Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.13 no.spe28 Texcoco Set./Out. 2022 Epub 13-Jan-2023
https://doi.org/10.29312/remexca.v13i28.3285
Articles
Iodine increases the concentration of phenolic compounds and photosynthetic pigments in three cultivars of Ficus carica L. subjected to salt stress
1Universidad Autónoma de Nuevo León-Facultad de Agronomía. Av. Francisco Villa s/n, col. Ex-Hacienda El Canadá, General Escobedo, Nuevo León, México. CP. 66050. (palanvf@hotmail.com; emolivares@gmail.com; nino.medina.g@gmail.com; crls-alonso@hotmail.com).
2 Universidad Autónoma de San Luis Potosí-Facultad de Agronomía y Veterinaria. Carretera San Luis-Matehuala km 14.5, ejido Palma de la Cruz, Soledad de Graciano Sánchez, San Luis Potosí, México. CP. 78321. (jcrodor@hotmail.com).
Iodine (I) is a non-essential element for plants; however, the application of the element has shown positive effects on plants grown in optimal conditions or under stress. The objective of this experiment was to evaluate the impact of iodine on the concentration of phenolic compounds, antioxidant capacity and photosynthetic pigments in leaves of three fig cultivars subjected to salt stress. Eight-month-old fig plants were established under a completely randomized experimental design with a 3x2x2 factorial arrangement: three fig cultivars (Ficus carica L.): Brown Turkey, Kadota and Black Mission; two levels of NaCl (0 and 100 mmol L-1) and two levels of iodine (0 and 10 mg L-1). The analyses of variance showed the impact of the main factors (cultivars, NaCl and I) and the interaction between them. The application of iodine on fig seedlings increased the concentration of chlorophyll a, regardless of the cultivar and the concentration of NaCl. The number of new leaves and their dry weight were affected by the interaction between NaCl and I, these variables increased with the presence of I in saline condition. The relative content of total phenols, total flavonoids, antioxidant capacity by DPPH and photosynthetic pigments (chlorophylls and carotenoids) showed interaction between the cultivars, the levels of NaCl and the concentration of I, where the values of these variables were increased by the presence of I under salinity conditions. Due to the above, iodine could be considered as an alternative to mitigate the stress caused by NaCl in Ficus carica L. plants.
Keywords: chlorophylls; fig; salinity; total flavonoids; total phenols
El yodo (I) es un elemento no esencial para las plantas; sin embargo, la aplicación del elemento ha demostrado efectos positivos en plantas cultivadas en condiciones óptimas o bajo estrés. El objetivo de este experimento fue evaluar el impacto del yodo sobre la concentración de compuestos fenólicos, capacidad antioxidante y pigmentos fotosintéticos en hojas de tres cultivares de higuera sometidas a estrés salino. Plantas de higuera de ocho meses de edad fueron establecidas bajo un diseño experimental completamente al azar con arreglo factorial 3x2x2: tres cultivares de higuera (Ficus carica L.): Brown Turkey, Kadota y Black Mission; dos niveles de NaCl (0 y 100 mmol L-1) y dos niveles de yodo (0 y 10 mg L-1). Los análisis de varianza mostraron impacto de los factores (cultivares, NaCl y I) y la interacción entre ellos. La aplicación de yodo en plántulas de higuera incrementó la concentración de clorofila a, independiente del cultivar y la concentración de NaCl. El número de hojas nuevas y el peso seco fue impactado por la interacción entre NaCl y I, incrementándose estás variables con la presencia de I en condición salina. El contenido relativo de fenoles totales, flavonoides totales, capacidad antioxidante por DPPH y pigmentos fotosintéticos (clorofilas y carotenoides) mostraron interacción entre los cultivares, los niveles de NaCl y la concentración de I, donde los valores de las variables fueron incrementados por la presencia de I en condiciones salinas. Por lo anterior, el I podría considerarse como alternativa para mitigar el estrés provocado por NaCl en plantas de Ficus carica L.
