INTRODUCTION
In the last few decades, antibiotic residuals and the development of bacterial strains resistant to antibiotics have become a problem1 due to the addition of antibiotics in animal feed formulations2,3,4. However, the prohibition of the use of antibiotics in animal feed in Europe in 2006 to improve the safety and security of the food chain caused significant health problems in poultry, such as increasing the incidence of intestine necrotic enteritis and clostridia. These in turn caused major complications related to decreasing animal welfare and increasing economic losses4,5. Hence prebiotics, probiotics, synbiotics, herbs, spices and essential oils has been investigated as an alternative to antibiotics because of their antibacterial, antioxidant, digestive and metabolic enhancing effects6.
Botanical compounds have been shown to be potential alternatives to antibiotics for poultry production7,8,9. Turmeric is a member of the Zingiberaceae family. It is mainly utilized in the food industry to enhance the palatability, preservation and appearance of food. Turmeric contains different bioactive compounds, such as curcumin, demethoxycurcumin, bisdemethoxycurcumin and tetrahydrocurcuminoids10,11,12. These bioactive compounds have antioxidant, anti-inflammatory and nematocidal activities10,13,14,15, protective effects against mutagenicity and hepatocarcinogenicity induced by aflatoxin2,16 and against coccidiosis5,17,18. In the literature, the effects of turmeric supplementation between 0 to 10 g/kg on chicken performance have been inconclusive. For example, broilers fed diets supplemented with turmeric at 5 g/kg feed exhibited improved performance but did not affect serum total protein, albumin, globulin, ALKP, ALT and AST enzymes1,6,19. On the other hand, turmeric powder did not significantly affect growth performance or the carcass yield of broiler chickens9,12,20. Turmeric powder at 0.6 and 0.9 g/kg alleviated the negative effect of aflatoxin B1 on serum total protein, albumin and globulin, boosted antioxidant defense enzymes, e.g. catalase and superoxide dismutase, and decreased MDA2. The levels of liver enzymes (ALT and ALKP) were substantially reduced by feeding broilers turmeric powder at 5 g/kg21.
Prebiotics are also possible alternatives, particularly mannan oligosaccharides (MOS) derived from Saccharomyces cerevisiae22,23. The mode of action of MOS involves supplying intestinal microflora by nutrients (as prebiotics) and inhibiting the attachment of pathogenic bacteria, i.e. E. coli and Salmonella enteritidis, to the intestinal mucosa by binding the mannose receptors on the type 1 fimbriae22. The positive effect of MOS on broiler performance was already reviewed22.
Oxytetracyclines (OTC) are broad-spectrum bacteriostatic agents derived from the bacteria Streptomyces. Oxytetracyclines prevent bacteria from multiplying while the host animal’s immune system deals with the original infection. The recommended dose is 5 to 50 g/t feed as a continuous feed additive. In the literature, oxytetracyclines have been used for the amelioration of the growth of broilers, but the results have been contradictory24,25. Thus this research aims to investigate the growth promoting effect of turmeric (Curcuma longa Linn.) and to compare it to OTC and MOS on growth performance, carcass characteristics, meat quality, serum biochemical constituents and health status during the 1st through 35th days of age of broiler chickens.
MATERIAL AND METHODS
Source of turmeric, fatty acid profiles and antioxidant indices
Turmeric purchased from the local market in a powder form was used in this experiment. The chemical analyses of the experimental diets were according to AOAC26, meanwhile, metabolizable energy value was calculated using the equation for vegetable/plant feedstuffs according to Janssen27. The fatty acids profile analysed according to Radwan28 (Table 1) after the extraction of lipids26. The total phenolic contents according to Balinsky et al29 and the antioxidant activity (%) inhibition, determined to Benzie et al methodology30.
