Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.13 no.3 Texcoco abr./may. 2022 Epub 08-Ago-2022
https://doi.org/10.29312/remexca.v13i3.2831
Articles
Polyphenols in five varieties of Euphorbia pulcherrima native to Mexico
1Instituto de Horticultura-Universidad Autónoma Chapingo. Carretera México-Texcoco km 38.5, Chapingo, Estado de México. CP. 56230. Tel. 229 1543388. (karlitauv@gmail.com; rosgar08@hotmail.com).
2Laboratorio de Productos Naturales-Área de Química-Departamento de PreparatoriaAgrícola- Universidad Autónoma Chapingo. Carretera México-Texcoco km 38.5, Chapingo, México. CP. 56230. Tel. 55 27197195 (dguerrar@chapingo.mx).
3Especialidad de Botánica-Colegio de Postgraduados-Campus Montecillo. Carretera México-Texcoco km 35.5, Montecillo, Estado de México. CP. 56230. Tel. 55 12958275. (msoto@colpos.mx).
4Facultad de Estudios Superiores Iztacala-Universidad Nacional Autónoma de México. Tlalnepantla de Baz, Estado de México. CP. 54090. Tel. 55 40559624. (maragarin@yahoo.com).
Poinsettia (Euphorbia pulcherrima Willd. ex Klotsch) grows wild in the Mexican tropical forests in a shrubby form and with striking red bracts and less often it has white bracts. The main use of the native poinsettia is ornamental, little has been studied about the nutritional or nutraceutical properties of the plant. The objective of the present study was to determine the profile of flavonoids and phenolic acids present in the methanolic-aqueous extracts in bracts of five varieties of sun poinsettia in order to know their nutritional properties and promote their consumption. Dry bracts (0.5 g per sample) were used. The extraction of the compounds was done from a solution of methanol/water (80:20 v/v). A profile of phenolic acids and flavonoids was made by high-performance liquid chromatography (HPLC) coupled to a diode-array detector. Gallic and syringic acids were the ones that had the highest concentrations in the varieties analyzed. The flavonoid rutin was found in high concentrations in all varieties, however, in the Juan Pablo variety, it was not possible to detect phlorizin and phloretin. These results suggest that poinsettia bracts may provide important antioxidants when consumed.
Keywords: euphorbiaceae; flavonoids; HPLC; phenolic acids
La nochebuena (Euphorbia pulcherrima Willd. ex Klotsch) crece de manera silvestre en los bosques tropicales mexicanos en forma arbustiva y con brácteas rojas llamativas y con menor frecuencia presenta brácteas blancas. El uso principal de la nochebuena nativa es el ornamental, poco se ha estudiado acerca de las propiedades nutrimentales o nutracéuticas de la planta. El objetivo del presente estudio fue determinar el perfil de flavonoides y ácidos fenólicos presentes en los extractos metanólico-acuosos en brácteas de cinco variedades de nochebuena de sol para conocer sus propiedades nutrimentales y promover su consumo. Se utilizaron brácteas secas (0.5 g por muestra). La extracción de los compuestos se hizo a partir de una solución de metanol/agua (80:20 v/v). Se llevó a cabo un perfil de ácidos fenólicos y flavonoides por cromatografía de líquidos de alta resolución (CLAR) acoplado a un detector de arreglo de diodos. Los ácidos gálico y siringico fueron los que presentaron las concentraciones más altas en las variedades analizadas. El flavonoide rutina se encontró en altas concentraciones en todas las variedades, sin embargo, en la variedad Juan Pablo no se logró detectar floridzina y floretina. Estos resultados sugieren que las brácteas de nochebuena pueden aportar antioxidantes importantes al consumirse.
Palabras clave: ácidos fenólicos; euphorbiaceae; flavonoides; HPLC
Introduction
Poinsettia (Euphorbia pulcherrima Willd. ex Klotsch) is a plant that grows wild and shrubby in the Mexican tropical forests, with a small stem and with striking red bracts and very rare with white bracts (Trejo et al., 2012). A variant of wild poinsettias is sun poinsettia. According to Galindo-García et al. (2012), sun poinsettia is an ornamental and traditional shrub that can have several colors such as red, pink, white, of great economic and social importance and has been produced conventionally in an open-air nursery for more than thirty years.