Palabras clave: clorofilas; fenoles totales; flavonoides totales; higo; salinidad
Introduction
Iodine (I) is a non-essential element for plants; however, it has been shown that the application of this element generates positive effects on the growth of various plants (Medrano-Macías et al., 2016). Small amounts of this element, in the range of microelement concentrations required by plants, have improved plant growth, development and productivity (Cakmak et al., 2017; Lyons, 2018; Duborská et al., 2020). This positive impact has been observed from modulation in gene expression to being a structural part of several proteins (Kiferle et al., 2021), as well as the increase in the concentration of essential minerals in the leaves (Cortés-Flores et al., 2016; Sularz et al., 2020).
Similarly, applications of I have shown diverse effects on antioxidant capacity in several species; this antioxidant capacity varies depending on sources, concentration and type of application (Halka et al., 2020; Sabatino et al., 2021). Contrary to the above, in some species, high levels of I in the nutrient solution have caused a marked reduction in leaf expansion and photosynthetic activity, which was attributed to a large accumulation of this element in leaf tissues (Incrocci et al., 2020).
On the other hand, low concentrations of I have improved the response to salinity stress, particularly caused by sodium chloride (NaCl). The effect of the application of I decreased the concentration of toxic ions such as Na+ and Cl-; in addition to increasing the concentration of soluble sugars (sucrose, glucose and fructose) that participate as osmoprotectants during osmotic adjustment, as well as causing the activation of antioxidant enzymes such as superoxide dismutase (SOD) and ascorbate peroxidase (APX), and the non-enzymatic antioxidant system in which phenolic compounds are found, in order to maintain reactive oxygen species (ROS), such as O2 and H2O2, at minimum levels (Leyva et al., 2011; Gonzali et al., 2017; Kiferle et al., 2019).
For these reasons, I can be considered as a beneficial element to help counteract the harmful effects of salinity stress, but it is necessary to investigate in a specific manner the impact on the various species and stages of development (Leyva et al., 2011; Pérez-Salas and Medrano-Macías, 2021). Considering the above, the objective of this experiment was to evaluate the impact of the application of iodine on the concentration of phenolic compounds, antioxidant capacity and photosynthetic pigments in leaves of three fig cultivars subjected to salt stress.
Materials and methods
Location of the experiment and plant material
The research was carried out at the Center for Protected Agriculture of the Faculty of Agronomy of the Autonomous University of Nuevo León, Mexico, inside a Spanish-type greenhouse. The average environmental conditions inside the greenhouse were 25 °C temperature and 70% relative humidity. Eight-month-old fig plants, with six leaves each, were transplanted into black polyethylene bags of a capacity of 10 L filled with perlite substrate. The experiment was established under a completely randomized experimental design with a 3x2x2 factorial arrangement: three fig cultivars (Ficus carica L.): Brown Turkey (BT), Kadota (K) and Black Mission (BM); two levels of NaCl (0 and 100 mmol L-1) and two levels of I (0 and 10 mg L-1) using potassium iodate (KI) as a source of iodine. Both factors, NaCl and I were applied by gravity irrigation.
The base irrigation water had the following characteristics: Ca: 3.5 Mg, 1.8, Na: 0.9, HCO3: 2.5, Cl: 1.35, SO4: 2.35; HCO3: 2.5 mEq L-1; pH: 7.5 and electrical conductivity (EC): 0.6 dS m-1. From the combination of the aforementioned factors, 12 treatments emerged, with six repetitions each. Each plant was considered as an experimental unit. The relative chlorophyll content, expressed in SPAD units, was measured using the portable SPAD-502 of Minolta (Konica Minolta, Osaka, Japan). SPAD units were measured in the middle lobe of leaves number 4, 5 and 6 from the base to the apex.
The values of this variable are the average of three measurements made 5, 10 and 15 days after the beginning of treatments. Also, after the last measurement, samples of four plants per treatment were collected for the determination of dry weight of new leaves, antioxidant compounds and pigments. The dry weight was obtained by means of a drying oven (Yamato model DX602C, Yamato Scientific America, Santa Clara, CA, USA) where the samples were subjected to a temperature of 60 °C for three days. The resulting material was weighed with an analytical balance with precision of 0.001 g.
