Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.13 no.spe28 Texcoco Set./Out. 2022 Epub 13-Jan-2023
https://doi.org/10.29312/remexca.v13i28.3284
Articles
Chemical synthesis of zinc oxide nanoparticles and their evaluation in Lactuca sativa seedlings
1Doctorado en Ciencias en Agua y Suelo-Tecnológico Nacional de México-Campus Instituto Tecnológico de Torreón. Carretera Torreón-San Pedro km 7.5, ejido Ana, Torreón, Coahuila, México. CP. 27170. (galindo@live.com; fortismanuel@hotmail.com; veronicadelarosa24@gmail.com; zermegon@yahoo.com.mx).
2Universidad Politécnica de la Región Laguna. Calle sin nombre, sin número, ejido Santa Teresa, San Pedro de las Colonias, Coahuila, México. CP. 27942.
There are currently studies on the different effects of nanomaterials in agriculture to improve crop germination and productivity, in order to ensure economic sustainability and the efficient use of production resources in agriculture. The ZnO nanoparticles applied in this study were synthesized by a chemical precipitation method and their characterization was performed by XRD, SEM, UV-visible spectroscopy and FTIR. The effect on the germination of lettuce (Lactuca sativa) seeds was determined by means of a completely randomized design with five ZnO-NPs treatments and a control treatment each with four repetitions. Physiological indices were measured, chlorophyll and carotenoid contents, and phenolic compound content in lettuce seedlings were quantified. The results indicate that applying doses of 50 mg L-1 ZnO-NPs, higher values of germination percentage (36.97%), fresh weight of plumule (23.91%), fresh weight of radicle (63.25%) and radicle length (50.58%) were achieved compared to the control groups. Likewise, the total phenol content increased (207.9%). Doses greater than 125 mg L-1 ZnO-NPs decrease the chlorophyll content, causing phytotoxic effects on L. sativa seedlings. As for the carotenoid content, the best treatment was 100 mg L-1 ZnO-NPs. The use of ZnO-NPs synthesized through a chemical precipitation method is a good alternative to be used as inducers in the biosynthesis of bioactive compounds in lettuce seedlings.
Keywords: Lactuca sativa; nanotechnology; toxicity
En la actualidad existen investigaciones sobre los diferentes efectos de nanomateriales en la agricultura para mejorar la germinación y la productividad de los cultivos, con la finalidad de garantizar la sostenibilidad económica y el uso eficiente de los recursos de producción en la agricultura. Las nanopartículas de ZnO aplicadas en este estudio fueron sintetizadas por un método de precipitación química y su caracterización se realizó por (XRD), (SEM), espectroscopía UV-visible y (FTIR). Se determinó el efecto sobre la germinación de semillas de lechuga (Lactuca sativa) por medio de un diseño completamente al azar con cinco tratamientos de NPs-ZnO y un tratamiento control cada uno con cuatro repeticiones. Se midieron índices fisiológicos, se cuantificó el contenido de clorofila y carotenoides, y el contenido de compuestos fenólicos en las plántulas de lechuga. Los resultados indican que aplicando dosis de 50 mg L-1 NPs-ZnO, se lograron mayores valores del porcentaje de germinación (36.97%), peso fresco de plúmula (23.91%), peso fresco de radícula (63.25%) y longitud de radícula (50.58%) respecto a los grupos control. Asimismo, se incrementó el contenido de fenoles totales (207.9%). Dosis superiores a 125 mg L-1 NPs-ZnO disminuyen el contenido de clorofila, causando efectos fitotóxicos en las plántulas de L. sativa. En cuanto al contenido de carotenoides el mejor tratamiento fue de 100 mg L-1 NPs-ZnO. El uso de NPs-ZnO sintetizadas a través de un método de precipitación química es una buena alternativa para ser utilizadas como inductores en la biosíntesis de compuestos bioactivos en plántulas de lechuga.
Palabras clave: Lactuca sativa; nanotecnología; toxicidad
Introduction
Nanotechnology has gained great attention over time and promotes the application of nanoparticles in a wide range of fields of the agricultural industry. Nanoparticles (NPs) have an average size <100 nm and are composed of carbon, metal, metal oxides or organic matter (Hasan, 2015). They can be synthesized by methods, such as the sol-gel method, chemical vapor deposition, precipitation, thermal decomposition or hydrothermal synthesis (Salama et al., 2019).