At present, nine varieties of sun poinsettia registered in the National Catalogue of Plant Varieties (Snics CNVV, 2021) are recognized: ‘Valenciana’ (red), ‘Juan Pablo’ (pink), ‘Rehilete’ and ‘Belén’ (red) and ‘Amanecer navideño’ (white), they are called varieties of public domain and are the result of the selection, reproduction and modifications made by some nurserymen from the native poinsettias from: Tetela del Monte, Tepoztlán, Oaxtepec, Jiutepec and Ahuatepec, in the state of Morelos (Colinas et al., 2015; CNVV, 2021). This study focused on the varieties: ‘Amanecer Navideño’, ‘Juan Pablo’, ‘Valenciana’, ‘Corona’ and one not yet registered, ‘Valenciana Superior’.
According to Steinmann (2002), many of the endemic species of the family Euphorbiaceae, to which poinsettia belongs, are cultivated for medicinal, industrial, food and ornamental use, however, there are very few studies about the nutritional or nutraceutical properties of sun poinsettia. Poinsettia leaves and bracts have begun to be introduced in different dishes. In Taxco Guerrero, where December 8 is celebrated as the day of poinsettia, there is a tasting of different dishes using the bracts of sun poinsettia.
Likewise, recipes using bracts are shared at the annual Christmas festival of the Ecological Park of Xochitla, State of Mexico. Edible ornamental plants can be attractive for their smell, color and taste; however, the evaluation of their nutritional properties is required to include them in the diet. The use of flowers for edible purposes has been reactivated through the consumption of different parts of the flower, such as stems and nectar, including them in the diet as spices, dyes and additives, in addition a great interest has been generated due to their nutraceutical properties (Janarny et al., 2021). Edible flowers and ornamental plants can be considered as a new source of nutraceuticals (Mlcek and Rop, 2011).
On the other hand, edible flowers may contain phenolic compounds with different chemical structures, mainly phenolic acids, flavonoids and anthocyanins, which can provide antioxidant capacity. Phenolic acids are distributed in different parts of plants, these aromatic secondary metabolites are responsible for providing color, flavor and astringency, thus contributing to the organoleptic characteristics of foods (Rashmi and Negi, 2020). The functions of phenolic acids have been the subject of many studies related to agriculture, biology, chemistry and medicinal studies. Mahomoodally et al. (2020), studying three species of Euphorbia (E. hirta, E. heterophylla and E. convolvuloides), report a high content of total phenols, between 35.84 and 441.9 mg g-1, and indicate their potential as antioxidants.
Flavonoids are phenolic substances widely distributed in all vascular plants and can be present in their free form, glycosylated or as methylated derivatives, they have a powerful antioxidant, anti-inflammatory, antimutagenic, antimicrobial and anticancer activity, in addition to abilities to trap free radicals and other medicinal properties related to the ability to modulate enzymatic functions (Karak, 2019). Phenolic compounds are an essential part of the human diet and are considered of great interest due to their antioxidant properties and potential health effect (Shahidi and Ambigaipalan, 2015). The objective of this study was to analyze the profile of phenolic acids and flavonoids of five varieties poinsettia of public domain, in order to know their nutritional properties and promote their consumption.
Materials and methods
Plant material
Bracts of five poinsettia varieties of public domain, free of mechanical or pathogens damage, were collected in January 2019, the varieties were: Amanecer Navideño (AN), Juan Pablo (JP), Valenciana (V), Valsu (VS) and Corona (C), which are kept under greenhouse conditions in the experimental field of the Chapingo Autonomous University (UACH, for its acronym in Spanish). The bracts were dehydrated at a low temperature in a freeze dryer (Free Zone 2.5 Liter Benchtop Labconco. USA), for their further analysis.
Method of extraction of phenolic acids and flavonoids
The obtaining of the extracts was carried out according to the methodology of Wang et al. (2010). In each poinsettia variety, 0.5 g of freeze-dried bracts were weighed and 10 ml of a methanol/water solution (80:20 v/v) was added. The mixture was stirred in a Vortex (VWE International) for 3 min at 3 000 rpm, sonicated for 15 min with a sonicator (Ultrasonic Cleaner 8890, Cole Palmer) and stirred for 30 min at 27 °C. This procedure was performed in triplicate. The extracts were stored in amber bottles for their further analysis.
