Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.7 no.4 Mérida oct./dic. 2016
Articles
Morphological and molecular characterization of sideoats grama (Bouteloua curtipendula) populations in Chihuahua, Mexico
a Facultad de Zootecnia y Ecología-Universidad Autónoma de Chihuahua. México.
b Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). C. E. Rosario Izapa, Chiapas. Km. 18. Carretera Tapachula-Cacahoatán, Tuxtla Chico, Chiapas. CP. 30780.
d Colegio de Posgraduados, Campus Montecillo. México.
e Sitio Experimental La Campana-INIFAP. México.
Sideoats grama [Bouteloua curtipendula (Michx.) Torr.] is an important native species for cattle production. However, inappropriate grazing practices have reduced its genetic diversity. The morphological and genetic diversity of 51 populations of sideoats grama in Chihuahua State was explored and analyzed. Transplanting was performed under natural conditions; two years later, morphological characterization was performed. To assess genetic variability AFLP molecular markers were used. Principal component analysis (PCA) showed that the first three components (PC) explained 73.83 % of variation. PC1 showed a significant correlation (P<0.0001) in dry matter yield (88 %), stem density (85 %), tiller diameter (83 %), height of foliage (82 %), plant height (79 %), and leaf length (65 %). An AFLP analysis with four pairs of primers detected 186 bands; 80.67 % (150 bands) had polymorphism. The highest percentage of polymorphism (93.75 %) and polymorphic bands (48) was obtained with the primer combination of EcoRI-ACT + MseI-CTG. Sideoats grama population presented a high morphological and molecular variability. Based on high forage potential, ecotypes (444, 359 y 557) were selected to be included in grassland restoration programs.
Key words: Bouteloua curtipendula; Morphological diversity; Molecular characterization; Populations
El pasto banderita [Bouteloua curtipendula (Michx.) Torr.] es una especie nativa de importancia ganadera; sin embargo, prácticas inadecuadas de pastoreo han reducido su diversidad genética. Se exploró y analizó la diversidad morfológica y genética de 51 poblaciones de pasto banderita del estado de Chihuahua. Las plantas se trasplantaron bajo condiciones de temporal. Dos años después se realizó la caracterización morfológica. Para evaluar la variabilidad genética se utilizaron marcadores moleculares AFLP. El análisis de componentes principales (ACP) mostró que los tres primeros componentes (CP), explican el 73.83 % de la variación. El CP1 presentó una correlación significativa (P<0.0001) con rendimiento de materia seca (88 %), densidad de tallos (85 %), diámetro de macollo (83 %), altura de follaje (82 %), altura de planta (79 %) y longitud de hoja (65 %). El análisis de AFLP con cuatro pares de iniciadores detectó 186 bandas; 80.67 % (150 bandas) presentó polimorfismo. El mayor porcentaje de polimorfismo (93.75 %) y bandas polimórficas (48) se obtuvo con la combinación de iniciadores EcoRI-ACT+MseI-CTG. Las poblaciones de banderita presentaron alta variabilidad morfológica y molecular. Se detectaron ecotipos (444, 359 y 557) con potencial forrajero de acuerdo a su variabilidad morfológica y molecular para ser incluidos en programas de restauración de pastizales.
Palabras clave: Bouteloua curtipendula; Variación morfológica; Caracterización molecular; Poblaciones
Introduction
In central and northern Mexico region 65 %, of native grasslands present erosion and reduction of important livestock forage species1. In the State of Chihuahua, overgrazing and invasion of exotic species have been among the main causes of this deterioration2 . Thus, it is important to collect and preserve native forage materials in the centers of origin, since it constitutes the foundation for ensuring feedstock for cattle3.
Sideoats grama [Bouteloua curtipendula (Michx.) Torr.] is a native species found in plains and rocky hillocks, produces abundant high quality forage for livestock, adapts to different types of soil and climatic conditions and possess drought tolerance4 . It has a good forage value and it is regarded as an excellent fodder for grazing and wildlife5; however, overgrazing has led to its reduction and disappearance of large populations of this species6. Sideoats grama is a species that adapts to different environments and several morphological-based ecotypes can be found, that is why it is important to carry out evaluations ex situ, where all ecotypes are placed in the same environment to detect characteristics of importance within the species7. In such a way, it is possible to identify exceptional characteristics by evaluating morphological descriptors in the diversity of native populations8.