Extraction of phenolic compounds
The extraction of phenolic compounds was carried out based on what was reported by Carballo-Méndez et al. (2019) with slight modifications. Four grams of fresh leaf tissue were suspended in 40 mL of water:methanol (20:80 v/v) (80%) and homogenized with an Oster blender (M4655-813/465-42, Sunbeam, Mexico City, Mexico) in a crystal glass for porridge for 30 s. It was then filtered with an organza fabric, placed in 50 ml Corning tubes and centrifuged at 4 500 rpm for 5 min at 25 °C. The supernatant was recovered and stored protected from light at -20 °C, until its further analysis.
Total phenols and flavonoids
The content of total phenols and total flavonoids was evaluated based on what was reported by Rodríguez-Salinas et al. (2020), with the following modifications: the total phenols were evaluated based on the colorimetric method of the Follin-Ciocalteu colorimetric reaction, with 200 μl of the methanolic extract in 2 600 μl of water, followed by the addition of 200 μl and alkalinization by adding 2 000 μl of Na2CO3, then it was left to react for 90 min and the absorbance was measured in an SP-830 Plus spectrophotometer (Barnstead, Turner, USA) at a wavelength of 750 nm.
The standard curve was prepared with gallic acid in concentrations from 0 to 200 mg L-1, 80% methanol was used as a blank. The results were expressed in milligrams of gallic acid equivalents per gram of fresh tissue (mgGAE g-1). The total flavonoids were measured by the method of aluminum chloride (AlCl3) and sodium hydroxide (NaOH), briefly, 200 μl of the methanolic extract was added to 3 500 μl of water, followed by 150 μl of a 5% solution of NaNO2 and it was left to stand for 5 min, then 150 μl of a 10% solution of AlCl3 was added and finally 5 min later, 1 000 μl of NaOH at 1 N was added.
It was left to react for 15 min and the absorbance of the samples was measured in an SP-830 Plus spectrophotometer at the length of 510 nm. Concentrations were calculated based on a catechin curve made at concentrations from 0 to 200 mg L-1. The results were expressed in milligrams of catechin equivalents per gram of fresh tissue (mg CE g-1).
DPPH antioxidant capacity
The determination of antioxidant capacity by the method of 2,2-diphenyl-1-picrylhydrazyl (DPPH) of the methanolic extracts of the fresh fig leaf tissue was carried out based on what was described by Rodríguez-Salinas et al. (2020), with slight modifications described below: DPPH, it was evaluated using the working stock solution prepared at 60 μM dissolved in 80% methanol, adjusted to an absorbance at 1 nm at a wavelength of 517 nm. The reaction was performed by adding 0.5 μl of the methanolic extract to 1.5 ml of the stock solution, it was left to stand for 30 min and the absorbance was measured on an SP-830 Plus spectrophotometer at a wavelength of 517 nm. As a standard curve, Trolox was used in concentrations from 0 to 500 μmol L-1. Results were expressed in Trolox equivalents per each gram of fresh tissue (μmol TE g-1).
The IC50 of DPPH was calculated for the amount of antioxidant needed to inhibit 50% of the oxidation of the radical, the absorbance adjusted to 1 in the working solutions was considered as 100% of oxidation and methanol was used as a control. The results were expressed in milligrams per gram of fresh leaf tissue (mg g-1).
Photosynthetic pigments
The photosynthetic pigments chlorophylls (a, b and total) and total carotenoids were extracted and calculated based on what was reported by Rajput and Patil (2017), with slight modifications. Briefly, on average 4 g of fresh leaf tissue was homogenized in an Oster blender with a crystal glass for porridge for 30 sec. in the presence of 40 ml of water:acetone (20:80 v/v) (80%) that contained 200 mg of Na2CO3. The homogenized was then passed through an organza fabric, placed in 50 ml capacity corning tubes and centrifuged at 6 000 rpm for 5 min at room temperature. The supernatant was recovered and stored protected from light at -20 °C, until its subsequent analysis by spectrophotometry.
