. SIAP. Servicio de Información Agroalimentaria y Pesquera. 2015. Resumen municipal pecuario. http://www.siap.gob.mx/ganaderia-resumen-municipal-pecuario/ . Consultado 1 Jul, 2016. [ Links ]
10. INIFAP. Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. Red Nacional de Estaciones Estatales Agroclimatológicas, Agroclima del Inifap. http://clima.inifap.gob.mx/redinifap/# . Consultado 6 Ene, 2016. [ Links ]
11. López-Santos A, Pinto-Espinoza J, Ramírez-López EM, Martínez-Prado MA. Modeling the potential impact of climate change in northern Mexico using two environmental indicators. Atm 2013;(26):479-498. http://www.journals.unam.mx/index.php/atm/article/view/32016/38321 . Consultado 18 Ago, 2015. [ Links ]
12. López SA, Pinto JE, Esquivel GA, Randeles VHR, Bueno PH. Escenarios climáticos locales basados en los MGCG del IPCC. 1ra ed. Durango, México: Universidad Autónoma Chapingo; 2015. ISBN: 978-607-12-0403-5. [ Links ]
13. Voldoire A, Sanchez-Gomez E, Salas y Mélia D, Decharme B, Cassou C, Sénési S. et al. The CNRM-CM5.1 global climate model: description and basic evaluation. Clim Dyn 2013;(40):2091-2121. doi: http://dx.doi.org/10.1007/s00382-011-1259-y. [ Links ]
14. Edenhofer O, Pichs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K. et al. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: IPCC. Intergovernmental Panel on Climate Change editor. Climate Change 2014 Mitigation of Climate Change. 1rst ed. United Kingdom and New York, NY, USA: Cambridge University Press; 2014. [ Links ]
15. Fernandez-Eguiarte A, Zavala-Hidalgo J, Romero-Centeno R, Trejo-Vázquez RI. Actualización de los escenarios de cambio climático para estudios de impactos, vulnerabilidad y adaptación. Centro de Ciencias de la Atmósfera. Universidad Nacional Autónoma de México. Instituto Nacional de Ecología y Cambio Climático. Secretaria de Medio Ambiente y Recursos Naturales de México. 2014. http://atlasclimatico. unam.mx:8550/geonetwork/srv/spa/main.home . Consultado 17 Mar, 2016. [ Links ]
16. Sukontason K, Piangjai S, Siriwattanarungsee S, Kabkaew L, Sukontason KL. Morphology and developmental rate of blowflies Chrysomya megacephala and Chrysomya rufifacies in Thailand: application in forensic entomology. Parasitol Res 2008;102(6):1207:1216. doi: http://dx.doi.org/10.1007/s00436-008-0895-6. [ Links ]
17. Stafford KC. A Guide to biology, dispersal, and management of the housefly and related flies for farmers, Municipalities, and Public Health Officials. 2008 Bulletin 1013. Conn Agr Exp Sta, New Haven Conn Agr Exp Sta, New Haven http://www.ct.gov/caes/lib/caes/documents/publications/ bulletins/b1013.pdf . Consultado 5 Nov, 2015. [ Links ]
18. WHO. World Health Organization. The housefly: Training and information guide. Vector control series Geneva; 1991. http://www.who.int/iris/handle/10665/58637 . Consultado 9 Oct, 2015. [ Links ]
19. Stanfford KC, Bay DE. Dispersion pattern and association of housefly, Musca domestica (Diptera: Muscidae), larvae and both sexes of Macrocheles muscaedomesticae (Acari: Macrochelidae) in response to poultry manure moisture, temperature, and accumulation. Environ Entomol 1987;16(1):159-164. doi: http://dx.doi.org/10.1093/ee/16.1.159. [ Links ]
20. Feldmeyer B, Kozielska M, Kuijper B, Weissing FJ, Beukeboom LW, Pen I. Climatic variation and the geographical distribution of sex-determining mechanisms in the housefly. Evol Ecol Res 2008;10:797-809. [ Links ]
21. Capinera JL. House fly, Musca domestica L. (Diptera: Muscidae). In: Capirera JL editor. Encyclopedia of entomology. 2nd ed. Springer; 2008:1877-1880. doi: 10.1007/978-1-4020-6359-6. [ Links ]
22. Mishra S, Kumar P, Malik A. Effect of temperature and humidity on pathogenicity of native Beauveria bassiana isolate against Musca domestica L. J Parasit Dis 2015;39(4):697-704. doi: http://dx.doi.org/10.1007/s12639-013-0408-0. [ Links ]
23. Tiwari PK, Archana J, Mohan DRK. Thermotolerance and heat shock response in Musca domestica. Curr Sci 1997;72(7):501-506. [ Links ]
24. Bong LJ, Zairi J. Temporal changes in the abundance of Musca domestica Linn (Diptera: Muscidae) in poultry farms in Penang, Malaysia. Trop Biomed 2009;26(2):140-148. [ Links ]
25. Dakshinamurty S. The common House-fly, Musca domestica, L., and its behaviour to temperature and humidity. Bull Entomol Res 1948;(39):339-357. doi: https://doi.org/10.1017/S000748530002246X. [ Links ]
26. Machtinger TE, Geden JCh, Kaufman EP, House MA. Use of pupal parasitoids as biological control agents of filth flies on equine facilities. J Integr Pest Manage 2015;6(1):1-16. doi: http://dx.doi.org/10.1093/jipm/pmv015. [ Links ]
27. Schou TM, Faurby S, Kjærsgaard A, Pertoldi C, Loeschcke V, Hald B, Bahrndorff S. Temperature and population density effects on locomotor activity of Musca domestica (Diptera: Muscidae). Environ Entomol 2013;42(6):1322-1328. doi: http://dx.doi.org/10.1603/EN13039. [ Links ]
28. Goulson D, Derwent lC, Hanley ME, Dunn DW, Abolins SR. Predicting calyptrate fly populations from the weather, and probable consequences of climate change. J Appl Ecol 2005;42(5):795-804. doi: http://dx.doi.org/10.1111/j.1365-2664.2005.01078.x. [ Links ]
29. Selem GS, El-Sheikh EA. Toxicity and biochemical effects of Neem Azal T/S, willow (Salix aegyptiaca L.) and Chasteberry (Vitex agnus-castus L.) on housefly, Musca domestica L. (Diptra: Muscidae). J Biopest 2015;8(1):37-44. ISSN: 0974-391X. [ Links ]
30. INEGI. Instituto Nacional de Estadística y Geografía. Vectorial de localidades amanzanadas y números exteriores, rurales, cierre de planeación del censo de población y vivienda. Jesús María, Aguascalientes. México. 2010. [ Links ]
31. Butler JF, Garcia-Maruniak A, Meek F, Maruniak JE. Wild Florida house flies (Musca domestica) as carriers of pathogenic bacteria. Fla Entomol 2010;93(2):218-223. doi: http://dx.doi.org/10.1653/024.093.0211. [ Links ]
Received: August 02, 2016; Accepted: March 05, 2018











 text in
text in