Morphological characterizations have been performed with native grasses within the genera Andropogon9, Panicum10,11, Hymenachne12, Bouteloua13, among others. In addition, the morphological characterization has detected some significant correlations between diversity of morphological characteristics and location of sampling sites14. Therefore, it is important to evaluate morphologic and genetic characteristics, as the environment influences the morphologic characteristics. Amplified fragment length polymorphism (AFLP) is a molecular marker that it is not affected by the environment. In addition, it can detect genotype level diversity and has been used with grasses15,16 to determine their genetic variability17. The aim of this study was to characterize the morphological and molecular diversity in populations of sideoats grama, collected from various locations in Chihuahua to detect populations with high forage potential.
Material and methods
During 2006, 135 ecotypes of sideoats grama were collected in cattle ranches in the state of Chihuahua (29 municipalities) and grouped into 51 populations, which were used for evaluations to understand and define the population structure. Table 1 shows the municipalities and some environmental variables of the collection sites.
Table 1 Environmental characteristics of 51 collection sites of sideoats grama populations (135 ecotypes) in Chihuahua, Mexico
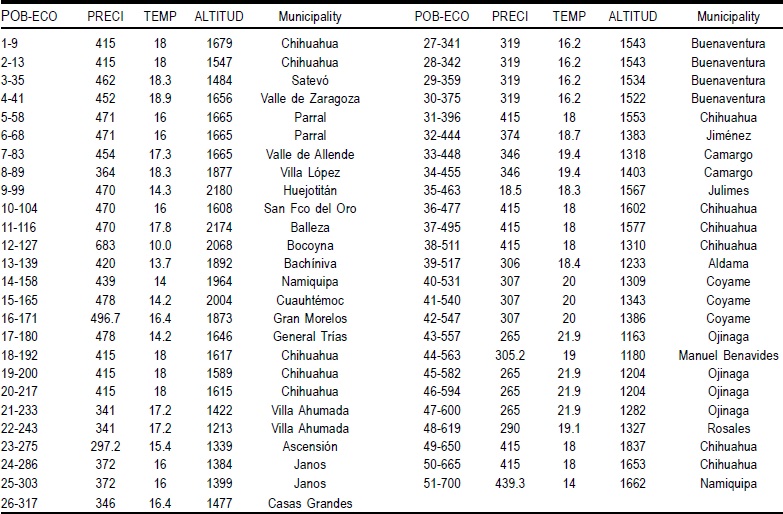
POB=population; ECO=ecotype: PRECI=precipitation; TEMP=temperature.
At each sampling site four plants were extracted with a diameter of 2.5 cm, including root. The aerial part was cut at a height of 15 to 20 cm, each plant was identified with a collection number, and each population was considered as a different ecotype. The plants were placed in plastic boxes, supplied with moist soil for transport and transplantation in the “Campo Experimental La Campana” of the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. The topography of the transplantation site is flat, while soils are of alluvial origin, sandy loam texture and pH of 6.5. The climate is temperate dry with warm summers BWk, annual average temperature of 16.5 °C and average annual rainfall of 355 mm18. Irrigation was provided at the time of transplant to promote their establishment. Subsequently, the plants were left in rainfed conditions (without irrigation).
Morphological characterization
During flowering and two years after plant establishment, the following morphological descriptors were evaluated: Total plant height (PH), foliage height (FH), stem density (SD), stem thickness (ST), leaf width (LW), leaf length (LL), inflorescence length (IL), tillering diameter (TD) and dry matter yield (DMY). PH was measured from the ground level to the tip of the highest inflorescence. The FH was measured from the ground to the height of the leaves. The ST was measured using a Vernier, randomly taking a stem of the central part of the plant. To measure LL and LW a random leaf was selected from the central part of the plant. The IL was measured by taking an inflorescence at random and measuring from the base to the tip of it. The TD was measured at the base, at ground level. The DMY was obtained by cutting the plants 5 cm above the soil and then forage was placed in paper bags and dried in a forced air oven at 70 °C for 48 h.
