In tropical grasses the fiber-bound protein (neutral detergent insoluble protein, NDIP) accounts for one third of its crude protein content1,2 which might represent a potential source of protein for ruminal degradation in bovines grazing under tropical conditions. The CNCPS version 6.53 assumes that the digestion rate of the PB2 fraction (fiber-bound protein) is similar to the CB3 fraction (digestible fiber) and Higgs4 validates this assumption assigning, for grass hay, an average digestion rate of 4.5 %/h for both fractions. Although Ogden5 showed that in C4-grasses the rate of digestion of the NDIP fraction is faster than its own NDF fraction (11.0 vs 7.8 %/h). In Australian tropical grasses measuring NDIP rates of digestion found a range between 4.7 and 7.9 %/h6. However, Singh2 for tropical grasses in India consider the Sniffen digestion rate of fiber-bound protein of less than 1.5 %/h7. Also, in Brazilian grasses8 reported digestion rates between 0.08 and 1.3 %/h for the PB3 fraction. At a given passage rate of 2.5 %/h Ogden5 or a range between 2.0-4.4 %/h Bowen6 it is critical to know if the NDIP digestion rate exceeds or not the passage rate. Because grasses frequently make up the only source of protein for many ruminants, information on the NDIP fraction of grasses is a prerequisite to modeling the N economy of ruminants.
Concentration, distribution and digestibility of protein in forages is affected by species9, N fertilization10, and cutting age8. Data on the effects of these management practices are incomplete. The goals were to examine in vitro the pool size, and extent and rate of digestion of the cell wall protein of four tropical grasses (Andropogon gayanus, Brachiaria brizantha, Cynodon plectostachyus, and Megathyrsus maximus), and to explore the effects of N fertilization on these variables.
The study was conducted at the experimental station La Posta, of the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Located in the State of Veracruz on the southeastern coast of Mexico at 19° 02' L and 96° 08' L, at 12 m asl, tropical subhumid Aw, average annual rainfall of 1,728 mm, 25 °C of average temperature and 81 % relative humidity. The soil is classified as Oxisol, a predominantly sandy loam with >15 % clay.
The grasses Andropogon gayanus, Brachiaria brizantha, Cynodon plectostachyus, and Megathyrsus maximus Var. Guinea, were selected because they are commonly used in tropical areas. At onset of the rainy season, each grass was grown in four plots (5×5 m). Two plots were fertilized with N (relationship equivalent to 100 kg N/ha) and other plots not. All plots were previously cut to a height of 5 cm. After 35 d of regrowth, one sample of 2 m2 from the center of each plot was clipped at a height of 10 cm. A sub-sample of 500 g of green material was frozen immediately at -15 °C, and other sub-sample of 500 g was placed in a forced air oven at 100 °C during 24 h for dry matter (DM) determination. This sample was discarded after DM determination. At the end of sampling (July 25th), four frozen samples from each grass were freeze dried, placed in 30 × 25 cm heavy duty freezer bags, and sent to Cornell University for laboratory analysis.
All samples were ground through a 1-mm screen in a Wiley mill (Model 4, Arthur H. Thomas Co. Philadelphia, PA). Dry matter was determined by direct oven-drying samples at 100 °C overnight. Crude protein (N × 6.25) was determined by Macrokjeldahl procedure11, modified by using boric acid at 4% concentration during distillation. The protein fractions were determined as described by Licitra12. The N and protein fractions of the grasses were partitioned according to Pichard and Van Soest13 and Van Soest14 as follow: all the nitrogen determinations were converted to protein values by multiplying the amount of N by 6.25. The non-precipitable fraction of the Tungstic acid precipitation method was used to measure non protein nitrogen (NPN) which is fraction A, and the borate-phosphate buffer method was employed to measure protein solubility (fraction B). The difference between the precipitable fraction in Tungstic acid and soluble in buffer is the true soluble protein (fraction B1 fast solubility). The difference between the insoluble fraction in buffer and soluble in NDF is the B2 fraction (medium solubility). The difference between the insoluble fraction in NDF and soluble in ADF is the B3 fraction (slow solubility). And the protein insoluble in ADF is the C fraction (indigestible protein).