Chlorophylls and total carotenoids were quantified in an SP-830 Plus spectrophotometer at wavelengths of 663-645 and 480-510 nm, respectively. The results were expressed in milligrams per gram of fresh tissue (mg g-1).
;
;
.
Where: A= absorbance obtained at the specified wavelength; V= volume of extraction; and SW= sample weight.
Statistical analysis
The values obtained were analyzed with the Levene test for homogeneity of variances and with the Kolmogorov-Smirnov test for the verification of normality. For the analysis of variance of the variables, the relative chlorophyll content covariate was considered, which was evaluated one day before the start of the treatments. In the variables where a significant difference was found, a comparison of means was made using the Tukey test (p< 0.05). For these analyses, the statistical package SPSS version 22.0, IBM, was used.
Results and discussion
The analysis of variance showed that the main factors (cultivars, NaCl levels, I levels) and the interaction between them was different in the variables evaluated (Table 1).
Table 1 F values and significance levels (P) observed in analyses of variance.
| Variables | Cultivar (C) | NaCl (S) | Iodine (I) | C*S | C*I | S*I | C*S*I | |
| Relative chlorophyll content | F | 14.271 | 0.022 | 17.485 | 2.021 | 1.992 | 3.896 | 7.734 |
| p | <0.001 | ns | <0.001 | ns | ns | ns | 0.003 | |
| Number of new leaves | F | 4.878 | 9.936 | 7.288 | 0.798 | 1.153 | 4.851 | 0.165 |
| p | 0.017 | 0.004 | 0.013 | ns | ns | 0.038 | ns | |
| Dry weight of new leaves | F | 0.425 | 16.245 | 15.845 | 0.37 | 0.496 | 4.567 | 0.099 |
| p | ns | 0.001 | 0.001 | ns | ns | 0.043 | ns | |
| Total phenols | F | 9.275 | 0.241 | 4.588 | 0.163 | 1.197 | 21.886 | 8.205 |
| p | 0.001 | ns | 0.043 | ns | ns | <0.001 | 0.002 | |
| Total flavonoids | F | 65.69 | 0.891 | 0.611 | 0.573 | 1.556 | 2.435 | 1.821 |
| p | <0.001 | ns | ns | ns | ns | ns | ns | |
| DPPH | F | 4.497 | 3.566 | 3.501 | 0.846 | 1.027 | 2.312 | 4.028 |
| p | 0.022 | ns | ns | ns | ns | ns | 0.032 | |
| IC50 | F | 5.544 | 3.068 | 4.905 | 0.401 | 6.231 | 2.146 | 0.677 |
| p | 0.011 | ns | 0.037 | ns | 0.007 | ns | ns | |
| Chlorophyll a | F | 20.8 | 23.283 | 7.974 | 0.657 | 2.656 | 0.198 | 2.377 |
| p | <0.001 | <0.001 | 0.01 | ns | ns | ns | ns | |
| Chlorophyll b | F | 47.641 | 68.021 | 11.578 | 19.475 | 2.202 | 0.157 | 3.927 |
| p | <0.001 | <0.001 | 0.002 | <0.001 | ns | ns | 0.034 | |
| Carotenoids | F | 14.557 | 23.262 | 1.079 | 7.702 | 0.695 | 0.005 | 1.275 |
| p | <0.001 | <0.001 | ns | 0.003 | ns | ns | ns |
ns= not significant.
