Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.7 no.1 Mérida ene./mar. 2016
Articles
Growth properties of DH82 and RF/6A cell lines under standard laboratory conditions
a Universidad Politécnica del Estado de Morelos. México.
b Unidad de Anaplasmosis, CENID Parasitología Veterinaria / INIFAP. Carretera Federal Cuernavaca-Cuautla No. 8534, Col. Progreso, C.P. 62550; Jiutepec, Morelos. México.
DH82 Cell line has been utilized to grow Ehrlichia canis and RF/6A for drug evaluation in short-term cultures, as well as for replicating Anaplasma marginale. However, specific in vitro culture development conditions are unknown. Several experiments were designed to solve inquiries of such procedure. Both cell lines were acquired from ATCC and put into culture. Na Pyruvate, NaHCO3 and fetal calf serum were used to enrich MEM culture media and incubated at 37 °C on 5% CO2 - air humid mixture atmosphere. First assays used 24 well plates and were focused on determination of a minimum initial cell concentration and cell density. In the former, averages of 62,500 cell/well for DH82 & 8,836 for RF/6A; were found. Later, experiments to identify a minimum cell density started with 5, 10, 20 & 40 cells/mm2, harvesting with a EDTA-Trypsin solution when confluence be reached. Growth indexes of 3,319.32, 1,956.70, 870.73 & 422.14 times and of 62.38, 63.51, 25.31 & 12.16 times, were respectively found for DH82 & RF/6A cell lines. For kinetics studies, 35 mm Ø sterile Petri dishes were used. Cultures were set with 20 cell/mm2 seed density for DH82 and 10 cell/mm2 for RF/6A. Dishes were randomly separated into two groups, with and without culture media periodical replacement. Maximum growth was observed at 336 and 315 h with 42.9 and 36.9 h doubling time in culture, respectively for DH82 & RF/6A cell lines. Generated data provided a model to define an in vitro growth pattern for future studies.
Keywords: Cell culture; DH82; RF/6A; Doubling time
La línea celular RF/6A ha sido utilizada en estudios de corto plazo evaluando fármacos o infecciones experimentales con Anaplasma marginale; en contraste, DH82 es utilizada para la multiplicación de Ehrlichia canis. No obstante, se desconocen condiciones específicas de su crecimiento, por lo que se diseñaron varios experimentos para resolver interrogantes de su propagación. Ambas líneas, se adquirieron de la American Type Culture Collection, mantenidas en Medio Mínimo Esencial suplementado con suero fetal bovino, piruvato de Na y NaHCO3 e incubadas en atmósfera de 5% de CO2 en aire, a 37 °C. Los primeros ensayos, en placas de 24 pozos, esclarecieron los valores de dosis mínima inicial, que fueron 62,500 y 8,836 células/pozo para DH82 y RF/6A; así como los de densidad de siembra; cultivos con concentraciones de 5, 10, 20 y 40 células por mm2, cosechados con solución Tripsina-EDTA al alcanzar >95% de confluencia. Los índices estimados fueron: 3,319.32, 1,956.70, 870.73 y 422.14 para DH82 y 62.38, 63.51, 25.31 y 12.16 veces con RF/6A. La cinética del crecimiento, en cajas de Petri de 35 mm Ø, incluyó la siembra de 20 células/mm2, cambio del medio cada 63 h y cosecha cada 21 h para DH82; para RF/6A; la siembra fue 10 células/mm2, cambio de medio cada 45 h y cosecha cada 15 h. El máximo crecimiento se observó hasta las 336 y 315 h con tiempos de duplicación de 42.9 y 36.9 h respectivamente para DH82 y RF/6A. Los datos permitieron proponer un modelo patrón de cultivo, para estudios futuros.
Palabras clave: Cultivo celular; DH82; RF/6A; Intervalo de duplicación
Introduction
Cell culture is the process by which animal or plant origin cells are propagated and maintained under in vitro conditions. Once they have been removed from an organism they may form a monolayer or remain in suspension keeping their functions1. Cell lines are those that become genetically and morphologically differentiated from lineages they were derived and are maintained in continuous culture where they can grow indefinitely2. Monkey (Macaca mulatta) RF/6A cell line3 has been widely used in toxicological studies for the evaluation of the effectiveness of several drugs4, as well as in infections caused by rickettsia Anaplasma marginale, due to its endothelial cell properties5. However, the published data are insufficient because they do not indicate specific conditions and characteristics of the cell lines, assays are short-term and their data are not homogeneous or they only show the number of passages6. The DH82 cell line, which derives from a 10-year-old male Golden Retriever (Canis lupus familiaris) with malignant histiocytosis6-8, has been used for the multiplication of Ehrlichia canis9,10 and for its interaction with erythrocytes infected with Anaplasma marginale11. Recently, a partial immunological characterization of cell line DH82 was described, which accentuated the need to generate further information concerning the properties of cell lines12.
