Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias pecuarias
versão On-line ISSN 2448-6698versão impressa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.6 no.4 Mérida Out./Dez. 2015
Technical notes
Effect of mushroom powder and flavophospholipol on carcass in broiler chickens
1 Department of Animal Science, Rasht Branch, Islamic Azad University, Rasht, Iran. Telephone: +989113313073.
2 Consejería Agricultura Castilla-La Mancha. Oficina Comarcal Agraria, 02630, La Roda (Albacete), España.
After showed its beneficial effects on chicken growth performance, a study was conducted to evaluate the effect of adding from birth and during the whole growth period, flavophospholipol and edible mushrooms powder on carcass characteristics, viscera weights and digestive tract measurements of male broiler chicken. A total of 300 one-dayold male broiler chicks were randomly distributed into 1 to 10 treatments with three replicates per treatment and 10 chicks per pen. The experiment consisted in a 2 x 5 factorial arrangement of treatments including five concentrations of mushrooms powder (0, 0.5, 1.0, 1.5 and 2.0 g/kg of diet) and the addition of 0.0 or 5.0 mg/kg of flavophospholipol from birth date to slaughter time at 42-d. Results show that the additions of dietary mushroom powder and flavophospholipol have a positive effect on weights of carcass parts with commercial importance as the breast and drumsticks. In contrast, there is no effect on wings weight. Regarding viscera weights, mushroom powder but not flavophospholipol or the interaction of both, affected the thymus, spleen, bursa of Fabricius and liver weights. Finally, despite weak statistically significant results on several measurements of the digestive tract, results show no effect of mushroom powder or the antibiotic addition on these variables.
Keywords: Broiler; Carcass; Flavophospholipol; Agaricus bisporus; Digestive tract
Tras demostrar efectos beneficiosos sobre el desarrollo del crecimiento de pollos, se realizó un estudio para evaluar el efecto de la adición de flavofosfolipol y harina de champiñón común desde el nacimiento y durante todo el período de crecimiento sobre las características de la canal, peso de vísceras y medidas del tracto digestivo en pollos de engorda. Se utilizaron 300 pollitos machos de un día de edad distribuidos al azar en 10 tratamientos con tres repeticiones por tratamiento y 10 pollitos por corral. El experimento consistió en un arreglo factorial de 2 x 5: cinco concentraciones de harina de champiñón (0, 0.5, 1.0, 1.5 y 2.0 g/kg de dieta) y la adición de dos concentraciones de flavofosfolipol (0.0 o 5.0 mg/kg), desde el nacimiento hasta el sacrificio a los 42 días de edad. Los resultados demostraron que la adición de harina de champiñón y flavofosfolipol tienen un efecto positivo sobre el peso de partes de la canal de importancia comercial como el pecho y muslos. En cambio, no hubo ningún efecto sobre el peso de las alas. En relación al peso de las vísceras, la harina de champiñón (pero no el flavofosfolipol o la interacción de ambos), afectó el peso del timo, bazo, bolsa de Fabricio e hígado. Finalmente, a pesar de escasos resultados significativos en varias medidas del tracto digestivo, no se encontró ningún efecto estadístico de la harina de champiñón o la adición del antibiótico en estas variables.
Palabras clave: Pollos; Canal; Flavofosfolipol; Agaricus bisporus; Tracto digestivo
The livestock sector in Asia is under pressure to adapt and expand1. These adaptations involve mainly a shift in livestock species and functions, with the greatest change being an increase in the number of monogastric animals (pig and poultry). The poultry industry has never been under a greater pressure than at the present time2. The production of poultry in Iran has been increasing annually at a rate of 8 % for the last decade reaching 1.589 million heads and 1.9 t of output3 in 2011, which is the largest in the Middle East.
The white button mushroom (Agaricus bisporus) with high content of polyphenols, ergothioneine, vitamins, minerals and polysaccharides4 has been demonstrated to possess valuable biological properties including antitumor, antiaromatase, antimicrobial, immunomodulatory, anti-inflammatory, and antioxidant activities5-11. Despite the largely known positive effects on human health12,13, there is little information about the effect of mushroom consumption in poults. However, on turkeys, it has been reported that A. bisporus, due to its polysaccharides content, has a prebiotic-like effect and improves the efficiency in digestibility and utilization of feed14,15.