The bicarbonate-phosphate buffer of Goering and Van Soest15 was employed in the in vitro medium. An equal amount of cysteine hydrochloride replaced the sodium sulfide. The medium was boiled to remove dissolved gases, cooled, cysteine added (0.625 mg/mL), and the pH was adjusted to 6.8 as necessary. Ruminal fluid was collected via ruminal cannula approximately 6 h after feeding from a 600 kg, mature, non-lactating Holstein cow that was fed ad libitum on average quality mixed hay in order to meet maintenance requirements in accordance with The Institutional Animal Care and Use Committee (IACUC, 2002) protocol.
At the onset of a fermentation, each 120 mL serum bottle contained 16 mL medium, 4 mL ruminal fluid, and 200 mg sample. Incubation times for determining NDIP disappearance were 0, 1.5, 3, 6, 9, 12, 24, 48, and 96 h. At each time point, the gas was released with a needle, the bottle was opened and the fermentation was briefly interrupted by adding 40 mL of NDF solution. The NDF16 and NDIP, measured by Kjeldahl analysis of the fiber residue, were determined at each time point. Because of the small size and low nitrogen concentration of the samples, Kjeldahl solutions were diluted by 50 % to increase sensitivity. Reducing agents such as cysteine HCl and sodium sulfide in the medium may affect the rate of protein degradation17 especially during the first 2 h of fermentation (unpublished data). To minimize this effect, blanks containing 200 mg of sample and medium with cysteine HCl but without rumen fluid were used. The amount of protein in the blanks digested after 2-h fermentation period was subtracted from the initial amount of protein in the experimental samples.
An exponential equation with lag was used to determine the rate of NDF disappearance18:
Y=a*(exp(-b*(X-c))) where Y=residual NDF/DM at time t; a=digestible NDF, %; b=rate of NDF disappearance, %/h; and c=lag time, h. Because no lag was observed in the NDIP disappearance, an exponential equation without lag was used for NDIP rate computations: Y=a*(exp(-b*X)) where Y=residual NDIP/DM at time t; a=digestible NDIP, %; b=rate of NDIP disappearance, %/h.
The goal of this study was to measure the digestion rate of the NDIP, not to determine whether ADIP is digested or not19. As a result, no ADIP corrections were made. All curves were fitted using Table Curve (version 2.0, Jandel Scientific, San Rafael, CA).
A complete randomized block design with factorial arrangement and two replicates per treatment was used, where the factors were grass species and N fertilization. A laboratory standard of guinea grass (M. maximus) was used to control for ruminal fluid variation among in vitro runs. A 4 x 2 factorial arrangement of forage species (A. gayanus, B. brizantha, C. plectostachyus, or M. maximus Var. guinea) and N fertilization (0 and 100 kg/ha) as factors was used. The grass*fertilizer interaction was used to test for the main effects. The grass*fertilization effects were tested with the rest of the experimental error. Planned comparisons among the forages were estimated using Tukey's W procedure. Results were deemed significant at P≤0.05 for the grass and for the fertilization effect. The ANOVA analyses were performed using the MINITAB, Version 10 (Minitab Inc., State College, PA). Because there were no interactions (grass*N fertilization) of the 4×2 factorial arrangement of treatments, means of mean factors (grass or N fertilization) are shown in separate tables.
Chemical composition, protein fractions, kinetic and potential degradation of tropical grasses are shown in Table 1. The average contents of CP (8.4 ± 2.21 %DM) and NDF (70.0 ± 3.58 % DM) are within the range of tropical grasses at similar stages of growth20. Most of the protein is in the buffer insoluble fraction (IP), and the major part of this is in the NDIP. Brachiaria brizantha is an exception because it has low NDIP and ADIP values with corresponding increases in NPN.