The interest of the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias to know about the propagation characteristics of these cell lines lies in establishing a study model for in vitro culture of rickettsia Anaplasma marginale, causative agent of bovine anaplasmosis, disease that has a negative effect on livestock economy in tropical zones of Mexico and worldwide13,14. In that matter, its purpose is to carry out experiments that allow studying the behaviour of DH82 and RF/6A cell lines before being infected with the microorganism. The objective of the study was to know how both cell lines grow and develop under standard laboratory conditions, in order to define their behaviour pattern.
Material and methods
Cell lines: Both lines, DH82 (Canis lupus familiaris) and RF/6A (Macaca mulatta), used in this study were commercially obtained in a frozen form from “American Type Culture Collection” (ATCC), CRL-10389 and CRL-1780™, respectively. Each was quickly thawed in water bath at 37 °C and suspended in Eagle’s minimum essential medium (MEM), with 2.0 mM L-glutamine (Sigma- ALDRICH QUÍMICA, SA de CV Edo. de México; México. No. de Cat. MO643-10x1L) supplemented with 1.0 mM sodium pyruvate, 1.5 g/L of NaHCO3 and 15 % (v/v) inactivated fetal bovine calf serum at 56 °C for the DH82 cell line (MEM 15i), and for RF/6A, 10 % (v/v) normal fetal calf serum (MEM 10) was used. Both cell lines were added onto a column of 2 mm height, equivalent to a volume of 2 μl/mm2 and maintained by successive passages in 25 cm2 flasks (Corning Inc, Corning, NY; EE.UU.; No. of Cat. 430372) with loosen caps, until experimentally used. In the process of maintenance and in the experimental assays, both cultures were incubated at 37 °C in 5.0 CO2 in air(v/v) atmosphere under saturated humidity15.
Minimum seed initial dose for DH82 cell line assay
For determining the minimum number of cells required to start cultures, a concentration of 5.0 × 105 cells (third passage), suspended in 350 μl of MEM15i complete cell culture media, was used; performing two-fold dilutions for reducing the inoculum to 2.5 × 105, 1.25 × 105 and 6.25 × 104 cells suspended in 350 μl of MEM15i. Cells were seeded in quadruplicate, inoculating 350 μxl/well, using two 24-well plates (CORNING Inc. Corning, NY; EE.UU. No of Cat. 35249). Cell culture media exchange was performed every 72 h until harvest. Which occurred when any of the wells of any treatment reached ≥95% confluence, collecting the contents of all wells by enzymatic dissagregation using 180 μl/well of Trypsin/EDTA 1X (Sigma- ALDRICH QUÍMICA, S.A. de C.V. Toluca, Edo. de México. No of Cat. 3924), stopping the reaction with an equal volume of complete medium with serum and transferring the cells into a sterile 1.5 ml Eppendorf tube. Subsequently, they were washed by centrifugation at 250 xg for 15 min at room temperature (microcentrifuge Hermle Model Z230MA), decanting the supernatant and resuspending the cell pellet in a volume of 180 μl of complete medium, using a Vortex Agitator. Cell viability and concentration was measured using a sample of 20 μl mixed with 20 μl of 0.15 % (p/v) sterile trypan blue solution in a 0.11 M NaCl solution; using the Neubauer® chamber, in a 40X bright-field Leica® microscope. Mean values, standard error and 95 % confidence interval, were determined using Microsoft Office Excel 200716 program.