Relative chlorophyll content and growth
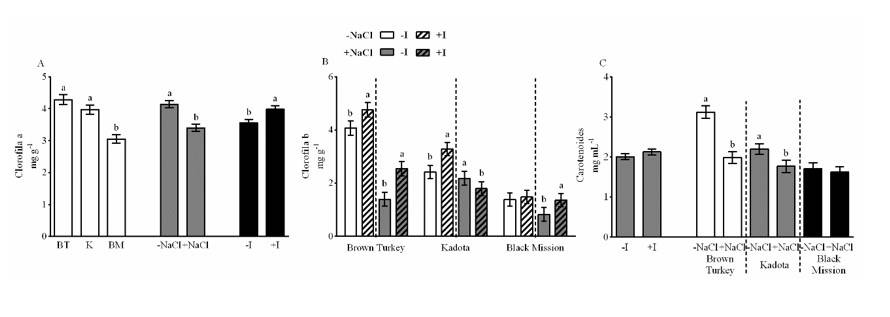
In the SPAD units, there was a triple interaction between cultivars, NaCl level and presence of I. In the BT cultivar, I reduced the SPAD units by 3% under conditions of low salinity, while in the condition of salinity, it increased them by 6%. For the K cultivar, the behavior was similar in both NaCl conditions, increasing these units by 11 and 3% under conditions of low and high salinity, respectively. In the BM cultivar, the effect of I was only present at the high level of salinity, with an increase of 13% (Figure 1A). These results found in fig leaves are similar to those reported by Cortes et al. (2016), who recorded that the presence of I increased by up to 5% the concentration of nitrogen related to the SPAD units, in leaves of bell pepper (Capsicum annum L.).
The number of new leaves showed a difference between the cultivars, with BT being the one with the highest quantity. For this same variable, there was an interaction between the level of NaCl and I, where the presence of I increased the number of leaves in the solution with NaCl by 204% (Figure 1B). The dry leaf weight only showed interaction between NaCl and I level, where I increased the dry leaf weight by 273% in the presence of NaCl (Figure 1C). Like our results, Salimpour et al. (2019) reported that different cultivars of fig show differences in leaf growth, as a consequence of genetic diversity. Kiferle et al. (2019) reported that, in non-saline conditions, the presence of I did not increase the dry matter in basil (Ocimum basilicum L.), while Leyva et al. (2011); Blasco et al. (2013) reported that, under salinity conditions, the presence of I increased the aerial biomass of lettuce (Lactuca sativa L.), which was attributed to the reduction of Na and Cl and the increase in the K/Na ratio.
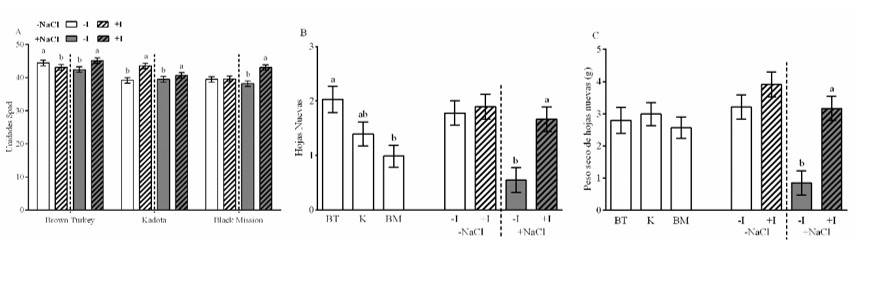
Figure 1 Effect of cultivars, NaCl and I, the relative chlorophyll content (SPAD) (A); on the number of new leaves (B); and dry weight of new leaves (C). The lines on the bars represent the standard error. The different letters on each bar mean that the treatments were statistically different (Tukey, p< 0.05).
Phenolic compounds and antioxidant capacity
The concentration of phenolic compounds was affected by the interaction of the different factors. The BR and K cultivars reduced the concentration of total phenols by 26 and 45% in the absence of NaCl, while with the presence of NaCl, these compounds increased by 25 and 17%, respectively. Contrary to the above, in the BM cultivar, the presence of I in saline situation reduced total phenols by 9% (Figure 2A). The concentration of total flavonoids was only affected by cultivars, where the BT cultivar exceeded K and BM; in general, 150% (Figure 2B). The antioxidant activity by DPPH showed interaction between the three factors. In the BT cultivar, the presence of I in the solution without NaCl decreased this activity by 11%, while in the presence of NaCl, I increased it by 455%. In the BM cultivar without the presence of NaCl, the presence of I increased this activity by 22% (Figure 2C).