Table 1: Chemical composition, protein fractions, kinetics and neutral detergent insoluble protein (NDIP) potential degradation of tropical grasses
|
Andropogon gayanus |
Cynodon plectostachyus |
Megathyrsus maximus |
Brachiaria brizantha |
SEM | |
|---|---|---|---|---|---|
| Chemical composition (% DM) | |||||
| NDF | 71.5a | 74.9a | 71.9a | 65.3b | 0.57 |
| ADF | 41.0a | 41.2a | 42.3a | 36.5b | 0.18 |
| CP (N × 6.25) | 9.1 a | 8.3ab | 7.2b | 9.0a | 0.12 |
| NPN (N × 6.25) | 1.5b | 1.0b | 1.1b | 2.5a | 0.06 |
| IP (N × 6.25) | 7.4 a | 5.6b | 5.2b | 5.3b | 0.11 |
| NDIP (N × 6.25) | 4.4 a | 3.1ab | 2.9b | 1.2c | 0.14 |
| ADIP (N × 6.25) | 0.6b | 0.8a | 0.6b | 0.3c | 0.02 |
| Protein fractions (% CP) | |||||
| A | 16.6b | 12.3b | 16.9b | 29.2a | 0.77 |
| B1 | 2.4c | 19.7a | 10.7b | 12.9b | 0.68 |
| B2 | 31.7c | 29.2c | 38.1b | 44.6a | 0.86 |
| B3 | 42.5a | 28.1b | 24.9b | 9.9c | 1.06 |
| C | 6.7b | 10.6a | 9.4a | 3.5c | 0.28 |
| NDIP kinetics and potential degradation | |||||
| Extent, % | 81.7a | 74.1b | 74.5b | 52.2c | 0.69 |
| Rate, %/h | 8.4a | 5.2b | 9.7a | 5.2b | 0.28 |
| Potentially indigestible, %DM* | 0.72b | 0.80a | 0.67b | 0.58c | 0.007 |
| Potentially digestible, %DM | 3.7a | 2.3b | 2.2b | 0.64c | 0.135 |
| Escape, %DM** | 1.3a | 1.1a | 0.75b | 0.32c | 0.040 |
| Escape, %NDIP** | 37.9b | 48.9a | 34.1b | 49.2a | 1.07 |
| Escape, %potentially digestible** | 30.8ab | 36.2a | 25.3b | 25.6b | 0.62 |
NDF=neutral detergent fiber; ADF=acid detergent fiber; CP=crude protein; NPN=non protein nitrogen; IP=protein insoluble in buffer; NDIP=neutral detergent insoluble protein; ADIP=acid detergent insoluble protein.
* NDIP residue after 96 h in vitro incubation.
** Insoluble potentially digestible NDIP escaping from rumen degradation.
a,b,c Means with different superscripts in row are different (P≤0.05); SEM=standard error of means.
When the protein fractions are expressed on a CP basis, intracellular soluble protein (A, B1, and B2) makes up 66 % of the total CP, and the extracellular structural protein (B3 and C) composes 34 % of the total. The largest pool (36 %) of the non-cell wall protein is the B2 fraction, which consists of proteins that make up the structure of cellular organelles and some enzymatic complexes21. The B3 fraction which is the NDIP - ADIP was 26 % of the total CP. Unlike the protoplasmic proteins, the structural proteins are glycoproteins (extensins) with high levels of hydroxyproline22.
Large variation was found within the protein fractions, primarily due to species. Brachiaria brizantha had a very different protein profile from the others, because it has more A and B2 and less B3 and C fractions, suggesting that this forage have a higher percentage of the forage N in an useable form for ruminants. Andropogon gayanus had about 50 % of its protein in the cell wall. However, C. plectostachyus and M. maximus Var. Guinea had similar protein profiles. Approximately 10 % of the protein in C. plectostachyus and M. maximus Var. Guinea was ADIP, a fraction often assumed to be unavailable or unused by the animal19. These variations pose a difficulty when trying to predict protein fractions on grasses with the same CP content. A situation that arise when NIRS technology is used to predict N fractions in forages. Even though, total N is well predicted by NIRS, and NDIP also has an acceptable coefficient of determination (only when there is a strong correlation with total N), the protein B3 fraction it is not well predicted given by the accumulation of errors caused by the subtraction process23. A recommendation is to generate NIRS calibration equations by grass specie.