The Iranian production of mushroom and truffles has increased from 10,000 t in 1997 to 82,000 in 2011 (FAO estimate), holding in this year the 8th place in the world mushroom production3. The mushroom industry produces significant quantities of mushroom wastage that are discarded during the mushroom production process. The recycling of this by-product as feed additive for broiler production should increase the efficiency of the Iranian food industry by reducing costs.
The positive impact of antibiotic as growth promoter (AGP) is well known in the poultry industry due to the suppression of harmful microorganisms16 and to inhibition of inflammation effects17. Association of bacterial resistance with the addition of AGP in the chicken feed may be questionable18,19 and it seems that is the precautionary principle the main reason for the banning of AGP in several countries20,21. In this sense, the use of antibiotics not used in humans therapy and that do not cause resistance could still be an alternative. This is the case of flavophospholipol, a phosphoglycolipid antimicrobial produced by several strains of Streptomyces that is not used for treatment of human infections, which is why it can still be legally added in poultry diets in many countries16.
Previous results from our group have shown that the addition of dietary mushroom powder and flavophospholipol has a beneficial effect on growth performance as it showed positive on weight gain, feed conversion ratio and body weight at 42-d of growth (Shamsi et al. under review). The aim of this current study is to demonstrate that these previous positive effects on chicken performance are directly linked to the carcass yield. In addition, the effect on viscera weight and digestive tract measurement have also been evaluated.
This study was performed in the Poultry Farm Facilities in Ramsar city (50°40' N and 36°54' E) and the Agriculture Faculty, Islamic Azad University, Rasht Branch, Iran, during the summer (warm) season, between July-September 2013, for a total of 42 d. The experiments were approved by the Scientific Board of the Islamic Azad University, and were conducted as per International Guidelines for research involving animals22.
Three hundred (300) one-day-old male Ross 308 broilers23 were randomly assigned to 10 treatments with three replicates per treatment. Each replicate consisted of 10 chicks housed in pens of 1.2 m2. Before the animals were placed in their pens, all the facilities were thoroughly cleaned and disinfected with 1:125 and 1:250 solution of Multifenelic. Other equipment including drinkers and feeders was disinfected with 20% Benzalkonium chloride - Germo Killer. All the windows and the ventilators were gasified with Formalex® solution.
The room temperature was adjusted to approximately 33 ºC on d 1, and then was gradually decreased to 24 ºC. The pens were fitted with electrical heaters. Lighting was provided with a program of 23L:1N (from 1900 to 2000 h). Humidity was maintained between 55 and 65 % in the early growing period, by spraying water on the floor. The chicks were vaccinated against Newcastle and Gumboro diseases. Within 24 h after vaccination, a multiple-vitamin and electrolytic solution (1:1000) was offered via drinking water to reduce stress.
Water and feed were supplied ad libitum during the entire experimental period. The nutritional requirements were based on Ross strain rearing catalogue23. The feed was formulated to be iso-proteic and iso-energetic for all treatments. Composition of the diets and their nutrient contents are presented in Table 1. Three types of feed formulations were used during the rearing period: the starter period (1-14 d of age (DOA), grower period (15-28 DOA) and the finisher period (29-42 DOA). Feed remaining in feeders was weighed and recorded weekly.
Table 1 Feed ingredients and nutrient analysis of diets used during the starter (1-14 d of age), grower (15-28 d of age), and finisher periods (29-42 d of age).