Table 1. Fatty acid (FA) composition as percentage of fatty acids and antioxidants indices of turmeric
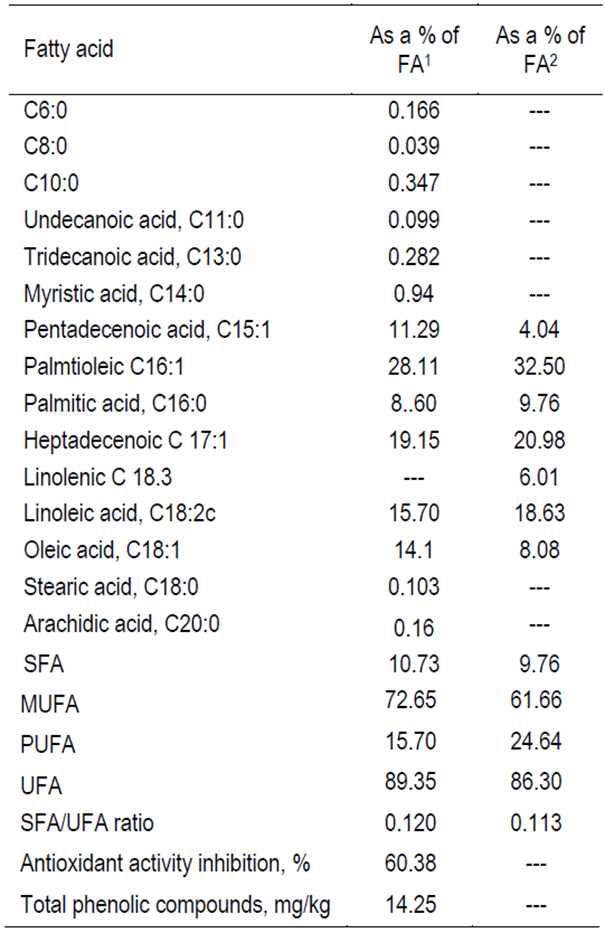
1 Represents the present sample of turmeric 2 according to Radwan28, SFA= Saturated fatty acids, MUFA= Mono unsaturated fatty acids, PUFA= polyunsaturated fatty acids, UFA= Unsaturated fatty acids.
Chicks, diets and experimental design
A total of 252 Hubbard broiler chicks 1 d of age were used in this experiment. They were fed the experimental diets (Table 2) according to a two-phase feeding system, with a starter-grower diet from d 1 to 27 and a finisher diet from d 28 to 35. The chicks were distributed randomly in a complete randomized design among six treatments. Each was replicated seven times with six unsexed chicks per replicate. The basal diet was formulated to be isocaloric and isonitrogenous and to meet the nutrient requirements31. The basal diet was administered without the tested supplements (the control group) or supplemented with turmeric at 0.5 (T_0.5), 1 (T_1), and 2 (T_2) g/kg diet. The concentrations of turmeric supplementation was chosen based in previous studies12,20,21 in which 0 to 10 g/kg was included in broiler diets with inconclusive results. The basal diet was also supplemented with mannan oligosaccharides (MOS; Alltech Inc., Nicholasville, KY, USA) at 1 g/kg diet or oxytetracycline (OTC) at 50 mg/kg diet. OTC is a broad-spectrum bacteriostatic agent derived from the bacteria Streptomyces. The recommended dose is 5 to 50 g/t feed as a continuous feed additive. Terramycin (OTC) is a registered trademark of Pfizer, Inc., USA. It is US FDA NADA (new animal drug application) #95-143, approved by the FDA 7870000 101-9010-07 and licensed to Phibro Animal Health Corporation for OTC HCl.
Table 2. Diets composition and nutrient profiles of the experimental diets percentage as fed basis

1Vit+Min mix. provides per kilogram of the diet: Vit. A, 12000 IU, vit. E (dl-⍺-tocopheryl acetate) 20 mg, menadione 2.3 mg, Vit. D3, 2200 ICU, riboflavin 5.5 mg, calcium pantothenate 12 mg, nicotinic acid 50 mg, Choline 250 mg, vit. B12 10 µg, vit. B6 3 mg, thiamine 3 mg, folic acid 1 mg, d-biotin 0.05 mg. Trace mineral (mg/ kg of diet): Mn 80 Zn 60, Fe 35, Cu 8, and Selenium 0.1 mg.