The extent of digestion at 96 h ranged from 52.2 to 81.7 % and the rates ranged from 5.2 to 9.7 %/h. These measured rates of NDIP digestion are faster than those in the Cornell Net Carbohydrate and Protein System (CNCPS version 6.5)4 feed library for the same grasses. The CNCPS is a nutritional model that evaluates the environmental and nutritional resources available in an animal production system and enables the formulation of diets that closely match the predicted animal requirements. Assuming a passage rate of 5 %/h, much of the NDIP will be degraded in the rumen if our rates are used. Data of the present work are in agreement with those found by Ogden5. In contrast, when slower rates are used2,9, the NDIP will make little contribution to the ruminal N supply. The B2 pool described by Messman24 was determined mathematically, using a 2-pool model, not by chemical fractionation. Their B2 rates ranged from 0.6 to 1.2 %/h, which are slower than rates of this work, and close to those proposed by Sniffen7 for the CNCPS version 5.0 B3 fraction.
There is wide variation in NDIP content, and in NDIP extent and rate of digestion due to species. A. gayanus and B. brizantha have similar CP content but their NDIP fraction behave differently. Brachiaria brizantha has less NDIP (15 %CP) and it is only 52.2 % digestible and its digestion rate is slow (5.2 %/h). On the other hand, half of the CP in A. gayanus is NDIP, but this NDIP is 81.7 % digestible with a digestion rate of 8.4 %/h. C. plectostachyus and M. maximus Var. Guinea contain the same amount of NDIP, but their rate constants were different (5.2 vs 9.7 %/h). Therefore, at a given passage rate, more of the NDIP from M. maximus would be ruminally available despite the fact that it had the same NDIP content as C. plectostachyus.
The species variation in the rate of digestion of the NDIP may be due to differences in the extent of glycosylation and amino acid profile of the structural proteins22. The role of moderately glycosylated extensin proteins; hyperglycosylated arabinogalactan proteins25; and hidroxiprolin/proline-rich proteins that may be no glycosylated at all as important components of cell wall proteins, play pivotal roles in cell wall signal and affects the amount of intra and intermolecular cross-linking26. Both glycosylation and cross-linking may affect ruminal degradation.
The first step in predicting the amount of undegradable protein in the NDIP was to evaluate how much remained undegraded after 96 h of in vitro fermentation. Between 16 % (A. gayanus) and 50 % (B. brizantha) of the NDIP remained after 96 h. Following the calculations of Anderson27 and using a fixed passage rate of 5 %/h, the NDIP escaping ruminal digestion ranged from 0.32 to 1.32 % of DM. Bowen6 found a range from 0.50 to 1.0 % of DM of rumen undegradable protein fraction actually measuring passage rate in C4 grasses. The escape value was highest for A. gayanus and lowest for B. brizantha. Expressing this fraction as a percentage of the NDIP indicates that between 25.3 to 36.2 % escaped the rumen and was available for digestion in the intestine.
The residual NDIP of B. brizantha after 96 h of fermentation was double the ADIP level. This implies that part of the NDIP would not be digested in the rumen even with long retention times. This observation may lead us to question the use of ADIP as an indicator of indigestible N in the rumen. Microbial contamination was not an interference due to its removal by either NDF or ADF solutions.
Means by effect of N fertilization on chemical composition, protein fractions, kinetics and potential degradation of tropical grasses are shown in Table 2. Nitrogen fertilization increased (P≤0.05) N content as has been reported in different studies28,29. Mass protein fractions (%DM): NPN, IP, NDIP and ADIP tended to follow the total protein mass fraction. Similar response in Lolium perenne found Hoekstra30.