The size of these materials allows them to manifest physical, chemical and biological properties different from those of the same material with larger particle sizes, mainly due to two reasons: nanoparticles have a greater specific surface area per unit volume and therefore greater reactivity (Ealia and Saravanakumar, 2017).
Zinc oxide nanoparticles (ZnO-NPs), due to their opto-electrical, physical and antimicrobial properties, have positive effects on plants (Faizan et al., 2020). Several studies suggest that ZnO-NPs have the ability to enhance growth in different plant species. In seed germination, it increases with the application of ZnO-NPs in low concentrations; however, in high concentrations, there is a negative effect on germination (Raskar and Laware, 2014; Afrayeem and Chaurasia, 2017). For example, Lin and Xing (2007) found that the application of high doses of ZnO-NPs >2 000 mg L-1 inhibited the germination of Lolium perenne seeds. Dhoke et al. (2013) observed that ZnO-NPs, as a micronutrient fertilizer, improved the growth of seedlings of Vigna radiata and Cicer arietinum at low concentrations in ranges of 1-20 mg L-1.
Lettuce (Lactuca sativa) belongs to the Asteraceae family and is one of the main crops in the world, considered to be the most important leafy vegetable and known as the most common fresh cut vegetable (Abdalla et al., 2021). Based on the above, the objective of this study was to synthesize and characterize ZnO nanoparticles by a conventional method of controlled chemical precipitation, as well as to evaluate the effect of these nanoparticles on the germination of lettuce (Lactuca sativa) seeds.
Materials and methods
The present study was developed at the Polytechnic University of the Laguna Region, Mexico. Located at 25° 46’ 59.8” north latitude, 103° 11’ 30.1” west longitude.
Synthesis of ZnO nanoparticles
They were synthesized by a modification of the method described by Aquino et al. (2018). Zinc acetate Zn (CH3COO)2 was used as a precursor and the nanoparticle production consisted of controlled precipitation with NaOH. NaOH 0.1 M was dissolved in 250 ml under constant stirring and at a temperature of 70 °C on a Labnet hot plate with magnetic stirring. After obtaining the desired temperature, 25 ml of Zn (CH3COO)2 0.5 M were added drop by drop with heating and it was stirred for 2 h. A white solution was obtained. This compound was filtered and washed several times with deionized water to remove any impurities. It was dried on an oven at a temperature of 70 °C for 24 h. The sample was calcinated at a temperature of 500 °C for 3 h in a Novatech MD-12-ESP muffle. The resulting white powder was crushed with a mortar to obtain ZnO-NPs.
Characterization of ZnO nanoparticles
The synthesized ZnO-NPs were characterized by various analytical techniques that include X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-visible spectroscopy, and Fourier transformed infrared (FTIR) spectroscopy. XRD analysis was carried out on a PANalytical Empyrean X-ray diffractometer, with copper Kα radiation with wavelength, λ= 1.5406Å for the purpose of knowing the purity of the sample. The goniometer was operated from 5° to 90° in 2θ, and the sweep speed was 30 seconds with a step of 0.026º. The phases present were identified using ICSD (Inorganic Crystal Structure DataBase) diffraction charts and HighScore Plus software.
The images for the determination of the surface morphology were made with a TESCAN MIRA scanning electron microscope with FEG field emission, the sample was coated with gold-palladium by sputtering. The optical properties of the sample were studied with a Jenway 7305 UV-visible spectrophotometer, using water as a reference solvent. The FTIR spectrum was recorded with a Thermo Scientific Nicolet IR 100 apparatus in an interval of 4 000-400 cm using the KBr tablet technique.
Seed germination test
The tests were performed using seeds of red sangria lettuce (Lactuca sativa) from the company Rancho Los Molinos. The seeds were disinfected with 75% ethanol for 5 min and washed with deionized water (Li et al., 2019). Germination tests were performed by placing 10 seeds per each 90 mm diameter Petri dish, which contained filter paper moistened with 5 ml of each prepared solution of ZnO-NPs.