Molecular characterization
Ecotypes were grouped into 51 populations for analysis. DNA extraction was performed following the method of Doyle and Doyle19) and for analysis of AFLP the protocol by Vos et al20, which included the following components:
Digestion: 1.5 µl of buffer reaction (RL) 10X, 2 µl of DNA were added to 50 ng/µl, 0.5 µl of enzyme Eco RI (10 U/ µl), 0.5 µl of enzyme Mse I (10 U /µl) and the reaction was brought to a volume of 12.5 µl with sterile deionized water. The mixture was centrifuged and incubated at 37 °C for 2 h and 15 min at 70 °C to inactivate the restriction enzymes. It was confirmed on an agarose gel at 1% stained with ethidium bromide.
Ligation of adapters: to the digestion reaction 0.3 µl of adapter Eco RI (50 pmol), 0.3. µl of adapter Mse I (50 pmol), 1.2 µl of ATP (10 mM, pH 7.0), 1.0 µl of solution was added buffer reaction (RL) 10X, 1.0 µl of T4 DNA ligase (5U/ µl) and 6.2 µl of sterile deionized water, mixed, centrifuged and incubated for 2 h at 16 °C.
Pre-amplification: 2.5 µl of digested DNA, ligated and diluted 1:10, 1.15 µl of Oligo Eco RI + A (50 ng/µl); 1.15 µl of Oligo Mse I + A (50 ng/µl), 0.5 µl dNTPs (10 mM); 2.5 µl of buffer enzyme reaction Taq DNA polymerase PCR (10X); 0.65 µl of MgCl2 (50 mM); 0.2 µl of Taq DNA polymerase (5 U/µl) and 16.85 µl sterile deionized water. This was mixed, centrifuged and placed in a thermocycler for 20 cycles at 94 °C for 30 s, for 1 min at 56 °C and 1 min at 72 °C, and finally maintained at 4 °C.
Selective amplification: 2.0 µl of DNA pre-amplified and diluted 1:40 with 4.9 µl of sterile deionized water; 1.1 µl of reaction buffer Taq DNA enzyme polymerase; PCR (10X), 0.3 µl of MgCl2 (50 mM); 0.5 µl of Taq DNA polymerase (5 U/µl); 1.0 µl of Oligo Mse I + 4 selective bases (30 ng/µl); 0.2 µl dNTPs (10 mM); 0.5 µl of Oligo Eco RI + 3 selective bases marked 700, 0.5 µl of Oligo Eco RI + 3 selective bases marked 800 were added. The thermocycler was programmed for one cycle at 94 °C for 30 s, 30 s at 65 °C and 1 min at 72 °C. Twelve cycles where subsequently carried out to reach the annealing temperature (65 °C) at a reduction rate of 0.7 °C per cycle, while the other temperatures remain the same. Followed by 23 cycles at 94 °C for 30 s, 30 s at 56 ºC and 72 °C for one minute, at the end, the reaction was kept at 4 °C. Electrophoresis was performed in acrylamide gel 6.5%, with 8M urea and 1X TBE (Tris 1M, 1M boric acid, 20 mM EDTA, pH 7.0). Separation of the amplified fragments was carried out in the DNA analyzer LI-COR Model 4200, with 0.8 µl of sample loading into a well and using the molecular weight marker 50 to 700 bp. Oligos or primers were used, fluorescently labeled, at different wavelengths (700 nm and 800 nm)20 were used. Chemiluminescent AFLP markers were used21.
Morphological data were analyzed with principal component analyses (PCA) and cluster analyses (CA), by Ward method22. PC- ORD and MINITAB v15 program were used to obtain the scatterplot and the dendrogram. A binary matrix was obtained for the presence and absence of bands with the banding pattern of the molecular data. Molecular data was statistically analyzed with NTSYSpc (v 2.1). Genetic similarity between ecotypes were also estimated using the program SIMQUAL with the Dice coefficient, and the Unweighted Pair Group Method with Arithmetic (UPGMA) was used as clustering method.
Results and discussion
Morphological characterization
Plant height ranged from 40 to 104 cm and foliage height 25 to 62 cm. The stem density and thickness ranged from 20 to 352 and 1.0 to 3.0 mm, respectively. The leaf width was 2.0 to 8.0 cm and leaf length was 6.0 to 25 cm. The inflorescence length varied from 12 to 37 cm and the tillering diameter ranged from 4 to 20 cm. Finally, the dry matter yield values were from 4 to 260 g/plant. Some of these morphological variables including foliage height, plant height, density of stems per plant, among others, agree with those values reported by Schellenberg et al23 when assessing the phenotypic variation of sideoats grama collections in Canada.