Table 2 Effect of N fertilization on average chemical composition, protein fractions, kinetics and NDIP potential degradation of tropical grassesN fertilization kg/ha
| N fertilization kg/ha | |||
|---|---|---|---|
| 0 | 100 | SEM | |
| Chemical composition (% DM) | |||
| NDF | 72.1 | 69.7 | 0.29 |
| ADF | 40.3 | 40.2 | 0.09 |
| CP (N × 6.25) | 5.9b | 10.9a | 0.06 |
| NPN (N × 6.25) | 1.2b | 1.8a | 0.03 |
| IP (N × 6.25) | 4.1b | 7.6 a | 0.06 |
| NDIP (N × 6.25) | 2.2b | 3.6 a | 0.07 |
| ADIP (N × 6.25) | 0.5b | 0.7 a | 0.01 |
| Protein fractions (% CP) | |||
| A | 20.8 | 16.7 | 0.38 |
| B1 | 9.3 | 13.5 | 0.34 |
| B2 | 33.4b | 38.4 a | 0.43 |
| B3 | 27.6 | 25.1 | 0.53 |
| C | 8.8 | 6.3 | |
| NDIP kinetics and potential degradation | |||
| Extent, % | 65.7 | 75.5 | 0.34 |
| Rate, %/h | 6.7 | 7.6 | 0.14 |
NDF=neutral detergent fiber; ADF=acid detergent fiber; CP=crude protein; NPN=non protein nitrogen; IP=protein insoluble in buffer; NDIP=neutral detergent insoluble protein; ADIP=acid detergent insoluble protein.
a,b,c Means with different superscripts in row are different (P≤0.05).
SEM=standard error of means.
The impact of N fertilization on the distribution of the protein fractions results in more true cellular protein (B2). Similar response found Johnson10 fertilizing C. dactylon in Florida at different rates of N, concluding that the nitrogen pool available for rumen microbes to be utilized in microbial protein synthesis is increased in tropical forages as fertilization rates increase. In contrast, with temperate grasses Hoekstra30 using L. perenne did not found any effect at all of the nitrogen fertilization on the nitrogen fractions suggesting that appears to be limited scope for the manipulation of grass-protein fractionation through grass fertilization. Because N-fertilized grasses had more N in the more digestible fractions, more N retention would be expected. The digestion rates for NDIP were not affected by N fertilization. In the literature review, it was not find evidence of N fertilization on digestion rate of the NDIP fraction in tropical forages.
Results for extent and rate of degradation of the NDF are presented in Figure 1. The range in the rates of the NDF disappearance is in agreement with those found elsewhere31 in some tropical forages (5 -6 %/h), and with those suggested by the CNCPS4 for grass hay (4.5 ± 1.0 %/h). The digestion coefficients for NDF were lower than those for the NDIP (P≤0.05). Also, Ogden5,32 shows that rates of digestion of NDIP fraction are faster than the NDF fraction and also found that the effective disappearance of NDIP is considerably higher than for NDF. Lignin (the major determinant of indigestibility)14 does not seem to affect the structural proteins the same way as it inhibits digestion of structural carbohydrates. For instance, glycoproteins may be more readily digested than cellulose or hemicellulose because there are no linkages between lignin and extracellular proteins. Besides, proline the most extensive amino acid in structural proteins26, is extremely soluble as 14 kg of it can be dissolved in 1 kg of water33. Weiss34 could not find evidence that lignin interferes directly with CP digestion.
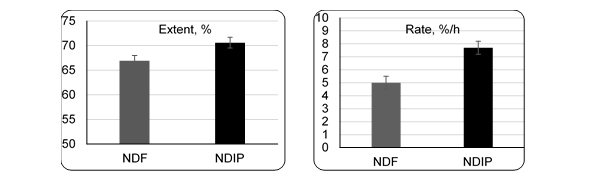
Figure 1 Comparison of extent and rate degradation of neutral detergent fiber (NDF) versus neutral detergent insoluble protein (NDIP) in tropical grasses
The NDIP as a percentage of the CP averaged 35 with a range of 10 to 60. The NDIP variation was primarily due to species but fertilization also was a factor. The NDIP is potentially ruminally available with an extent of 70.6 % at a rate of 7.1 %/h. Nitrogen fertilization had no influence on the kinetics of the NDIP digestion. The NDIP was digested faster than the NDF.











 nueva página del texto (beta)
nueva página del texto (beta)