Identification of phenolic acids and flavonoids
The analyses were performed in a high-performance liquid chromatograph (HPLC) (Hewlett Packard, mod. 1100) coupled to a diode-array detector. For the quantification of phenolic acids, a Nucleosil 100 column at 125 x 4 mm id 5 μm (Marcherey-Nagel, Fisher Scientific, USA) was used. The eluents used were: water (pH 2.5) with trifluoroacetic acid (A) and acetonitrile (B). For the mobile phase, the following elution gradient (v/v) was used: 85%A-15%B (0-10’), 65%A-35%B (20’), 65%A-35%B (23’). The elution flow was kept constant at 1 ml min-1 and the injection volume was 20 μl. The temperature of the column was kept at 30 °C. Retention times and spectra were obtained at 280 nm.
Regarding the quantification of flavonoids, a Hypersil ODS column 5μm particle diameter, 125 x 4 mm id. (Agilent Technologies, USA) was used. The eluents were: water (pH 2.5) with trifluoroacetic acid (A) and acetonitrile (B). For the mobile phase, the following elution gradient (v/v) was used: 85%A-15%B (0-10’), 65%A-35%B (20’), 65%A-35%B (23’). The elution flow was kept constant at 1 ml min-1 and the injection volume was 20 μl. The temperature of the column was kept at 30 °C. Retention times and spectra were obtained at 254, 316 and 365 nm. For both phenols and flavonoids, the identification was made based on a calibration curve, using the respective standards of the Merck brand, Dalmsdat, Germany.
Statistical analysis
The analyses were done in triplicate and were expressed in milligrams of phenolic acids or flavonoids per gram of sample in dry matter (mg g-1 dm). All data were analyzed with the SAS statistical package for Windows 9.0, statistical differences were determined in an analysis of variance (Anova). The means were expressed using Tukey’s HSD statistic with a minimum significant difference of p≤ 0.05.
Results and discussion
Phenolic acids
The phenolic acids identified in the methanolic-aqueous extracts of bracts of Euphorbia pulcherrima varieties are shown in Table 1. Their chemical structures vary according to the number and position of free radicals present in them (Figure 1).
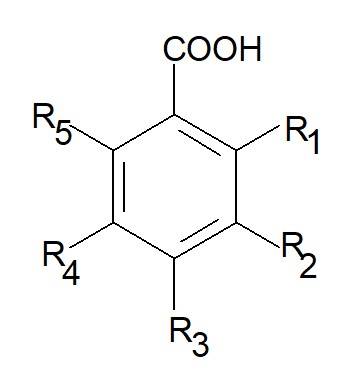
Figure 1 Position of the radical groups of phenolic acids present in varieties of Euphorbia pulcherrima.
Table 1 Phenolic acids identified in the bracts of five varieties of Euphorbia pulcherrima.
| Phenolic acid | IUPAC Name | Structure |
| Gallic | 3,4,5-trihydroxybenzoic | R3= R4= R5= OH |
| Chlorogenic | (1S,3R,4R,5R)-3-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5-trihydroxycyclohexanecarboxylic | R1= R4= R5= OH |
| Syringic | 4-hydroxy-3,5-dimethoxybenzoic | R4= OH; R3= R5= CH3O- |
| Vanillic | 4-hydroxy-3-methoxybenzoic | R4=OH; R3= CH3O- |
| P-hydroxybenzoic | 4-hydroxybenzoic | R4= OH |
| Caffeic | (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic | R3= R4= OH |
| Ferulic | (E)-3-(4-hydroxy-3-methoxy-phenyl)propyl-2-enoic | R4= OH; R3= CH3O- |
| P-coumaric | (E)-3-(4-hydroxyphenyl)-2-propenoic | R4= OH |
The concentrations of gallic and syringic acid were the highest in the five poinsettia varieties, with the Corona variety standing out with 50% more gallic acid than the Amanecer Navideño and Valenciana varieties, which rank second and third, respectively. While for syringic acid, the highest contents were in the Corona and Valenciana varieties. On the other hand, p-coumaric acid was found in lower concentrations (0.028-0.54 mg g-1 dm) in all varieties, particularly in Juan Pablo and Valenciana.