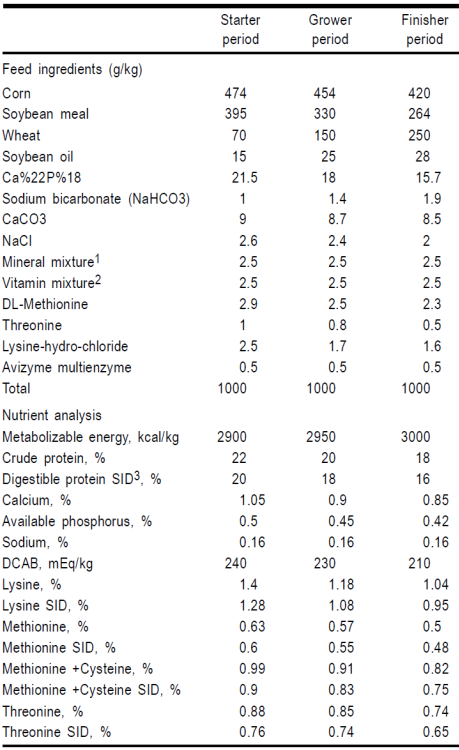
1 Calcium Pantothenate: 4 mg/g; Niacin: 15 mg/g; Vitamin B6: 13 mg/g; Cu: 3 mg/g; Zn: 15 mg/g; Mn: 20 mg/g; Fe: 10 mg/g; K: 0.3 mg/g.
2 Vitamin A: 5000 IU/g; Vitamin D3: 500 IU/g; Vitamin E: 3 mg/g; Vitamin K3: 1.5 mg/g; Vitamin B2: 1 mg/g.
3 SID (Standardized Ileal Digestible).
Mushrooms (A. bisporus) were obtained from a local mushroom producer (Pars Company, Ramsar, Iran). The whole mushrooms were dried at 60 ºC followed by grinding, and added to the experimental diets. Treatments were selected to determine five levels of mushroom powder (MP; i.e., 0, 0.5, 1.0, 1.5, and 2.0 g/kg), and two levels of antimicrobial flavophospholipol (Teif Azmoon Pars, Co., Tehran, Iran), 0 or 5.0 mg/kg.
At the end of the study, one bird per pen (totalling three birds per treatment) was selected based on the average weight of the group, weighed to obtain live body weight followed by killing and complete bleeding. Head, viscera and shanks were removed. The birds were dissected to remove and determine the yield of different viscera, digestive tract and carcass parts. Empty crop, empty proventriculus, empty gizzard, abdominal fat pad, thymus, spleen, bursa of Fabricius, liver, kidneys, pancreas, lungs, hearth and brain were weighed. Empty small intestine was divided into duodenum (from the gizzard outlet to the end of the pancreatic loop), jejunum (from the pancreatic loop to Meckel’s diverticulum) and ileum (from Meckel’s diverticulum to the ileo-caeco-colic junction) and these three different parts were weighted.
Length, width and diameter of different parts were measured. Empty large intestine was divided into right and left cecum, colon and rectum. These parts were weighted and measured. Relative weights were calculated as a percentage of live weight at slaughter. Carcass was left for one hour to remove excess water and allowed to sit overnight in a refrigerator at 4 ± 1 ºC using a ratio between the eviscerated weight and live weight x 100. Carcass was then portioned; breast, drumsticks, wings, neck and notarium were obtained and weighed. Data from these measurements were used to calculate the percentage of part to the carcass weight.
General Lineal Models (GLM, SPSS 15.0, Chicago, IL, USA) examined the effects of MP and flavophospholipol on carcass and viscera weights and on the measurements of the digestive tract in a 2×5 factorial arrangement using Yijk=ì+Aj+Bk+AjBk+ eijk statistical formula. Variables found significant with GLM were tested with one-way ANOVA to find differences between treatments, (different levels of MP addition or antibiotic vs no antibiotic addition groups). Tukey’s test was used to determine disparities among the groups.