Minimum seed initial dose for RF/6A assay
For the determination of the minimal number of cells required to start RF/6A culture, 5.0 × 105 cells in passage 543, suspended in 350 μl of complete culture medium MEM, were counted; performing two-fold serial dilutions to obtain a cell suspensions of 2.5 × 105, 1.25 × 105 and 6.25 × 104, in a first trial. For a second trial, the experiment began with a cellular concentration of 7.07 × 104 cells in passage 551, also performing two-fold serial dilutions to obtain cellular suspensions of 3.53 × 104, 1.77 × 104 and 8.84 × 103 in 350 μl of MEM10, already described. For both trials, cells in two 24-well plates were inoculated in duplicate with 350 μl/well of cell suspension. Cell culture media exchange was performed each 48 h, until harvest, which occurred when cells reached ≥95 % confluence in any well and then the totality of wells of each phase was collected. For cell count, the monolayer of confluent cells was disaggregated by enzyme action, using 200 μl/well of 1X Tripsyn-EDTA solution. After washed by centrifugation, cell viability and concentration were determined by trypan blue exclusion, recording obtained values as described in the first assay.
Seed density for DH82 assay
The aim was to know the time interval for reaching maximal growth, initiating the cultures with different cell concentrations. Fourth-passage cells were grown in two 24-well plates in duplicate, adding 350 μl/well of cell suspension, with density of 5, 10, 20 and 40 cells/mm2. As in the first assay, cell culture media exchange was performed every 72 h, until all treatments were harvested (≥95 % confluence). Cell viability and concentration were determined by trypan blue exclusion, as already described and mean values, standard error and 95 % confidence interval were recorded.
Seed density for RF/6A assay
After 547 passages, cells were grown in two 24-well plates using the same volume, with densities of 5, 10, 20 and 40 cells/mm2 in duplicate. Cell culture media exchange was performed each 48 h, until harvested, using the same criterion for reaching ≥95% confluence. The enzyme disaggregation was performed using 200 μl/well of 1X Tripsyn-EDTA solution, washing the cells by centrifugation. Similarly, viability and number of cells were measured by trypan blue exclusion.
Kinetics of DH82 cell line development
Cells were expanded up to passage 6. For this purpose, a cell suspension was prepared for seeding 64 Costar® Petri dishes - 35 mm Ø × 10 mm height (Corning Inc. Bellerica, NY, EE.UU. No of Cat. 14831), in volumes of 2,000 μl/dish, with a seed density previously determined in the second assay or third trial15; in terms of 20 cells/mm2 each, and two groups were formed. No cell culture media exchange was done to half of the dishes (32 units), whereas cell culture media exchange was performed in the remaining 32 dishes, at regular intervals of 63 h. Each 21 h and until 336 h of initiation of cultures, two dishes of each group were taken at random, harvesting its content as it has already been described. Finally, cell pellets were resuspended in 300 μl of complete medium and concentration and viability were determined, as previously stated; tabulating results as mean values.
Kinetics of RF/6A cell line growth
Cells were expanded up to passage 551. In the same way as the previous trial, enough cellular suspension was prepared for seeding 94 Petri dishes - dimension 35 mm Ø with the same passage, in volumes of 2000 μl/dish, with seed density determined in the fourth trial, as well as in cells/mm2 and divided into two groups of 46 dishes each. No cell culture media exchange was done to one group. Cell culture media exchange at regular intervals of 45 h were performed in the other half of the dishes. A pair of dishes from each group was randomly harvested each 15 h and until 345 h of initiating the culture, culture was processed according to the aforementioned for harvesting RF/6A cell line, resuspending cell pellet in 300 μl of complete medium, determining its concentration and viability and tabulating results as mean values according to the stated.
Doubling time determination
Using data from earlier trials, doubling time formula was applied (DT), suggested by ATCC17, which considers the log phase:
Where T= incubation period= interval [finalT - initialT] in hours; Xb= initial value of cell number; Xe= final value of cell number.
Results
There were no contamination problems due to strict management of asepsis, antisepsis and sterilization of components used in the culture of both cell lines. The morphology observed under the phase contrast microscope was apparently normal. Cell number was determined by total count of live and dead cells.
Minimum seed initial dose for DH82 cell line
This trial lasted 7 d from day zero, same time period that took the first treatment to reach ≥95 % confluence, which corresponded to the one started with 5.0 × 105 cells, whose maximum average concentration reached 1.07 × 107 cells, a growth 20.4 times the initial concentration. The maximum average concentrations for the remaining treatments were: 4.5 × 106, 2.49 × 106 and 7.38 × 105 cells, which is equivalent to a growth of 18, 19.9 and 11.8 times, respectively, for cultures started with 2.5 × 105, 1.25 × 105 and 6.25 × 104 cells/well. None of them demonstrated to be statistically significant. Table 1 shows values obtained for growth, standard deviations and upper and lower limits at 95% confidence interval (CI95%).