Considering the eight phenolic acids evaluated in this research, overall, the highest content of phenolic acids was obtained in the Corona variety, which with 57.14 mg g-1 dm represents the highest value, followed by the Valenciana variety with 33.08% and Amanecer Navideño with 37.73% less, respectively. Overall, the phenolic acid content was lower in the Valsu variety (11.48 mg g-1 dm).
Phenolic compounds are present in virtually all plant-based foods, because they provide useful building blocks with excellent functionality and biocompatibility, their use has been promoted as a replacement for synthetic antioxidants commonly used in the food industry (Dias et al., 2020). In the research conducted by Ertas et al. (2015), gallic acid was one of the most abundant phenolic acids in two species of Euphorbia (E. gaillardotii and E. macroclada) analyzed, a result similar to that reported in the poinsettia varieties of the present study.
Florkiewicz et al. (2019) analyzed the content of gallic acid in varieties of the genus Brassica, such as brussels sprouts (Brassica oleracea var. gemmifera) and broccoli (Brassica oleracea var. italica), obtaining concentrations of 0.0027 mg g-1 dm and 0.0025 mg g-1 dm respectively, while in the poinsettia varieties analyzed, the values of gallic acid are in a range of 0.13 mg g-1 dm to 23.76 mg g-1 dm. Gallic acid can also be found in beverages such as coffee, tea, and wine (Nayeem et al., 2016). According to Badhani et al. (2015), gallic acid provides efficient protection against oxidative damage caused by reactive species found in biological systems including hydroxyl, superoxide and peroxyl and non-radicals such as hydrogen peroxide and hypochlorous acid.
Another phenolic compound of great abundance in the plant kingdom is syringic acid and is present in olives, dates and spices, it also exhibits useful properties in the biomedical sector as it acts as an antioxidant, antimicrobial, anti-inflammatory and anticancer agent (Srinivasulu et al., 2018). Pioro-Jabrucka et al. (2011) analyzed the methanolic extracts of Euphorbia hirta, reporting values of syringic acid of 0.61 mg g-1 dm, a value lower than that found in the Juan Pablo, Valenciana, Valsu and Corona varieties of the present study. The Corona variety presented the highest concentrations of gallic, syringic and vanillic acids The latter is also known as 4-hydroxy-3-methoxybenzoic, it is a phenolic compound derived from edible plants and fruits (Prince et al., 2011), several studies have provided evidence of the effectiveness of vanillic acid in managing immune or inflammatory responses (Kim et al., 2010).
As for the Juan Pablo and Valenciana varieties, it was not possible to detect vanillic acid and in the Valenciana Superior variety, p-hydroxybenzoic and caffeic acids were not detected because they were found in very low concentrations (Table 2). Hydroxycinnamic acids are more commonly found in nature, compared to hydroxybenzoic acids, and are usually available either in their soluble form conjugated with sugars and organic acids or bound to cellular constituents. The most common hydroxycinnamic acids are p-coumaric, caffeic and ferulic acids (Rashmi and Negi, 2020). Jahan et al. (2013) analyzed five phenolic acids (gallic, chlorogenic, p-coumaric, ferulic and caffeic) present in methanolic extracts of Euphorbia tirucalli by liquid chromatography, gallic, chlorogenic and p-coumaric acids were the most representative; obtaining lower values of gallic (0.01 mg g-1 dm) and chlorogenic acids (0.04 mg g-1 dm) unlike those reported in the varieties analyzed in this study.
Table 2 Quantification of phenolic acids by HPLC in methanolic-aqueous extracts of Euphorbia pulcherrima.