The treatments were divided into: (control) deionized water, 25, 50, 75, 100 and 125 mg L-1 of ZnO-NPs to test how different concentrations can affect the viability of the seed. Four replications were prepared for each sample, respectively. The Petri dishes were sealed with Parafilm paper and placed in a Novatech CA-550 artificial growth chamber at 25 ±2 °C with a day/night cycle of 12 h for a period of 7 days (Liu et al., 2016).
Measurement of physiological indices
Physiological indices were measured, which include seed vigor (%), germination percentage (%), radicle length (mm), fresh weight of plumule and radicle (mg) (Li et al., 2019). The fresh weight of plumule and radicle were weighed on an AND HR-250AZ analytical balance and it was reported in milligrams per plumule.
Percentage of germination. After 7 days, the total number of germinated seeds was recorded, and the result was expressed as shown in the following equation:
1).
Seed vigor. On the fourth day after sowing, the first count was made for the collection of data on germinated seeds (seedlings that have well-developed radicle and plumule, with total development of 2 cm on average) (Ramírez-Rodríguez et al., 2021). To determine seed vigor, expressing the result in percentage according to the equation:
2).
Determination of chlorophyll (Chl) and carotenoid contents
Chlorophyll a (Chla) and b (Chlb) and carotenoids (Car) were determined after the appearance of 3 to 4 true leaves at 20 days of the development of seedlings by the method of Lichtenthaler and Wellburn (1983), suspending 0.5 g of fresh sample in 10 ml of 95% ethyl alcohol. The homogenate was centrifuged at 1500 × g for 20 min and the supernatant was collected. Absorbance readings were then recorded at 665, 649 and 470 nm on a Jenway 7305 UV-visible spectrophotometer. The content was reported according to the equations (Lichtenthaler and Wellburn, 1983): Chla= 13.95 A665 - 6.88 A649 3); Chlb= 24.96 A649 -7.32 A665 4); Car= (1 000 A470 - 2.05 Chla -114.8 Chlb)/245 5).
Total phenol content
Two grams of fresh sample (true leaves) were mixed in 10 ml of 80% ethanol, and were left in constant stirring for 24 h at 70 rpm and 5 °C. The extract was centrifuged at 3 000 rpm for 5 min and the supernatant was extracted for the analyses, Salas-Pérez et al. (2016). Total phenolic compounds were quantified by the Folin-Ciocalteu method (Singleton et al. (1999). Results were reported in mg of gallic acid equivalent per 100 g of fresh weight (mg GA equiv 100 g-1 FW).
Statistical analysis
The experiment was carried out by means of a completely randomized design with six treatments and four repetitions. The normality and homogeneity of variances of the data for each response variable were verified with the Bartlett and Kolmogorov-Smirnov tests. The results obtained were analyzed by analysis of variance and comparison of means with the Tukey test (p≤ 0.05) using the statistical package Statistical Analysis System Institute (SAS) version 9.4. The variables that are reported in percentage (vigor and germination) were normalized by applying the transformation of arcsine and square root.
Results and discussion
Synthesis of ZnO nanoparticles
The synthesis of ZnO-NPs was carried out following the precipitation mechanism. Zn(CH3COO)2 can be converted into colloidal Zn(OH)2 under an alkaline solution (pH= 13-14). During the hydrothermal process, part of the colloidal Zn(OH)2 dissociates to Zn2+ and OH-. When the concentration of Zn2+ and OH- reach the degree of super saturation, nuclei of ZnO are formed (Aquino et al., 2018). Calcination at 500 °C favors the formation of ZnO, in addition to the crystallization and growth of nanoparticles.
Characterization of ZnO nanoparticles
According to the diffractogram obtained by X-ray diffraction (Figure 1a), no impurities were detected in this pattern, the sample contains only zinc oxide in its zincite phase, whose structure is hexagonal (ICSD, 98-005-7478), which implies that pure ZnO-NPs were obtained.