Table 1 Minimum seed initial dose values determined for the DH82 cell line.

* Started with 5.00x105, 2.50x105, 1.25x105 y 6.25x105 cells/well, respectively.
§ Total cells/well.
† Average total cells/well.
SD= Standard deviation; SE= Standard error.
** Alpha value for 95 % confidence interval.
§§ Day to reach > 95 % confluence; NA= Not applicable.
Minimum seed initial dose for RF/6A
The assay had a duration of 10 d in any of the two trials, in which the first phase of treatment 1 and the second phase of treatment 5 reached ≥95% confluence. It was evident that there was a decrease in all first phase treatments. The biggest fall, represented by a reduction in cell number, occurred in treatment 1, reaching a value of -4.21 × 105 cells, followed by treatments 2, 3 and 4, with reductions of 1.7 × 105, 5.8 × 104 and 4.5 × 103 total cells. These data, together with statistical variables are shown in Table 2.1. Cell harvesting in the first assay reached a total of 7.9, 8.0, 6.7 and 5.8 × 104 average cells for treatments 1, 2, 3 and 4, respectively. The second experimental phase implemented with lower cell concentration, gave positive results. The maximum cell concentration was obtained in treatment 8, reaching on average 1.12 × 105 total cells. Treatments 7, 5 and 6 followed the performance of the cell concentration, obtaining on average 1.03 × 105, 8.48 × 104 and 8.35 × 104 total cells. The 95 % CI together with the statistical evaluation, which did not result significant, are shown in Table 2.2.
Table 2.1 Minimum seed initial dose values determined for the RF/6A cell line in first assay.

* Started with 5.00x105, 2.50x105, 1.25x105 y 6.25x105 cells/well, respectively.
§Total cells/well.
† Average total cells/well.
SD= Standard deviation; SE= Standard error.
** Alpha value for 95 % confidence interval.
§§ Day to reach > 95 % confluence; NA= Not applicable.
Table 2.2 Minimum seed initial dose values determined for the RF/6A cell line in a second assay.

* Started with 7.07x104, 3.53x104, 1.77x104 y 8.84x103 cells/well, respectively.
§ Total cells/well.
† Average total cells/well.
SD= Standard deviation; SE= Standard error.
** Alpha value for 95 % confidence interval.
§§ Day to reach > 95 % confluence; NA= Not applicable.
Seed density for DH82 cell line
Fourteen days after culture started, the first well that reached ³95 % confluence corresponded to the inoculum of 40 cells/mm2, followed by the inoculum of 20 cells/mm2 on d 15; d 16 corresponded to the inoculum of 10 cells/mm2 and the inoculum of 5 cells/mm2 on d 17. The highest average cell concentration at the moment of cell harvesting was obtained with the inoculum of 10 cells/mm2, reaching a value of 3.46 × 106 on d 17, followed by the inoculum started with 20 cells/mm2 with 3.08 × 106 on d 15, whereas for inocula of 40 and 5 cells/mm2, counts of 2.9 × 106 were obtained in both on d 14 and 17, respectively. The resulting values of this assay are shown in Table 3, where the standard error and 95 % CI are sown, since the average values of each treatment resulted to be statistically non-significant.
Table 3 Seed density values determined for the DH82 cell line.

* Started with 8.84x102, 1.77x103, 3.53x103 y 7.07x103 cells/well, respectively.
§ Total cells/well.
† Average total cells/well.
SD= Standard deviation; SE= Standard error.
** Alpha value for 95 % confidence interval.
§§ In number of cells/mm2.
†† Day to reach > 95 % confluence.
Seed density for RF/6A
Cultures began to reach progressive confluence in direct relation to the inoculum density on d 8, 9, 10 and 11, after culture of inocula of 40, 20, 10 and 5 cells/mm2 were initiated. The value of the maximum average cell concentration was 1.04 × 105 and it was obtained in the inoculum of 10 cells/mm2; the minimum average value of 5.6 × 104 was obtained with 5 cells/mm2, whereas the remaining inocula of 20 and 40 cells/mm2 both reached, on average, the intermediate concentration of 9.3 × 104. As in the previous assay, the resulting values of this experiment, were statistically non-significant, as shown in Table 4.
Table 4 Seed density values determined for the RF/6A cell line.

* Started with 8.84x102, 1.77x103, 3.53x103 y 7.07x103 cells/well, respectively.