The principal component analysis (PCA) showed that the first three principal components (PC) explain 73.83 % of the variation. Phenotypic variables that contributed most to PC1 were dry matter yield (0.430), stem density (0.414) and tillering diameter (0.402). These variables are related to forage production potential. For PC2, leaf width (0.68) and stem thickness (0.64) contributed the most and may be related to forage quality. Similar results were obtained by Schellenberg et al23, with positive correlations in stem density (0.51) and tillering diameter (0.50) with PC1 (Table 2).
Table 2 Characteristic vectors of the variables of higher descriptive value with respect to its principal component in 51 populations of sideoats grama
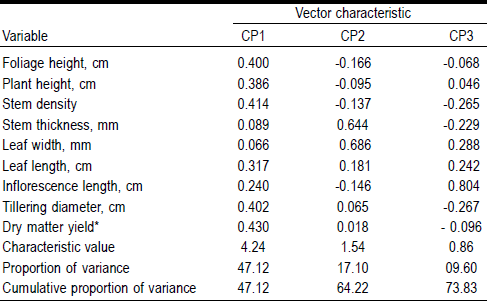
* (g/plant/35 d).
By correlating dry matter yield with other original variables, significant correlations were observed with stem density (r= 0.81; P<0.0001), tillering diameter (r= 0.71; P<0.0001), foliage height (r= 0.68; P<0.0001) and plant height (r= 0.56; P<0.0001). In addition, the nine variables were correlated with the first three principal components. PC1 showed a significant correlation (P<0.0001) with dry matter yield (88 %), stem density (85 %), tillering diameter (83 %), foliage height (82 %), plant height (79 %) and leaf length (65 %). PC2 showed a significant correlation (P<0.0001) only with leaf width (85 %) and stem thickness (80 %). Finally, PC3 presented only significant correlation (P<0.0001) with inflorescence length (74 %). Considering the above, it was found that ex situ collection of sideoats grama showed wide morphological diversity, probably due to environmental conditions in the sites of origin24, thus some of these ecotypes (B444, B359, B563, B557 and B455) are important to be considered in grassland restoration.
From the correlations obtained, ecotypes can be selected (B444, B359 and B563), considering the potential for protection and soil stability. These correlations and observed variances are consistent with those obtained by Schellenberg et al23, in assessing the behavior of phenotypic variation of several collections of sideoats grama, collected in several locations in Canada, where they report that the first two PC explained 91 % of the variance within the collection. In addition, other studies with grasses of the genus Lolium25, Panicum10, Bromus26 and Bouteloua24, concluded that the assessment of morphological variability is the basis for selecting material for various uses in ecological restoration or rehabilitation, forage production, materials with potential to retain soil, drought resistant ecotypes, among other purposes.
Figure 1 shows the extent of the existing morphological diversity in populations of sideoats grama. It was observed that the variables that contributed most were dry matter yield, stem density, forage height, plant height and tillering diameter. Morphological and genetic variability is useful in the search and selection of characters such as forage yield and seed quality. Since sideoats grama has the property of being an apomictic species, which allows their progeny to inherit these characteristics. With the observed variation, a gene bank can be established to preserve and evaluate this variation in breeding programs and create improved ecotypes according to these descriptors and potential for use in grassland rehabilitation programs.