Cold carcass weight was affected by both the inclusion of the antibiotic and MP (Table 2). The same variables affected positively too the dressed carcass weight (Model F3,26= 10.70, P<0.001, R2= 56.8 %; coefficients: MP= 7.4 ± 36.2, P<0.001, antibiotic= 212.4 ± 62.6, P<0.01, interaction between both factors= -196.7 ± 51.1, P<0.01). Inclusion of flavophospholipol in diet improved some of the measured carcass traits. In this sense, the addition of the antibiotic resulted in birds with greater breast weight as well as greater relative breast weight (Model F3,26= 6.23, P<0.05, R2= 18.2 %; coefficient: antibiotic= 2.0 ± 0.8, P<0.05). In contrast, dietary flavophospholipol showed a negative effect relative weight of wings (Model F3,26= 3.79, P<0.05, R2= 31.3 %; coefficients: antibiotic= -0.7 ± 0.2, P<0.01, interaction between MP and antibiotic= 0.03 ± 0.1, P<0.05) and neck (Model F3,26= 4.98, P<0.05, R2= 15.1 %; coefficient: antibiotic= -0.2 ± 0.1, P<0.05).
Table 2 Effect of flavophospholipol (Ab, 0.0 or 5.0 mg/kg) added to diets containing mushroom powder (MP) at 0.0, 0.5, 1.0., 1.5 or 2.0 g/kg on different carcass weights (g) of male broiler chicken at 42 d of age.
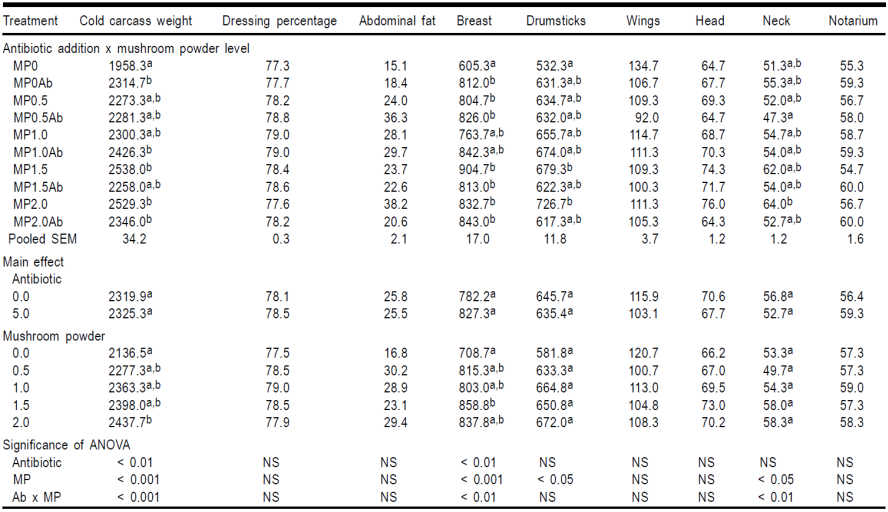
ab Means with the same letter are not significant different (P<0.05).
Feeding rations containing MP yielded the highest breast, drumstick and neck weights (Table 2). The effect of the two variables studied on carcass viscera weights are shown in Table 3. Inclusion of flavophospholipol in diet at 5.0% failed to improve the weight of any organ weighed. On the other hand, mushroom powder showed a positive effect on the weight of thymus, spleen, bursa of Fabricius, liver and brain. Finally, the effects of the antibiotic and MP diet addition on the gastrointestinal traits are showed in Tables 4.1, 4.2 and 4.3. There was no effect of the antibiotic on any of the gastrointestinal measurements taken and MP only showed statistically significance differences in 6 out of the 27 traits measured.
Table 3 Effect of flavophospholipol (Ab, 0.0 or 5.0 mg/kg) added to diets containing mushroom powder (MP) at 0.0, 0.5, 1.0., 1.5 or 2.0 g/kg on viscera weights (g) of male broiler chicken at 42 d of age.

ab Means with the same letter are not significant different (P<0.05).
Table 4.1 Effect of flavophospholipol (Ab, 0.0 or 5.0 mg/kg) added to diets containing mushroom powder (MP) at 0.0, 0.5, 1.0, 1.5 or 2.0 g/kg on digestive system (weights, g and measurements, mm) of male broiler chicken at 42 d of age.