The IC50 was only affected by the interaction of cultivars and I, particularly in the BM cultivar where the presence of this element reduced this concentration by 21% (Figure 2D). The increase in antioxidant capacity by the DPPH assay of plants reveals an increase in the synthesis of low molecular weight reducing molecules, mainly non-enzymatic antioxidants (Pérez-Salas and Medrano-Macías, 2021), such as phenolics (Leyva et al., 2011). Because DPPH bases its foundation on the ability to measure compounds with the ability to donate H atoms (Schaich et al., 2015).
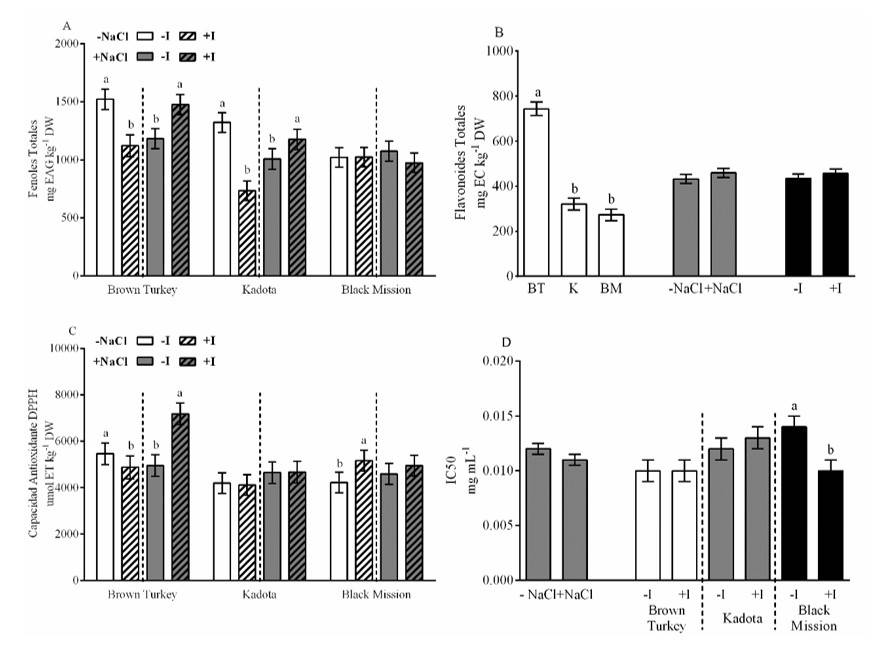
Figure 2 Effect of cultivars, NaCl and I on the concentration of total phenols (A); total flavonoids (B); DPPH antioxidant activity (C); and mean inhibitory concentration (IC50). The lines on the bars represent the standard error. The different letters on each bar mean that the treatments were statistically different (Tukey, p< 0.05).
Several authors have reported that applications of I increase the antioxidant capacity of plant tissues. Kiferle et al. (2019) mention that I increased the total phenols in basil leaves, as well as Blasco et al. (2013), who reported that, in lettuce subjected to salt stress, I increased the concentration of total phenols, without finding differences in the concentration of flavonoids. These results are consistent with what was observed in this experiment. Contrary to the above, Golubkina et al. (2018) reported that, in mustard (Brassica juncea L.) leaves, the application of I increased the concentration of flavonoids.
Similarly, Cortés-Flores et al. (2016) reported that foliar application of KI increased DPPH antioxidant activity in pepper seedlings under optimal development conditions, while Kiferle et al. (2019) reported the decrease in this activity in basil plants. In the present experiment, this effect was different between cultivars and salinity level. In non-saline conditions, the presence of iodine decreased the DPPH antioxidant activity in the BT cultivar, while it increased it in the BM cultivar. In saline situation, the presence of I only benefited the BT cultivar. Particularly for IC50, Incrocci et al. (2020) reported that, in basil, the presence of I reduced the IC50, although the impact was different between cultivars, a situation very similar to that shown in this experiment.