| Phenolic acid (mg g-1 dm) | Variety | ||||
| AN1 | JP | V | VS | C | |
| Gallic (1.635)* | 10.65a ±0.76 | 1.81cb ±0.16 | 11.5ba ±4.28 | 0.13b ±0.03 | 23.76a ±3.02 |
| Chlorogenic (2.491)* | 1.37bc ±0.11 | 2.54cb ±2.11 | 3.75ba ±6.3 | 4.57a ±4.5 | 2.75b ±3.2 |
| Syringic (3.00)* | 0.27c ±0.06 | 2.45b ±2.11 | 12.63a ±5.76 | 6.36b ±0.08 | 17.37ba ±0.91 |
| Vanillic (3.387)* | 0.28c ±0.09 | ND | ND | 0.17b ±0.26 | 12.3ba ±1.58 |
| P-hydroxybenzoic (11.271)* | 2.41bc ±0.21 | 1.65cb ±1.08 | 0.15ba ±0.14 | ND | 0.18b ±0.09 |
| Caffeic (3.387)* | 9.47ba ±3.1 | 8.7a ±0.34 | 9.8ba ±0.26 | ND | 0.37b ±0.15 |
| Ferulic (6.056)* | 2.49bc ±0.38 | 1.41cb ±0.63 | 0.32ba ±0.06 | 0.14b ±0.01 | 0.07b ±0.003 |
| P-coumaric* (5.289) | 0.54c ±0.08 | 0.03c ±0.02 | 0.09ba ±0.03 | 0.11b ±0.15 | 0.34b ±0.14 |
*= retention time. Values with the same letters within each column are statistically similar (Tukey’s HSD, p≥ 0.05); 1= standard deviation; AN= Amanecer Navideño; JP= Juan Pablo; V= Valenciana; VS= Valsu; C= Corona; ND= not detected.
P-coumaric acid had a value similar to that reported in the varieties analyzed, with a concentration of 0.09 mg g-1 dm. P-coumaric acid is present in plants and fungi, either in its free or conjugated form, it is also present in fruits (apples, pears, grapes, oranges, tomatoes and berries) and in vegetables (onions, beans and potatoes) or in cereals such as corn, oats and wheat. Several studies have shown that p-coumaric acid conjugates provide antioxidant, anti-inflammatory, antimutagenic, antiulcer and anticancer activity (Pei et al., 2016).
Flavonoids
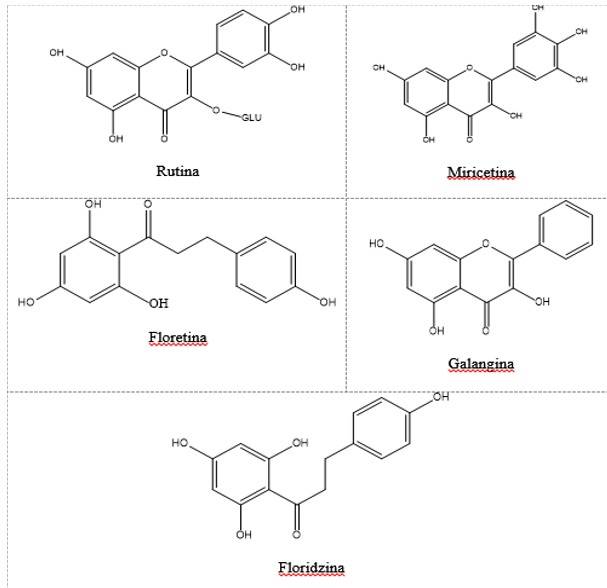
The flavonoids identified in the methanolic-aqueous extracts of bracts of the Euphorbia pulcherrima varieties are shown in Figure 2 and Tables 3 and 4. High concentrations of rutin were obtained in all the varieties analyzed, the Amanecer Navideño variety had the highest concentration of this flavonoid, followed by the Juan Pablo variety with 16.47% less and, in third place, Valsu with 14.53% less. Corona had the highest content of phlorizin (66.22 ±0.09 mg g-1 dm), followed by the Amanecer Navideño variety with 32.85% less. On the other hand, myricetin was found in greater concentration in the Amanecer Navideño variety, its content being very low in the other four varieties evaluated. The equipment did not detect the presence of the flavonoids phlorizin and phloretin in the Juan Pablo variety.
Table 3 Flavonoids identified in the bracts of five varieties of Euphorbia pulcherrima.
| Flavonoid | IUPAC Name | Structure |
| Rutin | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3- {[(2S,3R,4S, 5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-4H-chromen-4-one | R1= R2= R3= OH |
| Myricetin | 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone | R1= R4= R5= OH |
| Phloretin | 3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one | R4= OH |
| Galangin | 3,5,7-trihydroxy-2-phenylchromen-4-one | R4= OH; R3= R5= CH3O- |
| Phlorizin | 1-(2,4-dihydroxy-6-{[(2” S”,3” R”,4” S”,5 “S”, 6” R”)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phenyl)- 3-(4-hydroxyphenyl)propan-1-one | R4= OH |
Table 4 Quantification of flavonoids by HPLC in methanolic-aqueous extracts of Euphorbia pulcherrima.