§ Total cells/well.
† Average total cells/well.
SD= Standard deviation; SE= Standard error.
** Alpha value for 95 % confidence interval.
§§ In number of cells/mm2.
††Day to reach > 95 % confluence.
Kinetics of DH82 cell line growth
This assay had a duration of 336 h or 14 d. The lag or adaptation phase lasted 126 h in both groups. In the first, without cell culture media exchange, it reached an average cell concentration of 47,025; whereas in the second group, with a periodic cell culture media exchange, 59,875 total cells, equivalent to a density of 49 and 62 cells/mm2, respectively. Log phase lasted until 315 h with a value of maximum average cell concentration of 538 250 cells per well in the group without cell culture media exchange; but in the second one, the log phase lasted up to 336 h, when a maximum average cell concentration reached 1.77 × 106 (1’774,750 cells) and the assay was finished. Maximum densities reached were 559 and 1,845 cells/mm2 for treatments with and without cell culture media exchange. Figure 1 shows growth differences between both groups, as well as the log phase and time in culture medium.
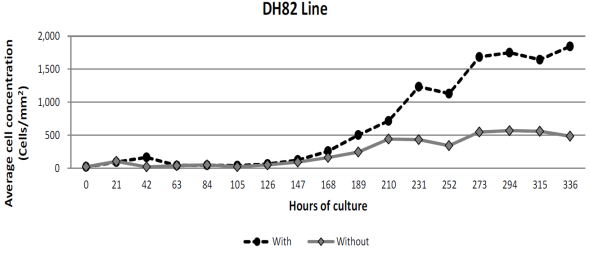
*Cultures started with a density of 20 cells /mm2. Maintained in minimum essential medium (Eagle formulation), with 2.0 mM L-glutamine supplemented with 1.0 mM sodium pyruvate, 1.5 g/l of NaHCO3 and 15 % (v/v) inactivated fetal bovine serum at 56 °C. Cell culture media was exchanged every 63 h. The exponential growth time period occurred between 126 and 336 h after culture.
Figure 1 Comparative cell growth of DH82 cell line of canine origin, with or without cell culture media exchange, having started on day 0*.
Kinetics of RF/6A cell line growth
This assay lasted 345 h, a little bit more than 14 d. For both treatments, with or without cell culture media exchange, the adaptation period had a duration of 90 h, from which log phase was observed. This was mild in the first case, reaching its maximum average value of 8.4 × 104 (83,950 cells) after 300 h culture started, equivalent to an average density of 87 cells/mm2. In a similar way, the second treatment reached its maximum value after 315 h with a maximum average concentration of 6.52 × 105 (651,600 cells), equivalent to an average density of 677 cells/mm2. Further measures revealed gradual decrease in cell number. Figure 2 shows growth differences between both groups, as well as log phase and time in culture medium.

* Cell cultures started with a density of 10 cells /mm2. Maintained in minimum essential medium (Eagle formulation), with 2.0 mM L-glutamine supplemented with 1.0 mM sodium pyruvate, 1.5 g/l of NaHCO3 and 10 % (v/v) fetal bovine serum. Cell culture media was exchanged every 45 h. The exponential growth time period occurred between 90 and 315 hours after culture.
Figure 2 Comparative cell growth of RF/6ª cell line derived from Rhesus monkey, with or without cell culture media exchange, having started on day 0*.
Doubling time
Based on data obtained from our experiments, results were tabulated, and shown in Table 5, as follows:
DT DH82 = 42.95 h, equivalent to 42 h 56’ real-time.
DT RF/6A = 37.00 h, equivalent to 36 h 59’ real-time.
Table 5 Cell development values of both cell lines, used in the doubling time (DT) determination formulae [16]
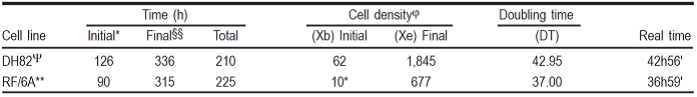
§DT= T ln2/ln(Xe/Xb).
φ= Cells/mm2.
* Time; hours of culture when log phase initiated.
§§ Time; hours of culture when log phase ended.
Ψ Isolated canine cell line (Canis lupus familiaris).