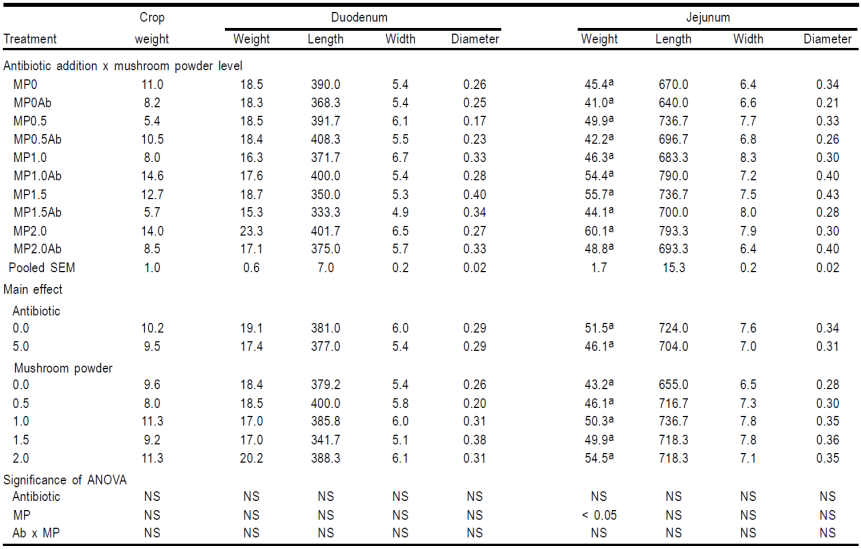
Means with the same letter are not significant different (P < 0.05).
Table 4.2 Effect of flavophospholipol (Ab, 0.0 or 5.0 mg/kg) added to diets containing mushroom powder (MP) at 0.0, 0.5, 1.0, 1.5 or 2.0 g/kg on digestive system (weights, g and measurements, mm) of male broiler chicken at 42 d of age.

Means with the same letter are not significant different (P < 0.05).
Table 4.3 Effect of flavophospholipol (Ab, 0.0 or 5.0 mg/kg) added to diets containing mushroom powder (MP) at 0.0, 0.5, 1.0, 1.5 or 2.0 g/kg on digestive system (weights, g and measurements, mm) of male broiler chicken at 42 d of age.

ab Means with the same letter are not significant different (P<0.05).
The results of the present study show that the effect of the antibiotic and MP are positive in the cold carcass weight. Although flavophopholipol had a negative effect on relative weights of neck and wings, however it had a positive effect on the eviscerated carcass weight. On the other hand, MP addition has a positive effect on carcass weight components that are economically important, e.g., breast and drumsticks. The combination of antibiotic and MP did not exert a cumulative effect on these carcass traits despite the effects shown as individual factors.
Greater weight of the immune organs (thymus, spleen and bursa) with MP suggests a better immune status of the MP treatment chicks since lymphoid organ weights reflect the body’s ability to provide lymphoid cells during an immune response. Thus, immunosuppressed or stressed birds usually have smaller lymphoid organs while higher bursa weight reflects better health status. The bursa of Fabricius has an extremely vital role in morphologic and functional integrity of the immune system. The weight of the bursa in broilers also reflects the anatomical response to immune system alteration due to stress24. In accordance with our results, other authors have also reported immune-enhancing benefits in broiler chickens fed different mushroom supplements25. Since the composition of mushrooms is complex and includes different proteins, such as fungal immunomodulating proteins (FIPs)26, edible mushrooms should not be considered as only a simple food.
The immunostimulant activity of A bisporus has been largely demonstrated; their polysaccharide extracts express an immunostimulating effect and induce synthesis of IFN-𝛿, which alters transcription of up to 30 genes producing a variety of physiological and cellular responses27. Furthermore, A. bisporus is also a good source of selenium28, which has been shown to improve humoral immune activity29.
The greater liver weight of chicks treated with mushroom extract could reflect the antioxidant effect of white button mushrooms7,8,9. It has been observed that the administration of ethanoic extract of A. bisporus significantly enhanced the activities of antioxidant enzymes in liver of mice25.