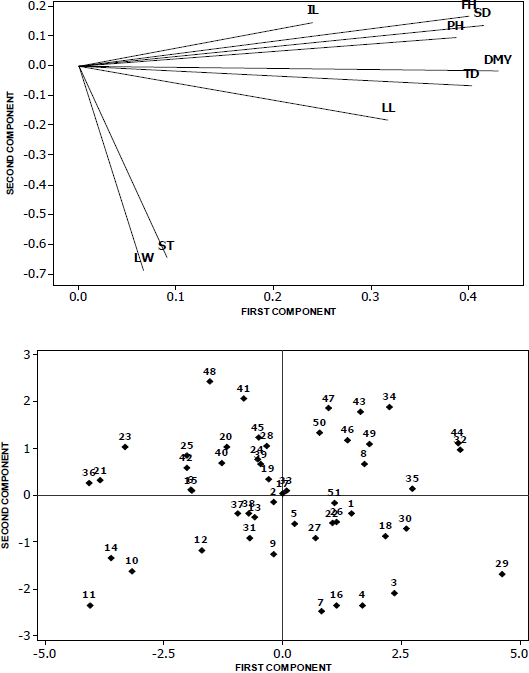
Figure 1 Distribution of the morphological diversity of 51 populations of sideoats grama based on the first two principal components obtained from the correlation matrix of the nine variables
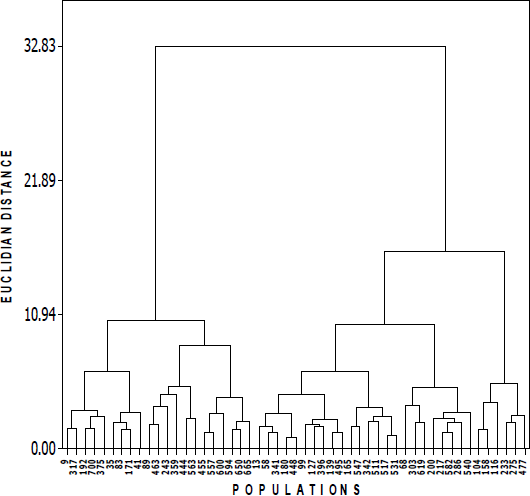
Cluster analysis combined three groups based on Ward method (Figure 2). Group I (GI) integrated 21 populations from 15 municipalities (Chihuahua, Casas Grandes, Namiquipa, Buenaventura, Satevó, Valle de Allende, Gran Morelos, Valle de Zaragoza, Villa Lopez, Julimes, Villa Ahumada, Jimenez, Manuel Benavides, Camargo and Ojinaga). These ecotypes are characterized by having the highest values of all nine variables evaluated. Subgroup I-a integrated nine populations and was characterized because it had the highest plant height (96 cm). The I-b subgroup combined six populations that had on average the highest stem density values (271), length of leaves (20 cm), inflorescence length (32 cm) tillering diameter (16 cm) and yield of dry matter (176 g/plant). The subgroup I-c also combined together six populations with the highest foliage height (60 cm), coming from municipalities of Ojinaga and Chihuahua. Group II (G-II) integrated 24 original populations from 15 municipalities, showing in general, intermediate values of morphological variables. Finally, group III (G-III) integrated six populations coming from the municipalities of San Francisco del Oro, Namiquipa, Balleza, Villa Ahumada, Ascensión and Chihuahua. These populations were characterized by the lowest values of foliage height (29 cm), plant height (58 cm), stem density (30), leaf length (12 cm), inflorescence length (23 cm) tillering diameter (6.2 cm) and dry matter yield (8.7 g/plant).
Molecular characterization
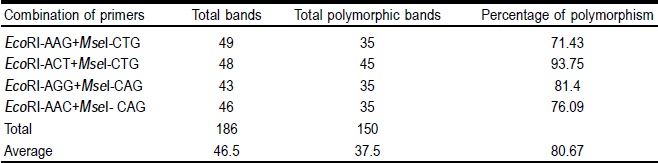
The AFLP analysis with the four pairs of primers detected 186 bands; 80.67 % (150 bands) showed polymorphism (Table 3). The number of polymorphic bands was 35, 45, 35 and 35 for the initiator combinations EcoRI-AAG+MseI-CTG, EcoRI-ACT+MseI-CTG, EcoRI-AGG+MseI-CAG and EcoRI-AAC+MseI-CAG, respectively. The highest percentage of polymorphism (93.75 %) and polymorphic bands (48) was obtained with the combination of primers EcoRI-ACT + MseI-CTG. These results in the number of bands obtained are consistent with other findings, with respect to efficiency to use this technique to generate greatest number of polymorphisms27. For future studies of genetic diversity in populations of sideoats grama, we recommend using the EcoRI-ACT+MseI-CTG combination because it detected the greatest variability. A study in Agrostis stolonifera obtained from 100 to 150 bands with 22 to 94 polymorphic bands28. However, in Schizachyrium scoparium it is reported 854 stem fragments and 653 in seed with 158 well-defined bands. In general, it was detected a greater than 91 % polymorphism in these populations29.