The SEM micrographs of the ZnO-NPs (Figure 1b) appear very uniformly, showing a hemispherical and polygonal morphology compatible with the crystalline nature of ZnO, whose size ranges between 67.37 and 71.1 nm. The optical properties of the ZnO-NPs studied by UV-visible spectroscopy (Figure 1c) show a peak of absorption centered at 378 nm, characteristic of the hexagonal structure of ZnO. The synthesized ZnO-NPs subjected to FTIR (Figure 1d) indicate the characteristic peaks of the functional group present, it is inferred that the first band is observed from 400 to 500 cm-1 due to the vibrations of the Zn-O bond.
Effect of ZnO-NPs on seed germination
Physiological indices
Table 1 shows the effect of ZnO-NPs exposure on germination of L. sativa seeds. The application of ZnO-NPs had significant effects between treatments and the control (p≤ 0.05). In the parameter of vigor, we can see that positive results were obtained when applying concentrations of 25 mg L-1. While applying 50 mg L-1 showed a decrease of 9.32% compared to the control, this small reduction can be considered as a growth inhibiting effect related to a high osmotic potential due to the doses of NPs (Hojjat et al., 2017; Tovar-Jimenez et al., 2020). In the percentage of germination, the treatments increased with respect to the control, except for the treatment of 125 mg L-1, which showed no significant difference. The best treatment was 50 mg L-1, increasing up to 36.97%. As for the fresh weight of the plumule and the fresh weight of the radicle, it increased significantly compared to the other treatments/control group, with the dose of 50 mg L-1.
Table 1 Comparison of means in germination, vigor, fresh weight of plumule, radicle and radicle length when ZnO-NPs are applied.
| ZnO-NPs (mg L-1) | Vigor (%) | Germination (%) | Fresh weight of plumule (mg) | Fresh weight of radicle (mg) | Radicle length (mm) |
| Control | 46.44 ±2.88 cd | 50.76 ±0 c | 9.2 ±0.38 cd | 1.66 ±0.29 c | 21.25 ±0.5 d |
| 25 | 60.11 ±3.83 a | 60.11 ±3.83 b | 8.32 ±0.3 d | 1.16 ±0.06 d | 25.5 ±1 b |
| 50 | 42.11 ±3.33 d | 69.53 ±4.06 a | 11.4 ±0.56 a | 2.71 ±0.09 a | 32 ±0.81 a |
| 75 | 52.27 ±3.01 bc | 65.46 ±4.06 ab | 9.78 ±0.14 bc | 2.10 ±0.11 b | 25 ±0.81 b |
| 100 | 53.77 ±3.47 ab | 65.46 ±4.06 ab | 9.79 ±0.52 bc | 1.23 ±0.15 d | 23.25 ±0.5 c |
| 125 | 46.44 ±2.88 cd | 50.76 ±0 c | 10.22 ±0.4 b | 1.29 ±0.27 cd | 10.25 ±0.5 e |
Values with different letters within the same column indicate significant differences according to the Tukey test (p≤ 0.05). The values are the average of four repetitions. Means (n= 24) ± standard deviation.
Such an effect could be attributed to an increase in the level of Zn within lettuce seeds and its interaction in biochemical processes (Rawashdeh et al., 2020). In the length of the radicle, there was also a significant difference, and the best treatment was 50 mg L-1 compared to the treatments/control group. However, due to the shape, size, surface charge, chemical composition and concentration of nanoparticles, they can cause different impacts on seed germination and seedling growth (Szőllősi et al., 2020).
The results obtained in this study indicate that the best treatment of application of ZnO-NPs was 50 mg L-1, compared to other authors who indicate a positive response when applying 25 mg L-1 ZnO-NPs on germination and biomass in L. sativa seeds, reported by Rawashdeh et al. (2020). NP-soaked lettuce seeds may show accelerated growth compared to untreated control seeds. Once the ions are inside the seeds, they modulate protein synthesis and metabolism, carbohydrate content, and increased antioxidant enzyme activity (Rawashdeh et al., 2020).