Broilers husbandry
Chicks were kept in battery brooders (40×45×60 cm) under similar managerial and hygienic conditions in semi-opened housing. Water and mash feeds were offered ad libitum. The brooding temperature was 34, 32 and 30 ºC during the 1st, 2nd and 3rd wk of age, respectively. During 21 to 35 d of age, the average ambient temperature and relative humidity (RH) were 30 ± 3 °C to 45 ± 4 %, respectively. The light-dark cycle was 23:1.
Data collection
Body weight was recorded at the 1st, 14th, 27th and 35th d of age; body weight gain, feed intake and the feed conversion ratio (FCR) were calculated for the periods 1-14, 1-27, and 1-35 d of age. At 35 d of age, seven chickens from each treatment representing all replicates were randomly taken and slaughtered to determine their carcass characteristics. In addition, the lymphoid organs, including the thymus, spleen and bursa of Fabricius, were removed and weighed. Meat quality traits (n= 7 samples/treatment) represented all replicates, such as chemical composition (dry matter, protein, lipid and ash) and physical characteristics (pH, colour of meat, water holding capacity [WHC] and tenderness) were carried out as previously reported32.
At d 35 of age, seven blood samples were collected in both not-heparinized and heparinized tubes from each treatment represented all treatment replicates. The serum was separated by centrifugation at 1,500 xɡ for 10 min at 4 °C and stored at -18 °C until analysis. The selected serum biochemical profile such as serum total protein and albumin concentrations (g/dL), alanine aminotransferase (ALT) and aspartate aminotransferase (AST), (µ/L), alkaline phosphatase (ALKP) enzymes, total antioxidant capacity (TAC) as an indicator of antioxidant status, and malnodialdehyde (MDA as a biomarker for lipid peroxidation respectively were determined using commercial diagnostic kits (Diamond Diagnostics Company, Cairo, Egypt)32,33. Globulin concentration (g/100 mL) was calculated as the difference between total protein and albumin. Red blood cell (RBCs) characteristics, including haemoglobin (Hgb), packed cell volume (PCV), mean corpuscular haemoglobin (MCH), mean corpuscular volume (MCV) and mean corpuscular haemoglobin concentration (MCHC), were measured as previously cited32,33. Haemagglutination (HINDV) inhibition for New Castle disease virus was determined according to Snyder et al34.
Upon necropsy, the intestine was removed, thoroughly washed with a physiological saline (0.9% NaCl) solution, blotted on filter paper and then buffered with formalin 10%. The fixed specimens were processed using a conventional paraffin embedding technique. From the prepared paraffin blocks, 5 mm thick sections were obtained and stained with haematoxylin and eosin for light microscopic examination35. In order to determine the length of the villi, 5 villi were measured on each segment for all groups. The villi lengths were measured from their base upwards to the end of the villus. The morphometric measurements were taken in a binocular microscope equipped with a clear Nikon camera and coupled with an image-analysing system from Optika36.
Statistical analysis
Data were analysed using the SAS software program37, using a completely randomized design, considering the replicate as the experimental unit according to the following model:
Yi, j=µ+Тi+εi(j)
With Yi,j= being any observation for which X1= i (i and j denote the level of the factor and the replication within the level of the factor, respectively); µ= general location parameter; Ti= is the effect of having treatment level I; εi(j)= is the random error.
Mean differences were tested by the Tukey’s studentized test37 using P≤0.05; although when P value was great than 0.05 and less than 10 was reported as trend. Before analysis, all percentages were converted to arc sin to normalize data distribution.
RESULTS
Chemical composition, fatty acids, antioxidant activity percentage inhibition and total phenolic compounds
Chemical composition of turmeric showed 89.7 % dry matter, 5.8 % crude protein, 4.7 % ether extract, 4.2 % ash 3.5 % crude fiber and 71.5 % nitrogen free extract and calculated ME value was found to be 3,664 kcal/kg turmeric. The results, as displayed in Table 1, describe the fatty acids content of turmeric, antioxidant activity inhibition and total phenolic compounds. The results indicate that linoleic acid is the dominant polyunsaturated fatty acid and palmitoleic acid is the dominant monounsaturated fatty acid. These indicate that turmeric is a good source of unsaturated fatty acids. The antioxidant activity inhibition and total phenolic compounds are 60.38 % and 14.25 mg/g, revealing a potential antioxidant activity.