Table 3 Level of polymorphism detected in 51 sideoats grama populations for each combination of primers used in the analysis of AFLP
The similarity values obtained using the Dice coefficient in pairwise comparisons of 51 sideoats grama populations varied between 0.267 and 0.96 (data obtained from genetic similarity matrix). Considering these similarity values, it is evident that there is high genetic diversity within this species, essential to meet the requirement to start breeding programs. The ranges obtained for the similarity coefficient in sideoats grama populations are consistent with those reported in some species of the genus Festuca and Cynodon transvaalensis30.
Cluster analysis based on molecular data using the Dice coefficient (Figure 3), apparently separated into a greater number of groups, compared to dendrogram analyses based on morphological variables as shown in Figure 2. There are some nodes that group grass populations from municipalities nearby. For example, analyzing in Figure 3 the tree from top to bottom, starts a node with B104 and B99 that are two neighboring towns (San Francisco del Oro and Huejotitán), located south of the state of Chihuahua, but not always do these cluster groupings occurred. Below, the node that groups the B200 and B217 populations (also on the right), is the node closest to 1 on the scale of the dendrogram, coinciding obviously with the highest value of similarity that was presented in populations (96 %, data obtained from genetic similarity matrix). These populations with high genetic similarity originated in the same municipality of Chihuahua, have the most genetic homogeneity, as they showed similarity in five of the descriptors evaluated. These populations were collected at an altitude of 1,589 and 1,615 m, respectively. Coming from the same municipality, Chihuahua, it is noted below in the tree that there is another subgroup integreted by B495, B511, B477 and B665 populations were collected at altitudes from 1,577, 1,310, 1,602 and 1,653 m, respectively.
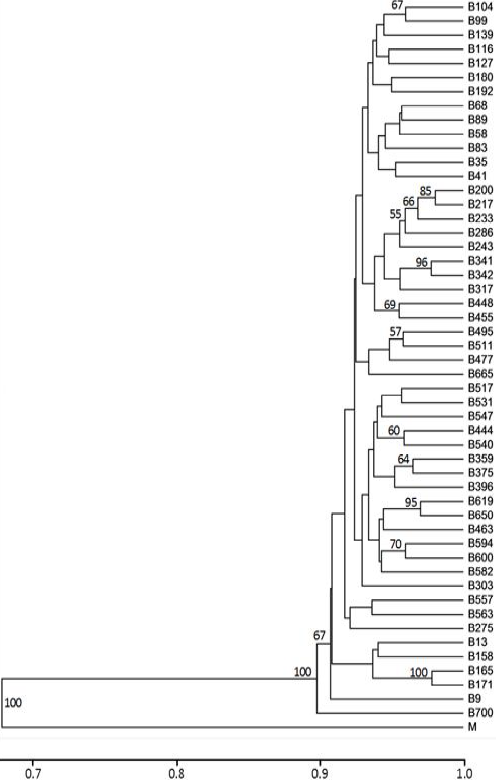
Figure 3 Cluster analysis of 51 populations of sideoats grama by Dice coefficient, using UPGMA-cluster method, based on 186 AFLP markers generated with four combinations of primers
Something similar happened with grass populations coming from the municipality of Buenaventura, where B341 and B342 were clustered with high bootstrap values (96), and showing almost the same five phenotypic descriptors of the characters evaluated. B359 and B375 populations had an acceptable bootstrap value (64) with collections acquired at almost identical altitudes from 1,522 to 1,543 m, with minimum oscillations, sharing similar phenotypic characteristic descriptors in their leaves (width and length) and close foliage heights. The municipality of Camargo also had two populations B448 and B455. Combined, they shared some similar phenotypic descriptors, although their altitudes varied (1,318 vs 1,403 m). Another example observed is a group of three populations from Ojinaga (B594, B600 and B582), also with some similar phenotypic characteristics among them, and that can be grouped with other grass populations of nearby municipalities, B463 from Julimes, B650 from Chihuahua and B619 from Rosales that are located in the central part of the state. This indicates that some populations are genetically close, since they are also correlated with morphology and location, although it is not a universal condition. Also, this graphical representation indicates the existence of genetic diversity, which is a requirement for the sustainable use of this phylogenetic resource. These results are reliable with respect to the effectiveness of using the AFLP technique for studies of genetic diversity in grasses, similar to that described by Roldán-Ruiz et al31. In addition, it is shown that this technique facilitates fast and efficient assessments of genetic diversity in this type of native grass populations32.