Chlorophyll and carotenoids
The chlorophyll content, including Chla and Chlb, of the seedlings treated with ZnO-NPs was quantified as shown in Figure 2a. The statistical analysis of the results of the Chla variable of the present work showed no significant difference (p≤ 0.05) in concentrations of 0, 25, 50, 75 and 100 mg L−1 of the ZnO-NPs applied; however, a change is observed with 125 mg L-1 ZnO-NPs, which reduced Chla up to 40.49% with respect to the control.
In addition, the application of ZnO-NPs significantly changed (p≤ 0.05) the content of Chlb, the mean comparisons showed that the highest Chlb was found in the treatment of 50 mg L-1 ZnO-NPs, even concentrations of 25 and 75 mg L-1 ZnO-NPs exceeded the control. However, at 100 and 125 mg L-1 ZnO-NPs, the Chlb content in the seedlings decreased by about 31.25% and 37.5%, respectively.

Figure 2 Content of chlorophyll (a) and content of carotenes (b) of L . sativa after each treatment. Data are shown as means (n= 24) ± standard deviation. Values with different letters indicate significant differences according to the Tukey test (p≤ 0.05).
Zn is a heavy element and, like other heavy metals, in large quantities is toxic to many plants, and the degradation of chlorophyll in these circumstances is evident (Mohsenzadeh and Moosavian, 2017). These results demonstrate that ZnO-NPs at high concentrations drastically decrease the chlorophyll content, it seems that perhaps the reduction in the amount of chlorophyll is due to the prevention or degradation of the precursors of these pigments.
The chlorophyll content is considered an important index of the total amount of the light-harvesting complex and electron transport components, it is positively related to the photosynthetic rate (Li et al., 2019), so it can be used as an indicator to measure the degree of stress caused by NPs (Yan et al., 2021). The photosynthesis of chloroplasts is altered, which causes oxygen to become an electron acceptor and reactive oxygen species to be produced (Yan et al., 2021). On the other hand, the treatment with ZnO-NPs significantly increased (p≤ 0.05) the carotenoid content in L. sativa seedlings (Figure 2b). The highest carotenoid content was found at the concentration of 100 mg L-1 ZnO-NPs, with an increase of up to 176.4% with respect to the control.
Carotenoids are present in the plastids of plant tissues and in environmental stress, they trigger oxidative stress, they are responsible for protecting photosynthetic tissues, especially chlorophyll. According to the results obtained in this study, it seems that a certain amount of zinc induces oxidative stress and causes the synthesis of carotenoids (Mohsenzadeh and Moosavian, 2017). This suggests that the reduction in photosynthesis is caused by the reduction of chlorophyll content. The results corroborate what was found by Wang et al. (2016) on the exposure of ZnO-NPs in biomass accumulation and photosynthesis in Arabidopsis.
Phenols
In this study, the application of concentrations of 75 and 125 mg L-1 ZnO-NPs showed no significant difference in the total phenol content of L. sativa seedlings. While the application of 25, 50 and 100 mg L-1 ZnO-NPs increased significantly with respect to the control treatment (p≤ 0.05) (Figure 3). The role of zinc in the use of carbon to produce phenolic compounds in the cycle of shikimic acid and acetate may be one of the reasons for this increase (Misra et al., 2006). Zinc induces oxidative stress and will lead to the production of free radicals in the plant, the plant will lead to stress and outperform these species.
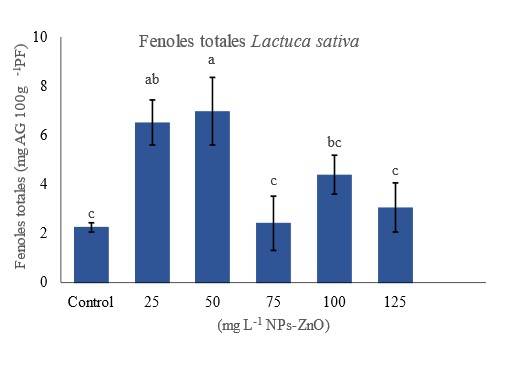
Figure 3 Content of total phenolic compounds of L . sativa after each treatment. Data are shown as means (n= 24) ± standard deviation. Values with different letters indicate significant differences according to the Tukey test (p≤ 0.05).