Growth performance
Data for broiler performance are shown in Table 3. The results showed that different supplements did not significantly affect BWG of chickens during different experiment period except for a trend for greater (P≤0.089) growth of T_0.5, and the MOS groups during d 15-27 and T_0.5 and T_1 (P≤0.095) during the whole experimental period (d 1-35 of age) in comparison to the control group, T_2 group and OTC groups and the control group, T_2 group, MOS and the OTC groups, respectively.
Table 3. Growth performance of broiler chickens fed diets supplemented with different concentrations of turmeric, mannanoligosacchride and oxytetracycline

MOS= Mannoligaosacchride; OTC=Oxytiteracycline; BWG= Body weight gain; SEM= Standard error of means.
a,b,c Differences among means within a column within each factor not sharing similar superscripts are significant (P<0.05)
Feed intake during the different experimental periods was significantly affected by the different treatments. During d 1-14 of age, the T_2 group consumed significantly less feed than the control and MOS groups. During d 15-27 of age, the T_2 and OTC groups reduced feed intake in comparison to the T_0.5 group. In addition, OTC groups reduced feed intake in comparison to the MOS group. For the 1-35 d period, the T_2 group significantly decreased feed intake in comparison to the T_0.5 and MOS groups.
During most of the experimental periods, the different supplements significantly affected FCR. During d 1-14 of age, the T_1, T_2 and OTC groups significantly improved FCR in comparison to the other groups, but the T_1 and T_2 groups had more favorable effects than the OTC group as the difference between OTC and control group was not significant. During d 15-27 of age, the OTC group significantly improved FCR in comparison to most of the experimental groups except for the T-0.5, T_1 and T_2 groups. During d 28-35 of age, there was a trend for improved FCR of groups on T_1 and T_2 in comparison to the other experimental groups. For the whole period, the T_1 and T_2 groups significantly boosted FCR in comparison to the other groups except for OTC.
The survival rate was 100 % in the different experiment groups. The European Production Efficiency Index of the T_1 group was significantly higher than that of the control and MOS groups. Other groups exhibited intermediate values.
Carcasses characteristics and inner body organs
The results for carcass traits, relative weight of internal organs, chemical composition and physical parameters of meat are presented in Table 4. Most of the traits were significantly affected by the treatments with the exception of the relative weight of the abdominal fat, liver, pancreas (although F value was significant P≤0.039 for only pancreas) and intestine. Dressing percentage significantly decreased due to T_0.5 and OTC supplements in comparison to the control group. It was found that the T_1 group significantly decreased proventriculus in comparison to the control and MOS groups, and also decreased rate of gizzard in comparison with the control, T_0.5 and T_2 groups. The heart percentage was significantly lower in the T_0.5 group than in the control and T_2 groups. Most supplemented groups, except the MOS group, significantly increased intestinal villi length in comparison to the control one, with the OTC group displaying the greatest effect.
Table 4. Carcass characteristics, inner body organs and meat quality of broiler chickens fed diets supplemented with different concentrations of turmeric, mannanoligosacchride and oxytetracycline
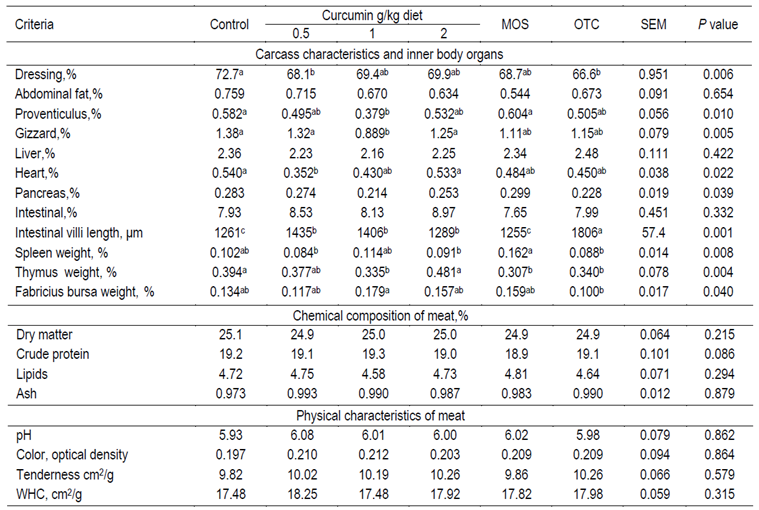
SEM= Standard error of means, MOS= Mannoligaosacchride, OTC=Oxytiteracycline, pH= hydrogen power; WHC= Water holding capacity.