Photosynthetic pigments
The concentration of chlorophyll a showed main effects of the three factors. The BT and K cultivars exceeded BM by 35%. The presence of NaCl reduced these compounds by 18%, while the presence of I increased them by 12% (Figure 3A). The concentration of chlorophyll b was affected by the interaction of the three factors. In the BT cultivar, the presence of I increased them, the concentration of chlorophyll b by 17% without the presence of NaCl and by 83% in the presence of said salt. In the K cultivar, the effect of I was different since, in the absence of NaCl, chlorophyll b increased 36%, while, in the presence of salt, these compounds decreased by 17%.
For the BM cultivar, the presence of I in salinity condition increased these pigments by 65% (Figure 3B). The carotenoid concentration was only affected by the interaction between the cultivar and NaCl. In BT and K cultivars, the presence of NaCl reduced carotenoid concentration by 36% and 20%, respectively (Figure 3C). The results shown above differ partially from those reported by Pérez-Salas and Medrano-Macías (2021), who point out that, in tomato (Solanum lycopersicum L.) plants subjected to salt stress, the presence of I did not modify the concentration of chlorophyll a, but it did increase the concentration of chlorophyll b.
Conclusions
The application of 10 mg L-1 of iodine in fig seedlings increased, in general terms, the relative content of chlorophyll, number and dry weight of new leaves, as well as the antioxidant activity and the concentration of chlorophylls, under salt stress condition. Therefore, iodine can be considered as an alternative to mitigate the stress caused by NaCl in Ficus carica L. However, in particular it is necessary to consider that there are differences in the impact of iodine between cultivars, so it is necessary to know the effect of the application of this element in species and varieties of specific interest.
Literatura citada
Blasco, B.; Leyva, R.; Romero, L. and Ruiz, J. M. 2013. Iodine effects on phenolic metabolism in lettuce plants under salt stress. J. Agric. Food Chem. 61(11):2591-2596. [ Links ]
Cakmak, I.; Prom-u-thai, C.; Guilherme, L. R. G.; Rashid, A.; Hora, K. H.; Yazici, A.; Savasli, E.; Kalayci, M.; Tutus, Y.; Phuphong, P.; Rizwan, M.; Martins, F. A. D.; Dinali, G. S. and Ozturk, L. 2017. Iodine biofortification of wheat, rice and maize through fertilizer strategy. Plant and Soil. 418(1):319-335. [ Links ]
Carballo, M. F. J.; Olivares, S. E.; Bolivar, D. M.; Antonio, B. A.; Vázquez, B. M. E. and Nino, M. G. 2019. Effect of silicon on germination of Moringa oleifera Lam. in different types of salts. Fresenius Environmental Bulletin. 28(11):8823-8830. [ Links ]
Cortés, F. C.; Rodríguez, M. M. N.; Benavides, M. A.; García, C. J. L.; Tornero, C. M. y Sánchez, G. P. 2016. El yodo aumenta el crecimiento y la concentración de minerales en plántulas de pimiento morrón. Agrociencia. 50(6):747-758. [ Links ]
Duborská, E.; Urík, M. and Šeda, M. 2020. Iodine Biofortification of Vegetables Could Improve Iodine Supplementation Status. Agronomy. 10(10):1574. [ Links ]
Golubkina, N.; Kekina, H. and Caruso, G. 2018. Yield, quality, and antioxidant properties of Indian mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants. 7(4):80-89. [ Links ]
Gonzali, S.; Kiferle, C. and Perata, P. 2017. Iodine biofortification of crops: agronomic biofortification, metabolic engineering and iodine bioavailability. Current Opinion in Biotechnol. 44:16-26. [ Links ]