In response to this stress, plants release several defensive compounds known as antioxidant secondary metabolites, including different groups of polyphenols (Paramo et al., 2020). Thus, this parameter related to the antioxidant system of seedlings improves, which is consistent with what was reported by other authors (Faizan et al., 2018; Paramo et al., 2020; Ramírez-Rodríguez et al., 2021).
Conclusions
The characterization made showed that the synthesis of ZnO-NPs by a chemical process allowed obtaining particle sizes of 67.31-71.1 nm. This study analyzed ZnO-NPs solutions in a concentration range of 25 to 125 mg L-1 in lettuce seedlings and provides valuable data and evidence to increase crop productivity by increasing the concentration of useful compounds, such as antioxidants and secondary metabolites. ZnO-NPs could be a good alternative to improve the quality of seedlings; however, more research is needed to clarify the effects of ZnO-NPs as there are factors that depend on the species and concentration.
Acknowledgements
This research was funded by the National Technological Institute of Mexico (TecNM, for its acronym in Spanish). Project: 13989.22-P-Campus Technological Institute of Torreón (2022). Alma Patricia Galindo Guzmán appreciates the financial support provided by the National Council of Science and Technology (CONACYT, for its acronym in Spanish) of Mexico for postgraduate studies.
REFERENCES
Abdalla, M. A.; Li, F.; Wenzel-Storjohann, A.; Sulieman, S.; Tasdemir, D. and Mühling, K. H. 2021. Comparative metabolite profile, biological activity, and overall quality of three lettuce (Lactuca sativa L., Asteraceae) cultivars in response to sulfur nutrition. Pharmaceutics. 5(13):713. https://doi.org/10.3390/pharmaceutics13050713. [ Links ]
Afrayeem, S. M. and Chaurasia, A. K. 2017. Effect of zinc oxide nanoparticles on seed germination and seed vigour in chilli (Capsicum annuum L.). J. Pharmacogn. Phytochem. 6(5):1564-1566. [ Links ]
Aquino, P.; Osorio, A. M.; Ninán, E. y Torres, F. 2018. Caracterización de nanopartículas de ZnO sintetizadas por el método de precipitación y su evaluación en la incorporación en pinturas esmalte. Rev. de la Sociedad Química del Perú. 84(1):5-17. [ Links ]
Dhoke, S. K.; Mahajan, P.; Kamble, R. and Khanna, A. 2013. Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnol. Development. 3(1):e1-e1. https://doi.org/10.4081/nd.2013.e1. [ Links ]
Ealia, A. M. and Saravanakumar, M. P. 2017. A review on the classification, characterization, synthesis of nanoparticles and their application. In IOP Conference series: materials science and engineering. 263(3):032019 https://doi.org/10.1088/1757-899X/263/3/032019. [ Links ]
Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S. T. and Hayat, S. 2018. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica. 56(2):678-686. https://doi.org/10.1007/s11099-017-0717-0. [ Links ]
Faizan, M.; Hayat, S. and Pichtel, J. 2020. Effects of zinc oxide nanoparticles on crop plants: a perspective analysis. Sustainable Agriculture Reviews. (41):83-99. https://doi.org/10.1007 /978-3-030-33996-8-4. [ Links ]
Hasan, S. 2015. A review on nanoparticles: their synthesis and types. Res. J. Recent Sci. 4 (ISC-2014):9-11. [ Links ]
Hojjat, S. S. and Kamyab, M. 2017. The effect of silver nanoparticle on Fenugreek seed germination under salinity levels. Russian Agric. Sci. 43(1):61-65. https://doi.org/ 10.3103/S1068367417010189. [ Links ]
Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S. K.; Sun, Y.; Hu, J. and Yin, H. 2019. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Inter. J. Biol. Macromol. 126:91-100. https://doi.org/10.1016/j.ijbiomac.2018.12.118. [ Links ]