a,b,c Differences among means within a column within each factor not sharing similar superscripts are significant (P<0.05).
Lymphoid organs such as the spleen, thymus and Fabricius bursa were significantly affected by the dietary supplementations. The MOS group exhibited significantly greater spleen weight than the T_0.5, T_2 and OTC groups. The thymus percentage was greater of the control and T_2 groups than those of the other groups except for T_0.5 group. The Fabricius bursa of the T_1 group was significantly higher than that of only the OTC group.
Meat quality
Table 4 shows the content of the dry matter, protein, lipids and ash of the meat, as well as the physical traits such as pH, color, WHC and tenderness. These traits were not significantly affected by the different supplementations, but there was a trend (P≤0.086) for higher CP of T_1 group than that of MOS group.
Blood biochemical, liver leakage, antibody titer and red blood cells characteristics
The blood serum biochemical components are shown in Table 5. Different supplements significantly affected serum total protein, globulin, ALKP, ALT, AST, AST/ALT ratio, HI NDV and TAC. It was found that the T_0.5, MOS and OTC groups had a significant increase in the total protein in comparison to the other groups except for the control group. The latter group had also greater total protein than T_1 group. Differences in serum globulin were not significant among different means of groups. ALKP was significantly higher for the groups supplemented with T_1, T_2 and MOS in comparison to the control group; the T_1 group showed the greatest effect and T_0.5 and OTC exhibited intermediate values.
Table 5. Serum biochemical, liver leakage markers, antioxidant indices and red blood cell parameters of broiler chickens fed diets supplemented with different concentrations of turmeric, mannanoligosacchride and oxytetracycline

MOS= Mannoligaosacchride; OTC=Oxytiteracycline; SEM= Standard error of means; ALT= Alanine amino transferase; AST= Aspartate amino transferase; HINDV=haemagglutination inhibition for new castle disease virus; TAC= Total antioxidant capacity; MAD= Malnodialdehyde; Hgb=Hemoglobin; PCV= Packed cell volume; MCV= Mean cell volume; MCH= Mean cell hemoglobin; MCHC= Mean cell hemoglobin concentration.
a,b,c Differences among means within a column within each factor not sharing similar superscripts are significant (P<0.05).
The antibiotic supplemented group had significantly decreased ALT in comparison to the other groups, while the T_0.5 group had significantly decreased AST in comparison to only the control group. In addition, the AST/ALT ratio of the turmeric groups was significantly lower than that of the OTC group. HINDV was the highest of T_1 group while the lowest was from the control and OTC groups. Most of supplemented groups except that T_2 group had significantly higher TACs than that of the control group, with the T_0.5 group exhibiting the greatest TAC. There were no significant differences in MAD among the different groups.
The different treatments had a significant effect on most of the hematological traits except for RBCs (P≤0.071). Hgb, PCV, MCV, MCH and MCHC were the lowest in the MOS group, while Hgb, PCV, MCV of the T_1 group had the highest values. MCH and MCHC were the highest in the control group but did not significantly differ from the T_1 group.
DISCUSSION
The present results indicate that turmeric is a potential source of nutrients, poly-unsaturated fatty acids and antioxidants38, and T_1 improved growth performance and the European production efficiency index. This indicates that 1 g/kg turmeric is adequate as an alternative growth promoter that could replace OTC and have a better impact on productive performance than MOS for both FCR and the European production index.