Broadly speaking, supplementation of MP showed a very weak effect on the different measurements of the gastrointestinal tract. It was expected greater differences since it has been showed that the mushrooms affect intestinal morphology. Thus, researchers have found that mushroom intake influences mucosal architecture resulting in greater duodenal, jejunal and ileal villus height, changes that affect the capacity of the birds to absorb nutrients from the feed15. More surprising is the fact that it was not find any positive effect of including flavophospholipol, as AGP cause changes in the gross structure of the gastrointestinal system. Most of these changes are the result of reduction in overall weight of the small intestine due more to changes in the thickness of the intestinal wall rather than changes in the intestinal length30,31,32. Some authors have indicated that dietary inclusion of antibiotics, given as growth promoters, reduces intestine weight by thinning the intestinal wall33 and some have found lower small intestine weight and shortening of the gut in poults treated with virginiamycin34. A plausible explanation is that the negative effects of an AGP and the positive effects of the components of the A bisporus could be overlapping. Further research is needed to arrive at a definitive conclusion.
In conclusion, the use of flavophospholipol and MP had a positive effect on the growth performance of chicks. These effects were translated into greater carcass components with economic relevance such as the breast and drumsticks. Results also show an overall increment in the immune related organs which reflects a better immune status of the broilers fed MP. An increment in the liver weight was also associated with antioxidative properties of the white mushroom button. Finally, regarding the effect on gastrointestinal parameters, despite the weak influence observed, further research is needed for a definitive conclusion.
Literatura citada
1. Steinfeld H. Livestock production in the Asia and Pacific region-current status, issues and trends. World Anim Rev 1998;(90):14-21. [ Links ]
2. Shariatmadari F. Poultry production and the industry in Iran. World Poultry Sci 2000;(56):55-65. [ Links ]
3. FAOSTAT. FAO Statistics. Food and Agriculture Organization of the United Nations 2014. [ Links ]
4. Tian Y, Zeng H, Xu Z, Zheng B, Lin Y, Gan C, et al. Ultrasonicassisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr Polym 2012;88(2):522-529. [ Links ]
5. Chen S, Oh SR, Phung S, Hur G, Ye JJ, Kwok SL, et al. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res 2006;66(24):12026-12034. [ Links ]
6. Kohno K, Miyake M, Sano O, Tanaka-Kataoka M, Yamamoto S, Koya-Miyata S, et al. Anti-inflammatory and immunomodulatory properties of 2-Amino-3Hphenoxazin- 3-one. Biol Pharma Bull 2008;31(10):1938-1945. [ Links ]
7. Tsai SY, Huang SJ, Lo SH, Wu TP, Lian PY, Mau JL. Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem 2009;113(2):578-584. [ Links ]
8. Jeong SC, Jeong YT, Yang BK, Islam R, Koyyalamudi SR, Pang G, et al. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr Res 2010;(30):49-56. [ Links ]
9. Kozarski M, Klaus A, Niksic M, Jakovljevic D, Helsper JP, Van Griensven LJ. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem 2011;129(4):1667-1675. [ Links ]
10. Moro C, Palacios I, Lozano M, D’Arrigo M, Guillamón E, Villares A, et al. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem 2012;130(2):350-355. [ Links ]
11. Liu J, Jia L, Kan J, Jin CH. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem Toxicol 2013;(51):310-316. [ Links ]
12. Jong SC, Birmingham JM. Medicinal benefits of the mushroom Ganoderma. Adv Appl Microbiol 1992;(37):101-134. [ Links ]
13. Meschino JP. Reishi mushroom extract and immune support. Dyn Chiropractic 2002;20(12):1-8. [ Links ]