** Derived from Rhesus monkey fetus (Macaca mulatta).
Discussion
Characteristics and properties of DH82 cell line of canine origin and RF/6A line, derived from Rhesus monkey, were defined. Additionally, both lines behaved very different from each other, which increases the value of this study by highlighting their inequality. This is to be expected, since DH82 is myeloid origin, monocyte-macrophage type6,7, in contrast to RF/6A which is endothelial type2,5.
For the first DH82 cell line assay, a first dose of 5.0 × 105 cells was established, which is two times less than 1.0 × 106, with which cultures are traditionally started in 25 cm2 flasks at the laboratory. For the determination of a dose of 6.25 × 104, it was established that cell cultures can be started even at doses 16 times lower. Moreover, cultures started in flasks with the highest dose of 1.0 × 106 have a density of 400 cells per unit area (mm2); in contrast, the ones that are started with a lower dose of 6.24 × 104, the density is 354 cells per unit area, which is not a great difference; but that is not indicative of the advantage for initiating cultures with comparatively high doses. This led to the conclusion that cell lines, once adapted to laboratory conditions, should be started based on a density determination, which will indicate the dose to be used independently of the container size.
Although in the case of RF/6A cell line, whose initial assay had the same expectation as the earlier trial, the results were very different. First of all, the concentrations previously defined and considered high, resulted in limited assay conditions and that is why none of the established treatments managed to increase their values; on the contrary, they decreased. According to these results, the second phase was defined, reducing even more the total initial cell concentration to just over 141 times. This result proves that for this cell lineage, the initial dose of seeding inoculum can start from smaller quantities than the traditionally recommended. Likewise, in this assay it is shown that possibly large amounts of inoculum failed to adapt under culture conditions, which can be associated with lack of nutrients, enough to satisfy requirements for the total population. Although this should rather be referred to some key nutrient at the moment of adhesion to the container walls . It should be remembered that the selection criterion to define the end of the experiment is almost complete confluence. This is a subjective visual measurement and it will not always give the same results, specifically based on its degree of subjectivity. Areas near the border of the wells seemed not to have same cell density, in comparison to the central areas, where cells appeared to have more contact between them and more uniform in appearance. As a disparity reflex, the reported by Kasai et al18 and Shen et al was performed, using 4.8 × 103 and 1.0 × 105 cells per well, in 96-well plates to start RF/6A cell culture, respectively. This represents a difference, with respect to each other, of 20 times the concentration. As a result of this, the reliability of the results is in doubt, because at the moment of starting the cultures with high cell concentrations, these conglomerations will be sub-optimal for a cell-based assay, because instead of growing and multiplying, their concentration decreases.
Based on the experience of the previous assays, seed density trials were designed, initially aiming at determining whether the closeness of the cells to each other could be a conditioning factor for their growth. The initial concentration of 1.0 × 106 cells in culture flasks surface area 25 cm2, is equivalent to a density of 400 cells/mm2. So the following pair of trials would be started with quantities varying from 80 times less, which means 5 cells/mm2, recorded as treatment 1, up to 10 times less, which is equivalent to 40 cells/mm2, described as treatment 4. For the case of DH82 line, it was found that the maximum growth values reached, in each of the treatments, were not statistically different between them, which is interpreted as uniform but not equal. Partly because the subjective criterion of confluence for their harvesting coincided in all cases with the discrepancy of interval that corresponds to the incubation period that was indeed different.
With respect to seed density of RF/6A cell line results were discordant with the canine origin line. Values were much lower, both in cell performance and the required interval to reach confluence. A simple explanation is that the size of the cells tended to be greater, indicated by the density obtained, that in no case it was on average higher than 645 cells/mm2. Likewise, they reached confluence in shorter incubation intervals, because the maximum value reached was 11 d. The average growth differs with the inoculum of 5 cells/mm2, although the obtained with the other three treatments is not statistically significant. Considering, as a simple explanation, the necessity of cell proximity, being a cell line of epithelial origin, it would be easier to understand that it is a tissue that requires a certain degree of organization and structure to operate efficiently17,18, speculating that the communication between cells is favored by being physically closer together. Monocytes/macrophages expressed in DH82 cell line tend to function individually and cell-cell interactions are rather with other cell lineages, as antigen-presenting cells would be in the immune response. However, this study does not pretend to compare cell lines used, but rather discover differences with the aim to find their properties.