The potential effect of turmeric on growth performance and the production index of broilers are in line with those reported elsewhere11,20,39. The aforementioned authors concluded that turmeric supplementation at the rate of 1 to 10 g/kg improved growth performance of broiler chickens without adverse effects on mortality. In addition, turmeric supplementation at 5 g/kg feed improved the growth of chickens exposed to aflatoxins40,41, and alleviated the negative influences of Eimeria infection18,19,42 and of heat stress39. These potential effects of turmeric could be attributed to its curcuminoids (3 to 5 %, as found in turmeric powder), bisdemethoxy curcumin and demethoxy curcumin, the principle active compounds in turmeric43. These compounds show a wide spectrum of biological activities including antioxidant, antibacterial, antifungal, antiprotozoal, antiviral, anticoccidial and anti-inflammatory properties, digestion- and absorption-enhancing effects, and protection effects against coccidiosis and toxins5,19,44. Turmeric also improves liver and bile functions through increased bile secretions, protects the stomach from ulcers and reduces liver toxins. These improvements can enhance digestion, metabolic processes and nutrient utilisation for growth through stimulation of protein synthesis by the chicken enzymatic system6,11. Turmeric has been observed to enhance the intestinal lipases, amylase, trypsin and chymotrypsin secretions45. This is similar to our findings regarding the increase in the length and width of villi in the intestinal, which are also similar to other findings45. Therefore, the improvement in the growth performance due to turmeric supplementation to broilers’ diets can be partly attributed to improving the ecology and function of the digestive tract of chickens. On the other hand, turmeric did not show constant effects on growth performance as had been reported21. No significant positive effect of turmeric powder at between 3.03 and 10 g/kg diet on the growth performance of broiler chickens9,12. This inconsistency in the reviewed results can be attributed to the different qualities of feed, breeder and age of the broilers, statistical design, doses of turmeric and the sanitary and environmental conditions. The improved production index by 15.6 % due to inclusion of turmeric powder at 1 g/kg is in the range cited in the literature of 1.5 %39 and 11.8 %6 when turmeric was supplemented at 5 g/kg feed.
Turmeric, particularly at 1 g/kg feed, induced adaptive changes in the different body organs. The decreased proventriculus and gizzard and increased intestinal villi length indicated enhanced digestive function that can explain the increased performance, meat protein and somewhat decrease in meat lipid of the broilers on the T_1 treatment. On the other hand, turmeric at 1 and 2 g/kg diet had no negative effects on carcass traits. Meat quality is an important concept in broiler production nowadays and improved postharvest quality and shelf life is essential32. The present findings indicate that turmeric is a beneficial feed additive due to phytochemicals, such as curcumin, AR-turmerone, methylcurcumin and other active compounds that could improve carcass quality and reduce spoilage1,5. This increase in the quality of the carcass traits of broilers could be attributed to its antimicrobial effect, which improves the shelf life of the carcasses1,6,11.
Despite the absence of a significant effect of turmeric in this study on the relative weight of abdominal fat, liver and intestines, there was a numerical decease in percentage abdominal fat of 11.7 % and 16.5 % and in liver of 8.5 and 4.7 % due to turmeric supplementation at 1 and 2 g/kg, respectively. The positive effect of turmeric in abdominal fat and liver could be attributed to its negative influence on liver fatty acid synthesis as manifested by an increase in meat CP and the decrease in meat lipid of T_1 group. In literature, liver triacylglycerol and plasma triacylglycerol in the VLDL fraction and liver cholesterol significantly decreased, but the activity of hepatic acyl-CoA oxidase increased9,46. In addition, turmeric at a rate of 3 g/kg feed reduced the meat fat content and increased the carcass quality of broilers11,47,48.