14. Giannenas I, Pappas IS, Mavridis S, Kontopidis G, Skoufos J, Kyriazakis I. Performance and antioxidant status of broiler chickens supplemented with dried mushrooms (Agaricus bisporus) in their diet. Poultry Sci 2010;(89):303-311. [ Links ]
15. Giannenas I, Tsalie E, Chronis EF, Mavridis S, Tontis D, Kyriazakis I. Consumption of Agaricus Bisporus mushroom affects the performance, intestinal microbiota composition and morphology, and antioxidant status of turkey poults. Anim Feed Sci Techol 2011;(165):218-229. [ Links ]
16. Pfaller MA. Flavophospholipol use in animals: Positive implications for antimicrobial resistance based on its microbiologic properties. Diagn Microbiol Infect Dis 2006;56(2):115-121. [ Links ]
17. Niewold TA. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poultry Sci 2007;(86):605-609. [ Links ]
18. Cox NA, Craven SE, Musgrove MT, Berrang ME, Stern NT. Effect of sub-therapeutic levels of antimicrobials in feed on the intestinal carriage of Campylobacter and Salmonella in turkeys. J App Poultry Res 2003;(12):32-36. [ Links ]
19. Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poultry Sci 2003;(84):634-643. [ Links ]
20. WORLD HEALTH ORGANIZATION (WHO). First joint FAO/OIE/WHO expert workshop on non-human antimicrobial usage and antimicrobial resistance: Scientific assessment. Geneva, Switzerland, 2003. http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFK_2004.7.pdf . Accesed Dec 2, 2014. [ Links ]
21. WORLD HEALTH ORGANIZATION (WHO). Second joint FAO/OIE/WHO expert workshop on non-human antimicrobial usage and antimicrobial resistance: Management options. Olso, Norway 2004. http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFK_2004.8.pdf . Accesed Dec 2, 2014. [ Links ]
22. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 of the protection of animals used for scientific purposes. Official Journal of the European Union of 20th October 2010. L276/33. [ Links ]
23. Aviagen. Ross 308 broiler: Nutrition Specification. 2007. http://www.natchix.co.za/pdf/nutrition_specifications.pdf . Accesed Dec 2, 2014. [ Links ]
24. Pope CR. Pathology of lymphoid organs with emphasis on immunosuppression. Vet Immunol Immunopathol 1991;30(1):31-44. [ Links ]
25. Guo FC, Savelkoul HFJ, Kwakkel RP, Williams BA, Verstegena MWA. Immunoactive, medicinal properties of mushroom and herb polysaccharides and their potential use in chicken diets. World Poultry Sci J 2003;59(4):427-440. [ Links ]
26. Borchers AT, Krishnamurthy A, Keen CL, Meyers FJ, Gershwin ME. The immunobiology of mushrooms. Exp Biol Med 2008;(233):259-276. [ Links ]
27. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferongamma: An overview of signals, mechanisms and functions. J Leuk Biol 2004;(75):163-189. [ Links ]
28. Vetter J, Lelley J. Selenium level of the cultivated mushroom Agaricus bispours. Acta Aliment 2004;(33):297-301. [ Links ]
29. Saad MB, Gertner LR, Bona TD, Santin E. Selenium influence in the poultry immune response. Recent Pat Food Nutr Agric 2009;(1):243-247. [ Links ]
30. Henry PR, Ammerman CB, Campbell DR, Miles RD. Effect of antibiotics on tissue trace mineral concentration and intestinal tract weight of broiler chicks. Poultry Sci 1987;(66):1014-1018. [ Links ]
31. Izat AI, Thomas RA, Adams MH. Effects of dietary antibiotic treatment on yield of commercial broilers. Poultry Sci 1989;(68):651-655. [ Links ]
32. Izat AI, Colberg M, Reiber MA, Adams MH, Skinner JT, Cabel MC, et al. Effects of different antibiotics on performance, processing characteristics and parts yield of broiler chickens. Poultry Sci 1990;(69):1787-1791. [ Links ]
33. Visek WJ. The mode of growth promotion by antibiotics. J Anim Sci 1978;(46):1447-1469. [ Links ]
34. Rahimi S, Teymouri-Zadeh Z, Karimi-Torshizi MA, Omidbaigi R, Rokni H. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial populations in broiler chickens. J Agric Sci Technol 2011;(13):527-539. [ Links ]
Received: December 09, 2014; Accepted: February 19, 2015











 texto em
texto em 