Kinetic assay of DH82 cell line was based on the use of specific cell density of 20 cells/mm2, which was associated with certain expectations, foreseen beforehand, in terms of reaching the expected confluence after 15 d in culture. This would confirm our speculation of obtaining a pre-established growth, independently of the container’s size. The intermediate sampling interval of 21 h was based on an arithmetic estimation of generation time, according to the data calculated (not shown) in the first assay; and based on that estimation, cell culture media exchange was proposed every 63 h, which is three times that period of time. In compliance with the premise of reaching confluence after 15 d, representing 360 h in culture and having fallen short for one day, since biological material prepared for it ran out. The individual observation of the graphs allows to distinguish that the adaptation time period coincided between both treatments, only highlighting the log phase, while it decreased in the group without culture media exchange, the same did not occurred in the group with cell cultured media exchange, which would appear to be growing although not in the same reproductive rate.
Following the same reasoning for the kinetic assay for the development of RF/6A line derived from Rhesus macaque, initial seed density of 10 cells/mm2 was established to reach the expected confluence after 10 d or 240 h, independently of the size of the container. As in the previous assay, the interval of 30 h was calculated (data not shown), which corresponds to a generation time period, based on data obtained in the second phase of the initial seeding dose assay. Samplings were programmed every 15 h, considering it as half of the time between generations. For cell culture media exchange, the interval of 45 h was considered, 1.5 times the calculated for the generation time period, but very similar to the 48 h used in continuous cultures and previous assays. Now, the experience was different, since cell growth exceeded the expected time period of 10 d by extending the log phase up to 315 h, equivalent to 13.125 d. Cell density reached at that date was 677 cells/mm2, similar or closer to 645 cells/mm2 obtained in the seed density assay after 10 d. This phenomenon is biologically compatible with the functionality of the cell line, considering that it is not likely that only one layer is formed but several layers will pile up, characteristic of the tissue from where this cell line derived8.
It is globally accepted that in every cell culture there are adaptation and log phase time periods1. One point this paper emphasizes is that in both cell lines these assays have demonstrated a similar behavior on what the adaptation time period refers to, regardless of whether or not they received fresh media culture, the intervals coincide, since the ups and downs shown (data not shown) at the beginning of the previous cultures, on the definition of intervals confirm this.
The results of the DT determination were surprising, because they give the appearance of being too high. The DT for DH82 of almost 43 h, as well as 37 h for RF/6A seem to coincide given the culture conditions used. Likewise, they differ from the initially calculated doubling time periods of 21 and 30 h, respectively. This verified information could give rise to studies for decreasing or even increasing its efficiency, using new culture medium formulations. MEM is one of the most commonly used of all cell culture media; it has a very simple formulation, compared to the components of other media chemically defined, such as Medium 199 or RPMI 1640, commercially available, for which it would be interesting to perform assays to identify some growth-restricting components.
Indirect inference obtained by preparation and staining of imprints (data not shown) allows to assert that cells from RF/6A lineage are polymorphic, in contrast to the description of cells reported by Luna Castro et al14, who mentioned that RF/6A cells present a mosaic shape. Additionally, it was observed that cells from this cell lineage show different sizes. Nonetheless, cells in general are very big, in comparison with other cell lines, such as BUVEC E6E715,20 and DH827,8, cell lines that are uniform in shape and have an inferior size, in contrast to RF/6A cell line.
Conclusions and implications
Data obtained allowed to generate a cell culture pattern model for further studies. In this way it will be more practical and simple to predict times and dates, for eventually obtaining cell cultures with high degree of confluence on a particular day. Equally relevant is the finding of cell cultures that have little growth when they are started with large number of cells, in contrast to the widespread view that cell cultures should be started with the largest number of cells as possible.
Acknowdgement
Special thanks to INIFAP-Recursos fiscales for the financing of Project No. 14531319812.