In partial agreement with the present results, turmeric supplementation at 5 g/kg feed did not significantly affect percent dressing, liver, gizzard and heart, but significantly increased the proventriculus6. In addition, turmeric at the same dose significantly increased percent dressing, weight of the breast and thigh, but did not affect percent liver, heart and gizzard6. In other studies, turmeric supplementation did not significantly affect the weight of the carcass, heart, pancreas or intestine9, gall bladder11, the ready-to-cook carcass, liver, pancreas, heart, gizzard, proventriculus, abdominal fat and length of the entire small intestinal, duodenum, jejunum and ileum12. However, turmeric decreased the abdominal fat and liver percentages9,11 and increased percentage of the entire small intestinal and the ileum weight12.
The effect of turmeric, MOS and OTC on meat quality are in partial agreement with those reported elsewhere11, who showed that curcuma longa did not affect crude protein or extracts of breast and thigh meat as well as organoleptic tests (smell, flavour, colour and tenderness).
Lymphoid organs, antibody level, antioxidant status and blood metabolites are a good markers of health status of the animal. The impact of turmeric concentrations on lymphoid organs indicates that different concentrations of turmeric did not affect spleen, thymus and Fabricius bursa percentages, but MOS increased percent spleen and decreased thymus and did not significantly affect Fabricius bursa and 28-d HINDV titer in comparison with the control and antibiotic groups. In the literature, the inclusion of turmeric powder increased the spleen weight and did not affect the Bursa and thymus weight index11, spleen and bursa of Fabricius9,12 and relative weight of the spleen, bursa or thymus20. These results reveal that turmeric is a safe phytogenic feed supplement for chickens and may enhance their immune response as measured by specific antibody titres, as reviewed by others5.
The changes in serum metabolites indicate that, except for the decrease in serum total protein, turmeric supplementation at different doses did not affect serum albumin, globulin and albumen to globulin and indices of hepatocellular leakage (ALT, AST and AST/ALT). There were, however, numerical decreases in the AST and AST/ALT ratio, which show a potential decease in hepatocellular leakage markers that could be attributed to the significant increase in TAC (antioxidants index) of broilers fed turmeric supplemented-diets. Similarly, serum ALT and AST were not affected by turmeric powder49. In addition, turmeric powder at 0.6 and 0.9 alleviated the negative effect of aflatoxin B1 on serum total protein, albumin and globulin, boosted antioxidant defence enzymes, e.g. catalase and superoxide dismutase, and decreased MDA2. The levels of liver enzymes (ALT and ALKP) were substantially reduced by feeding broilers turmeric powder22. On the other hand, turmeric at 5 g/kg feed did not affect serum total protein, albumin, globulin, ALKP, ALT and AST enzymes20.
The increase in the Hgb and PCV of broilers supplemented with turmeric at 1 g/kg feed indicates an improvement in health status. This can be attributed to the antioxidant capacity of turmeric and its digestive-enhancing effect that may improve iron absorption. Similar results were reported by for RBCs11 and for PCV12. Also, found that turmeric improved the health status of broilers20. In accordance with the present results, no mortality up to 35 d of age in broilers was observed when turmeric was supplemented at 0.5 % in the broiler diet6, and less mortality was observed at 0.1 % inclusion39.
It was found that turmeric at 1 g/kg feed had effects comparable to MOS and OTC on growth, dressing percentage and lymphoid organs, and was better by 8.7 % than MOS for FCR. The lower feed utilization of the MOS group might have been due to their lowest villi length. OTC induced the highest increase in intestinal villi, with no difference in FCR and production index from the turmeric groups. The effect of OTC seems to be related to the anti-inflammatory action of OTC rather than to its antibiotic effect26,50,51. On the other hand, MOS and OTC increased serum total protein compared to the intermediate and highest turmeric doses. These results indicated that turmeric had antibiotic-like effects due to its antimicrobial and anti-inflammatory effects.
CONCLUSION AND IMPLICATIONS
Turmeric can be used at 1 kg/t feed as a phytogenic feed additive as an alternative to OTC or MOS without negative effects on the productive and economic traits of broilers. There were no differences from using OTC and MOS, while there was an increase in the European production efficiency index and the broilers’ health status.











 nova página do texto(beta)
nova página do texto(beta)