| Flavonoid mg g-1 dm | Variety | ||||
| AN1 | JP | V | VS | C | |
| Rutin (5.734)* | 32.68a ±1.43 | 27.3a ±2.58 | 22.74a ±3.68 | 27.93a ±1.14 | 26.57ba ±0.7 |
| Phlorizin (9.056)* | 44.47a ±0.37 | ND | 0.37b ±0.1 | 0.32b ±0.06 | 66.22a ±0.09 |
| Myricetin (9.190)* | 9.4a ±0.06 | 0.21b ±0.12 | 0.21b ±0.02 | 0.17b ±0.01 | 0.14b ±0.02 |
| Phloretin (16.263)* | 0.28a ±0.2 | ND | 0.04b ±0.01 | 0.28b ±0.22 | 0.22b ±0.01 |
| Galangin | 0.4a ±0.01 | 0.03b ±0.06 | 0.42b ±0.04 | 0.33b ±0.004 | 0.04b ±0.005 |
*= retention time. Values with the same letters within each column are statistically similar (Tukey’s HSD, p≥ 0.05); 1= standard deviation; AN= Amanecer Navideño; JP= Juan Pablo; V= Valenciana; VS= Valsu; C= Corona; ND= not detected.
Considering the total content of the five flavonoids evaluated, the highest content was obtained in the Corona variety with 93.19 mg g-1 dm, followed by Amanecer Navideño with 87.23 mg g-1 dm. While the other three varieties Valsu (29.03 mg g-1 dm), Juan Pablo (27.54 mg g-1 dm) and Valenciana (23.78 mg g-1 dm) had approximately 50% less total flavonoids than the previous varieties.
Flavonoids are an integral part of the diet because they are plant-specific phytochemicals and cannot be synthesized by humans, which is why they must be obtained from different sources, mainly of plant origin (Mahomoodally et al., 2020). According to research conducted by Ertas et al. (2015), one of the main constituents of two Euphorbia species analyzed was rutin, a result similar to that obtained in the five varieties of Euphorbia pulcherrima analyzed in this study.
The flavonoid rutin is constituted by a glycoside of quercetin (flavonol) and rutinose (disaccharide), which is commonly found in the diet and in beverages of plant origin such as tea and wine, it also has various pharmacological properties such as anti-inflammatory, neuroprotective and anticarcinogenic (Imani et al., 2020). Jahan et al. (2013) reported myricetin as one of the main flavonoids present in the methanolic extracts of E. tirucalli, obtaining a value of 0.82 mg g-1 dm, higher than those obtained in most of the varieties of E. pulcherrima analyzed here, except for the Amanecer Navideño variety, in which a value of 9.4 mg g-1 dm was found.
Myricetin is a phenolic compound very common in berries, vegetables, teas and wines, it is also produced by several plants, this compound shows a variety of pharmacological activities and acts as an anti-inflammatory, analgesic, antitumor and hepatoprotective agent (Semwal et al., 2016). Mustafa et al. (2022) analyzed the content of quercetin and myricetin in the fruits of commercial blueberry (Vaccinium spp.), finding values of 0.069 mg g-1 and 0.003 mg g-1 respectively, while in the poinsettia varieties analyzed, the values of myricetin are in a range of 0.14 mg g-1 dm to 9.4 mg g-1 dm.
On the other hand, chalcones are yellow pigments partly responsible for the color of flowers and fruits (Peñarrieta et al., 2014). This fact could explain why high concentrations of phlorizin (44.47 ± 0.37 mg g-1 dm) were found in the Amanecer Navideño variety, since its bracts are yellow. According to Haider et al. (2020), phloretin is commonly present in apple trees and Prunus mandshurica, it also shows various bioactivities such as antioxidant, anticarcinogenic, anti-inflammatory and immunomodulatory. In the study conducted by Zielinska et al. (2019), they analyzed the content of phloretin and phlorizin in apple (Malus sp., Rosaceae var. Sunrise) in concentrations of 0.0014 and 0.062 mg g-1 respectively, being values lower than those found in the poinsettia varieties analyzed in this study.