Halka, M.; Smoleń, S. and Ledwożyw, S. I. 2020. Antioxidant potential and iodine accumulation in tomato (Solanum lycopersicum L.) seedlings as the effect of the application of three different iodobenzoates. Folia Hortic. 32(2):203-219. [ Links ]
Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Kiferle, C.; Perata, P. and Pardossi, A. 2019. Iodine accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves grown in floating system technique. Front. Plant Sci. 10:1494. Doi: 10.3389/fpls.2019.01494. [ Links ]
Kiferle, C.; Ascrizzi, R.; Martinelli, M.; Gonzali, S.; Mariotti, L.; Pistelli, L.; Flamini, G. and Perata, P. 2019. Effect of Iodine treatments on Ocimum basilicum L.: biofortification, phenolics production and essential oil composition. PLoS ONE. 14(12):0226559. Doi:10.1371/journal.pone.0226559. [ Links ]
Kiferle, C.; Martinelli, M.; Salzano, A. M.; Gonzali, S.; Beltrami, S.; Salvadori, P. A.; Hora, K.; Holwerda, H. T.; Scaloni, A. and Perata, P. 2021. Evidence for a nutritional role of iodine in plants. front. Plant Sci. 12:616868. Doi: 10.3389/fpls.2021.616868. [ Links ]
Leyva, R.; Sánchez, R. E.; Ríos, J. J.; Rubio, W. M. M.; Romero, L.; Ruiz, J. M. and Blasco, B. 2011. Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci. 181(2):195-202. [ Links ]
Lyons, G. 2018. Biofortification of cereals with foliar selenium and iodine could reduce hypothyroidism. Front. Plant Sci. 9:730. Doi:10.3389/fpls.2018.00730. [ Links ]
Medrano, M. J.; Leija, M. P.; González, M. S.; Juárez, M. A. and Benavides, M. A. 2016. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 7:1146. Doi:10.3389/fpls.2016.01146. [ Links ]
Pérez, S. S. and Medrano, M. J. 2021. Uso del yodo como inductor a la tolerancia en plántulas de tomate bajo condiciones de estrés por salinidad. Rev. Científica de la Universidad Autónoma de Coahuila. 15(25):14-22. [ Links ]
Rajput, R. D. and Patil, R. P. 2017. The comparative study on spectrophotometric analysis of chlorophyll and carotenoids pigments from non-leguminous fodder crops. Inter. J. Innov. Sci. Eng.Technol. 7:140-148. [ Links ]
Rodríguez, S. P. A.; Zavala, G. F.; Urías, O. V.; Muy, R. D.; Heredia, J. B. and Niño, M. G. 2020. Chromatic, nutritional and nutraceutical properties of pigmented native maize (Zea mays L.) genotypes from the Northeast of Mexico. Arabian J. Sci. Eng. 45(1):95-112. [ Links ]
Sabatino, L.; Di, G. F.; Consentino, B. B.; Rouphael, Y.; El-Nakhel, C.; Bella, S.; Vasto, S.; Mauro, R. P.; D’Anna, F.; Iapichino, G.; Calderella, R. and Pasquale, C. 2021. Iodine biofortification counters micronutrient deficiency and improve functional quality of open field grown curly endive. Horticulturae. 7:58. Doi:10.3390/horticulturae7030058. [ Links ]
Salimpour, A.; Shamili, M.; Dadkhodaie, A.; Zare, H. and Hadadinejad, M. 2019. Evaluating the salt tolerance of seven fig cultivars (Ficus carica L.). Adv. Hortic. Sci. 33(4):553-565. [ Links ]
Schaich, K. M.; Tian, X. and Xie, J. 2015. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Functional Foods. 14:111-125. [ Links ]
Sularz, O.; Smoleń, S.; Koronowicz, A.; Kowalska, I. and Leszczyńska, T. 2020. Chemical Composition of Lettuce (Lactuca sativa L.) Biofortified with Iodine by KIO3, 5-Iodo-, and 3.5-Diiodosalicylic Acid in a Hydroponic Cultivation. Agronomy . 10(7):1022-1029. [ Links ]
Received: May 01, 2022; Accepted: August 01, 2022











 texto em
texto em