Conclusions and implications
Genetic resources of sideoats grama collected in the state of Chihuahua, showed high morphologic and molecular variability. Morphological variables with more variation were dry matter yield, stem density, tillering diameter, foliage height, plant height and leaf length. The combination of primers EcoRI-ACT+MseI-CTG showed the greatest amount of polymorphism, revealing high levels of genetic variation. Based on their morphological and genetic characteristics, B444, B359 and B563 ecotypes can be selected for its high potential and outstanding attributes to be included in grass breeding programs and grassland rehabilitation.
Acknowledgements
The Consejo Nacional de Ciencia y Tecnología (CONACYT) and Chihuahua state government for financial support (key project: CHIH-2005-C01-23250) Mixed Fund. Sincere thanks to Dr. June Simpson, Dr. Corina Hayano and technician Emigdia Alfaro of CINVESTAV-Irapuato for their support in the AFLP analysis and the use of the sequencer.
REFERENCES
1. Gauthier DA, Lafon A, Toombs TP, Hoth J, Wiken E. Grasslands toward a North American conservation strategy. Canadian Plains Research Center. Montreal, Canada. 2003:99. [ Links ]
2. Valerio AE, Carreon A, Lafon JM, Ochoa P, Calderon DM, Soto C, Chacon EF. Distribución, extensión espacial y condición de los pastizales en el estado de Chihuahua. Protección de la fauna mexicana, A.C. The Nature Conservancy. Chihuahua, Mexico. 2005. [ Links ]
3. Do Valle CB. Genetic resources for tropical areas: achievements and perspectives. Proc XIX Int Grassland Cong. São Pedro, São Paulo, Brazil. Brazilian. Soc Bras Zoot 2001;477-482. [ Links ]
4. Willard EE, Schuster JL. An evaluation of an interseeded sideoats gramma stand four years after establishment. J Range Manage 1971;(24):223-226. [ Links ]
5. Stubbendieck J, Hatch SL, Kju KJ. North American range plants. 2nd ed. Lincoln, NE: University of Nebraska Press; 1982. [ Links ]
6. Holecheck JL, Pieper RD, Herbel CH. Range management principles and practices. New Jersey, USA: Regents Prentice-Hall, Inc; 1989. [ Links ]
7. Erickson VJ, Mandel NL, Sorensen FC. Landscape patterns of phenotypic variation and population structuring in a selfing grass, Elymus glaucus (blue wildrye). Can J Bot 2004;(82):1776-1790. [ Links ]
8. Steiner JJ, Piccioni E, Falcinelli M, Liston A. Germplasm diversity among cultivars and the NPGS crimson clover collection. Crop Sci 1998;(38):263-271. [ Links ]
9. Smart AJ, Moser LE, Vogel KP. Morphological characteristic of big bluestem and switchgrass plants divergently selected for seedling tiller number. Crop Sci 2004;(44):607-613. [ Links ]
10. Casler, MD. Ecotypic variation among switchgrass populations from the Northern USA. Crop Sci 2005;(45):388-398. [ Links ]
11. Das MK, Fuentes RG, Taliaferro ChM. Genetic variability and trait relationships in switchgrass. Crop Sci 2004;(44):443-448. [ Links ]
12. Enríquez F, Quero A, Hernández A, García E. Azuche Hymenachne amplexicaulis (Rudge) Nees forage genetic resources for floodplains in tropical Mexico. Gen Res Crop Evol 2006;(53):1405-1412. [ Links ]
13. Morales NC, Quero AR, LeBlanc O, Hernández A, Pérez J, González S. Caracterización de la diversidad del pasto nativo Bouteloua curtipendula (Michx) Torr., mediante marcadores de AFLP. Agrociencia 2006;(40):711-720. [ Links ]
14. Peter-Schmid MK, Kèolliker R, Boller B. Value of permanent grassland habitats as reservoirs of Festuca pratensis Huds. and Lolium multiflorum Lam. populations for breeding and conservation. Euphytica 2008;(164):239-253. [ Links ]