Conclusions
The concentrations of gallic and syringic acids were the highest, particularly in the Corona variety. It was detected that p-coumaric acid was the one with the lowest concentration in the Juan Pablo variety. The Corona variety stands out in the vanillic acid content. As for flavonoids, high concentrations of rutin were found in all varieties, with Amanecer Navideño standing out. Phlorizin was found in high levels in Amanecer Navideño and Corona. In the Juan Pablo variety, it was not possible to detect phlorizin and phloretin. It is considered that the bracts of the Corona and Amanecer Navideño varieties, having a high level of phenolic acids and flavonoids, have the potential to be included in the diet.
Literatura citada
Badhani, B.; Sharma, N. and Kakkar, R. 2015. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Advances. 5(35):27540-27557. Doi: 10.1039/c5ra01911g. [ Links ]
Colinas, M. T.; Espinosa, A.; Mejia, J.; Rodríguez, M. A.; Pérez, M. L. y Alia-Tejacal, I. 2015. Cultivars of Euphorbia pulcherrima from Mexico. Acta Hortic. 1104:487-490. Doi: 10.17660/ActaHortic.2015.1104.70. [ Links ]
Dias, R.; Oliveira, H.; Fernendes, I.; Simal-Gandara, J. and Perez-Gregorio, R. 2020. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Critical Reviews in Food Science and Nutrition. 61(7):1130-1151. Doi: 10.1080/10408398.2020.1754162. [ Links ]
Ertas, A.; Yilmaz, M. A. and Firat, M. 2015. Chemical profile by LC-MS/MS, GC/MS and antioxidant activities of the essential oils and crude extracts of two Euphorbia species. Natural Product Res. 29(6):529-534. Doi: 10.1080/14786419.2014.954113. [ Links ]
Florkiewicz, A.; Socha, R.; Florkiewicz-Filipiak, A. and Topolska, K. 2019. Sous-vide technique as an alternative to traditional cooking methods in the context of antioxidant properties of Brassica vegetables. Sci. Food Agric. 99(1):173-182. Doi.org: 10.1002/jsfa.9158. [ Links ]
Galindo-García, D. V.; Alia-Tejacal, I.; Andrade-Rodríguez, M.; Colinas-León, M. T.; Canul-Ku, J. y Sainz-Aispuro, M. J. 2012. Producción de nochebuena de sol en Morelos, México Sun-poinsettia production in Morelos, México. Rev. Mex. Cienc. Agríc. 3(4):751-763. [ Links ]
Haider, K.; Haider, R. M.; Neha, K. and Yar, M. S. 2020. Free radical scavengers: an overview on heterocyclic advances and medicinal prospects. Eur. J. Medicinal Chem. 204(112607):1-16. Doi:10.1016/j.ejmech.2020.112607 [ Links ]
Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S. and Maleki-Dizaj, S. 2020. Molecular mechanisms of anticancer effect of rutin. Phytotherapy Res. 35(5):1-14. Doi.org: 10.1002/ptr.6977. [ Links ]
Jahan, N.; Ur-Rahman, K.; Ali, S. and Rafiq-Asi, M. 2013. Phenolic acid and flavonol contents of gemmo-modified and native extracts of some indigenous medicinal plants. Pakistan J. Bot. 45(5):1515-1519. [ Links ]
Janarny, G.; Gunathilake, K. D. P. P. and Ranaweera, K. K. D. S. 2021. Nutraceutical potential of dietary phytochemicals in edible flowers a review. J. Food Biochem. 45(4):1-20. doi:10.1111/jfbc.13642. [ Links ]
Karak, P. 2019. Biological activities of flavonoids: an overview. Inter. J. Pharm. Sci. Res. 10(4):1567-1574. Doi:10.13040/IJPSR.0975-8232.10(4).1567-74. [ Links ]
Kim, S. J.; Kim, M. C.; Um, J. Y. and Hong, S. H. 2010. The beneficial effect of vanillic acid on ulcerative colitis. Molecules. 15(10):7208-7217. Doi: 10.3390/molecules15107208. [ Links ]