REFERENCES
1. Cossío BR, Rojas MC, Miranda ME, Álvarez MJA, Figueroa MJV, Vega YMCA. Cultivo in vitro de células animales y sus aplicaciones. 1ª ed. Morelos, México: CENID-PAVET / INIFAP; 2011. [ Links ]
2. Lou D, Hu F. Specific antigen and organelle expression of a long-term rhesus endothelial cell line. In vitro Cell Dev Biol 1987;23:75-85. [ Links ]
3. Grigsby JG, Parvathaneni K, Almanza MA, Botello AM, Mondragon AA, Donald MA, Tsin TCA. Effects of tamoxifen versus raloxifene on retinal capillary endothelial cell proliferation. J Ocular Pharma Therap 2011;27(3):225-233. [ Links ]
4. Munderloh U, Lynch MJ, Herron MJ, Palmer AT, Kurtti TJ, Nelson RD, Goodman LJ. Infection of endotelial cells with Anaplasma marginale and A. phagocytophilum. Vet Micro 2004;101:53-64. [ Links ]
5. Machuca FS. Implementación de un cultivo de células endoteliales de origen primate [tesina licenciatura]. Jiutepec, Morelos: Universidad Politécnica del Estado de Morelos; 2014. [ Links ]
6. Dawson JE, Rikihisa Y. Growing Ehrlichia species in a continuous cell line. US patent 5,192,679; Mar 9, 1993. [ Links ]
7. Wellman ML, Krakowka S, Jacobs RM, Kociba GJ. A macrophage-monocyte cell line from a dog with malignant histiocytosis. In vitro Cell Dev Biol 1988;24(3):223-229. [ Links ]
8. Granjeno CG, Machuca FS, Rodríguez CSD, Vega YMCA. Mantenimiento de las líneas celulares RF/6A del mono Rhesus y DH82 del perro doméstico. 1ª ed. Morelos, México. CENID-PaVet / INIFAP; [EN PRENSA] 2015. [ Links ]
9. Hegarty BC, Levy MG, Gager RF, Breitschwrdt RF. Immunoblot analysis of the immunoglobulin G response to Ehrlichia canis in dogs: An international survey. J Vet Diag Invest 1997;9:32-38. [ Links ]
10. Keysary A, Trevor W, Strenger C, Harrus S. Cultivation of Erlichia canis in a continuous BALB/C mouse macrophage cell culture line. J Vet Diag Invest 2001;13:521-523. [ Links ]
11. Granjeno CG, Machuca FS, Rojas REE, Amaro EI, Preciado DLTJF, Rodríguez CSD, Vega YMCA. Interacción entre eritrocitos infectados con Anaplasma marginale y las estirpes celulares permisivas DH82 y RF/6A [resumen]. Reunión Nacional de Investigación Pecuaria. Mérida, Yucatán. 2014:4. [ Links ]
12. Heinrich F, Bono-Contioso V, Stein VM, Carlson R, Tipold A, Ulrich R, et al. Passage-dependent morphological and phenotypical changes of a canine histiocytic sarcoma cell line (DH82). Vet Immunol Immunopathol 2015;163:86-92. [ Links ]
13. Fragoso SH. La anaplasmosis bovina en México. En: Vega YMCA, et al. editores. 2º Seminario Internacional de Parasitología Animal. Oaxtepec, Morelos. 1993:153-160. [ Links ]
14. Vega YMCA. Actualidad e importancia de las enfermedades causadas por los hemoparásitos En: Vega YMCA et al. editores. 2º Seminario Internacional de Parasitología Animal. Oaxtepec, Morelos. 1993:144-152. [ Links ]
15. Luna CGS, Rodríguez CSD, Ramírez NP, Preciado DLTJF, Rojas REE, Mosqueda GJJ, Vega YMCA. Cultivo in vitro de Anaplasma marginale en líneas celulares endoteliales. Rev Mex Cienc Pecu 2010;1(4):373-390. [ Links ]
16. Microsoft® Office Excel 2007: Funciones estadísticas. [ Links ]
17. ATCC. ATCC® Animal Cell Culture Guide, tips and techniques for continuous cell lines. https://www.atcc.org/~/media/PDFs/CultureGuides/AnimCellCulture_Guide.pdf Accessed: 29 Jan, 2014. [ Links ]
18. Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Baba A. Apelin is a novel angiogenic factor in retinal endotelial cells. Biochem Biophysical Res Com 2004;325(2):395-400. [ Links ]
19. Shen S, Yu H, Chen P, Yin J, Xiong YK. Fatty acids in tea shoots (Camellia sinensis (L) O. Kuntze) and their effects on the growth of retinal RF/6A endotelial cell lines. Mol Nutrition Food Res 2007;51:221-228. [ Links ]
20. Cajero-Juárez M, Avila B, Ochoa A, Garrido-Guerrero E, Varela-Echavarria A, Martínez DLEE, Clapp C. Immortalization of bovine umbilical vein endothelial cells: a model for the study of vascular endothelium. Eur J Cel Biol 2002;81:1-8. [ Links ]
Received: December 19, 2014; Accepted: May 06, 2015











 texto en
texto en 