15. Puecher DI, Robredo CG, Ríos R, Rimieri P. Genetic variability measures among Bromus catharticus Vahl. Populations and cultivars with RAPD and AFLP markers. Euphytica 2001;(121):229-236. [ Links ]
16. Renganayaki K, Read JC, Fritz AK. Genetic diversity among Texas bluegrass genotypes (Poa arachnifera Torr.) revealed by AFLP and RAPD markers. Theor Appl Gen 2001;(102):1037-1045. [ Links ]
17. Meudt HM, Clarke AC. Almost forgotten or latest practice? AFLP applications, analyses and advances. Tren Plant Sci 2006;(12):1360-1385. [ Links ]
18. Royo MM, Lafón A. Descripción fisiográfica, diversidad vegetal y vertebrados del rancho experimental La Campana. Chávez A. Carrillo R editores. Rancho Experimental La Campana 50 Años de Investigación y Trasferencia de Tecnología en Pastizales y Producción Animal. INIFAP. Chihuahua, Chih. 2008. [ Links ]
19. Doyle JJ, Doyle JL. A rapid total DNA preparation procedure for fresh plant tissue. Focus 1990;(12):13-15. [ Links ]
20. Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M. et al. AFLP: A new technique for DNA fingerprinting. Nucl Ac Res 1995;(23):4407-4414. [ Links ]
21. Hoisington D, Khairallah M, González de-León D. Laboratory Protocols: CIMMYT Applied Molecular Genetics Laboratory. Second ed. México, DF: CIMMYT; 1998. [ Links ]
22. Statistical Analysis System (SAS). Institute Inc. User’s guide. Statistics. Version 8. Sixth edition. SAS Inc. Cary, North Carolina, USA. 1999. [ Links ]
23. Schellenberg MP, Biligetu B, McLeod GJ, Wang Z. Phenotypic variation of side-oats grama grass [Bouteloua curtipendula (Michx.) Torr.] collections from the Canadian prairie. Can J Pl Sci 2012;(92):1043-1048. [ Links ]
24. Morales NC, Quero CA, Melgoza CA, Martínez SM, Jurado GP. Diversidad forrajera del pasto banderita [Bouteloua curtipendula (Michx.) Torr.], en poblaciones de zonas áridas y semiáridas de México. Téc Pecu Mex 2009;(47):231-244. [ Links ]
25. Bennett SJ, Hayward MD; Marshall DF. Morphological differentiation in four species of the genus Lolium. Gen Res Crop Evol 2000;(47):247-255. [ Links ]
26. Ferdinandez YSN, Coulman BE. Genetic relationships among smooth bromegrass cultivars of different ecotypes detected by AFLP markers. Crop Sci 2004(44):241-247. [ Links ]
27. Valdés-Infante, J. Utilización de caracteres morfoagronómicos y de marcadores de ADN para el desarrollo de una metodología que contribuya al mejoramiento genético del guayabo (Psidium guajava L.) en Cuba [Tesis doctorado]. La Habana, Cuba: Universidad de la Habana; 2009. [ Links ]
28. Vergara GV, Bughrara S. Genetic differentiation of tetraploid creeping bentgrass and hexaploid redtop bentgrass genotypes by AFLP and their use in turfgrass breeding. Crop Sci 2004;(44):884-890. [ Links ]
29. Fu YB, Ferdinandez YSN, Phan AT,Coulman BE, Richards KW. Genetic diversity in natural populations and corresponding seed collections of little bluestem as revealed by AFLP markers. Crop Sci 2004(44):2254-2260. [ Links ]
30. Mian MA, A Hopkins, Zwonitzer J. Determination of genetic diversity in Tall Fescue with AFLP markers. Crop Sci 2002;(42):944-950. [ Links ]
31. Roldán-Ruiz I, Dendauw J, Van Bockstaele E, Depicker A, De Loose M. AFLP markers reveal high polymorphism rates in ryegrass (Lolium spp.). Mol Breed 2000;(6):125-134. [ Links ]
32. Hammer KA. Paradigm shift in the discipline of plant genetic resources. Gen Res Crop Evol 2003;(43):337-341. [ Links ]
Received: April 13, 2015; Accepted: August 13, 2015











 texto en
texto en