Mlcek, J. and Rop, O. 2011. Fresh edible flowers of ornamental plants a new source of nutraceutical foods. Trends Food Sci. Technol. 22(10):561-569. Doi: 10.1016/j.tifs.2011.04.006. [ Links ]
Mahomoodaly, M. F.; S. Dall-Acqua, K.; Sut, I.; Ferrarese, O.; Katinan, N.; Bibi, G.; Ak, G. and Zengin. 2020. Phenolic compounds analysis of three Euphorbia species by LC-DAD-MS and their biological properties. J. Pharm. Biom. Analy. 189:1-9. https://doi.org/10.1016/ j.jpba.2020.113477. [ Links ]
Mustafa, A. M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S. and Caprioli, G. 2022. A new HPLC-MS / MS method for the simultaneous determination of 36 polyphenols in blueberry , strawberry and their commercial products and determination of antioxidant activity. Food Chem. 367:1-11. doi:10.1016/j.foodchem.2021.130743. [ Links ]
Nayeem, N.; Smb, A.; Salem, H. and Ahei-Alfqy, S. 2016. Gallic acid: a promising lead molecule for drug development. J. Appl. Pharm. 8(2):1-4. Doi: 10.4172/1920-4159.1000213. [ Links ]
Pei, K.; Ou, J.; Huang, J. and Ou, S. 2016. Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 96(9):2952-2962. Doi: 10.1002/jsfa.7578. [ Links ]
Peñarrieta, J. M.; Tejeda, L.; Mollinedo, P.; Vila, J. L. y Bravo, J. A. 2014. Compuestos fenólicos y su presencia en alimentos. Rev. Boliviana de Química. 31(2):68-81. Doi: 10.13140/RG.2.1.5018.1840. [ Links ]
Pioro-Jabrucka, E.; Pawelczak, A.; Baczek, K. and Weglarz, Z. 2011. Accumulation of phenolic and sterol compounds in Euphorbia hirta (L.). Herba Polonica. 57(2):30-37. [ Links ]
Prince, P. S. M.; Rajakumar, S. and Dhanasekar, K. 2011. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur. J. Pharm. 668:233-240. Doi: 10.1016/j.ejphar.2011.06.053. [ Links ]
Rashmi, H. B. and Negi, P. S. 2020. Phenolic acids from vegetables: a review on processing stability and health benefits. Food Res. Inter. 136:2-14. doi.org: 10.1016/j.foodres. 2020.109298. [ Links ]
Semwal, D. K.; Semwal, R. B.; Combrinck, S. and Viljoen, A. 2016. Myricetin: a dietary molecule with diverse biological activities. Nutrients. 8(90):1-31. Doi: 10.3390/nu8020090. [ Links ]
Shahidi, F. and Ambigaipalan, P. 2015. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects a review. J. Functional Foods. 18:820-897. Doi: 10.1016/j.jff.2015.06.018. [ Links ]
Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C. M. and Suresh-Kumar, C. 2018. Syringic acid (SA) a review of its occurrence, biosynthesis, pharmacological and industrial importance. Bio. Pharm. 108:547-557. Doi:10.1016/j.biopha.2018.09.069. [ Links ]
Steinmann, V. W. 2002. Diversidad y endemismo de la familia euphorbiaceae en México. Acta Botánica Mexicana. 61(3):61-93. Doi: 10.21829/abm61.2002.909. [ Links ]
Trejo, L.; Arroyo, T. P. F.; Olsen, K. M.; Eguiarte, L. E.; Arroyo, B.; Gruhn, J. A. and Olson, M. E. 2012. Poinsettia’s wild ancestor in the mexican dry tropics: historical, genetic, and environmental evidence. Am. J. Bot. 99(7):1146-1157. Doi: 10.3732/ajb.1200072. [ Links ]
Wang, W.; Bostic, T. R. and Gu, L. 2010. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 122(4):1193-1198. Doi: 10.1016/j.foodchem.2010.03.114. [ Links ]
Zielinska, D.; Llopis-Laparra, J. M.; Zielinski, H.; Szawara-nowak, D. and Giménez-Bastida, J. A. 2019. Role of apple phytochemicals, phloretin and phloridzin, in modulating processes related to intestinal inflammation. Nutrients . 11(1173):1-14. Doi: 10.3390/nu1105117. [ Links ]
Received: January 01, 2022; Accepted: March 01, 2022











 texto en
texto en