Lichtenthaler, H. K. and Wellburn, A. R. 1983. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. 11(5):591-592. https://doi.org/ 10.1042/bst0110591. [ Links ]
Lin, D. and Xing, B. 2007. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environmental Pollution. 150(2):243-250. https://doi.org/10.1016/j.envpol.2007. 01.016. [ Links ]
Liu, R.; Zhang, H. and Lal, R. 2016. Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: nanotoxicants or nanonutrients? Water Air and Soil Pollution. 227(1):1-14. https://doi.org/10.1007/s11270-015-2738-2. [ Links ]
Misra, A.; Dwivedi, S.; Srivastava, A. K.; Tewari, D. K.; Khan, A. and Kumar, R. 2006. Low iron stress nutrition for evaluation of Fe-efficient genotype physiology, photosynthesis, and essential monoterpene oil (s) yield of Ocimum sanctum. Photosynthetica . 44(3):474-477. https://doi.org/10.1007/s11099-006-0054-1. [ Links ]
Mohsenzadeh, S. and Moosavian, S. S. 2017. Zinc sulphate and nano-zinc oxide effects on some physiological parameters of Rosmarinus officinalis. Am. J. Plant Sci. 8(11):2635-2649. https://doi.org/10.4236/ajps.2017.811178. [ Links ]
Paramo, L. A.; Feregrino-Pérez, A. A.; Guevara, R.; Mendoza, S. and Esquivel, K. 2020. Nanoparticles in agroindustry: applications, toxicity, challenges, and trends. Nanomaterials. 10(9):1-19. https://doi.org/10.3390/nano10091654. [ Links ]
Ramírez-Rodríguez, S. C.; Ortega-Ortiz, H.; Fortis-Hernández, M.; Nava-Santos, J. M.; Orozco-Vidal, J. A. y Preciado-Rangel, P. 2021. Nanopartículas de quitosano mejoran la calidad nutracéutica de germinados de triticale. Rev. Mex. Cienc. Agríc. 12(4):579-589. https://doi.org/10.29312/remexca.v12i4.2929. [ Links ]
Raskar, S. V. and Laware, S. L. 2014. Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Inter. J. Current Microbiol. Appl. Sci. 3(2):467-473. [ Links ]
Rawashdeh, R. Y.; Harb, A. M. and AlHasan, A. M. 2020. Biological interaction of zinc oxide nanoparticles; lettuce seed as case of study. Heliyon. 6(5):e03983. https://doi.org/10.1016/ j.heliyon.2020.e03983. [ Links ]
Salama, D. M.; Osman, S. A.; Abd El-Aziz, M. E.; Abd-Elwahed, M. S. A. and Shaaban, E. A. 2019. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatalysis and Agricultural Biotechnology. (18):101083. https://doi.org/10.1016/j.bcab.2019.101083. [ Links ]
Salas-Pérez, L.; Gaucín-Delgado, J. M.; Preciado-Rangel, P.; Fortis-Hernández, M.; Valenzuela-García, J. R. y Ayala-Garay, A. V. 2016. Efecto del ácido benzoico en la capacidad antioxidante de germinados de trigo. Rev. Mex. Cienc. Agríc. 3(17):3397-3404. [ Links ]
Singleton, V. L.; Orthofer, R. and Lamuela-Raventós, R. M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 299(7):152-178. https://doi.org/10.1016/S0076-6879(99)99017-1. [ Links ]
Szőllősi, R.; Molnár, Á.; Kondak, S. and Kolbert, Z. 2020. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs phytotoxicity. Plants. 9(12):1745. https://doi.org/10.3390/plants9121745. [ Links ]
Tovar-Jimenez, G. I.; Flores, S.; Suarez, J.; González, G. and Briceño, S. 2020. Biogenic synthesis of iron oxide nanoparticles using Moringa oleifera and chitosan and its evaluation on corn germination. Environmental Nanotechnology, Monitoring Management. (14):100350. https://doi.org/10.1016/j.enmm.2020.100350. [ Links ]
Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Gao, X.; Wang, L. and Wang, S. 2016. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Frontiers in Plant Science. (6):1243. https://doi.org/10.3389/fpls.2015.01243. [ Links ]
Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X. and Ye, W. 2021. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biology. 21(1):1-11. https://doi.org/10.1186/s12870-021-02929-3. [ Links ]
Received: June 01, 2022; Accepted: August 01, 2022











 texto em
texto em 



