Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.13 no.2 Texcoco Fev./Mar. 2022 Epub 01-Ago-2022
https://doi.org/10.29312/remexca.v13i2.2906
Essays
Phosphites and their applications in agriculture
1Posgrado en Ciencias Agropecuarias y Recursos Naturales-Universidad Autónoma del Estado de México-Campus Universitario El Cerrillo. Piedras Blancas, Toluca, Estado de México. CP. 50200. (emoralesm374@gmail.com).
2Instituto de Ciencias Agropecuarias y Rurales-Campus Universitario El Cerrillo-Universidad Autónoma del Estado de México. Piedras Blancas, Toluca, Estado de México. CP. 50200. (amartinezc@uaemex.mx).
3Centro de Investigación y Estudios Avanzados en Fitomejoramiento-Campus Universitario ‘El Cerrillo’-Universidad Autónoma del Estado de México. Piedras Blancas, Toluca, Estado de México. CP. 50200. (mrubia@uaemex.mx; jalopezsa@uaemex.mx).
4Departamento de Fitotecnia-Universidad Autónoma Chapingo. Carretera México-Texcoco km 38.5, Chapingo, Texcoco, Estado de México. CP. 56230. (anasofiacasg@hotmail.com).
Phosphites are compounds derived from phosphorous acid that regularly combine with ions such as potassium, sodium, calcium or ammonium. The chemical difference between phosphates and phosphites lies in an oxygen atom, which is replaced by a hydrogen atom. Due to their structural similarity, phosphites are considered to be analogs of phosphates. Although the use of phosphites is currently accepted for their plant biostimulant action, as well as for their auxiliary action in the control of phytoparasites such as oomycetes, protozoa, fungi, bacteria and nematodes, their use as a source of phosphorus for plant nutrition is still debated. Both phosphites and phosphates can be taken up by plants through leaves or roots; however, phosphites cannot be reduced within the plant cell to a lower oxidation state. Nevertheless, phosphites can be oxidized to phosphates if applied directly to the soil. The ability of soil microorganisms to be able to oxidize phosphites to phosphates opens up a possibility that phosphites can be applied as a complementary source of nutrition to phosphate fertilizers. The document prepared is a review of studies that addresses the role that phosphites play in agriculture nowadays, their uses as biostimulators, fungicides and their possibility of use as phosphate fertilizer, as well as a compilation of the most relevant studies on these uses and the results.
Key words: biostimulator; fertilizer; fungicide; phosphorous acid
Los fosfitos son compuestos derivados del ácido fosforoso que regularmente se combinan con iones como potasio, sodio, calcio o amonio. La diferencia química entre fosfatos y fosfitos radica en un átomo de oxígeno, el cual es sustituido por uno de hidrógeno. Debido a su similitud estructural, los fosfitos son considerados como análogos de los fosfatos. Si bien en la actualidad es aceptado el uso de los fosfitos por su acción bioestimulante vegetal, así como auxiliar en el control de fitoparásitos como oomycetes, protozoos, hongos, bacterias y nematodos, es aún debatido su uso como fuente de fósforo para la nutrición vegetal. Tanto fosfitos como fosfatos pueden ser absorbidos por las plantas mediante las hojas o las raíces; sin embargo, los fosfitos no se pueden reducir dentro de la célula vegetal a un estado de oxidación más bajo. No obstante, los fosfitos pueden verse oxidados a fosfatos si se aplican directamente al suelo. La capacidad de microorganismos del suelo de poder oxidar los fosfitos a fosfatos abre una posibilidad de que estos puedan ser aplicados como fuente de nutrición complementaria a los fertilizantes fosfatados. El documento elaborado es una revisión de las investigaciones que aborda el papel de los fosfitos dentro de la agricultura en la actualidad, sus usos como bioestimulador, fungicida y su posibilidad de uso como fertilizante fosfatado, así como una recopilación de las investigaciones más relevantes sobre estos usos y los resultados.
Palabras clave: ácido fosforoso; bioestimulador; fertilizante; fungicida
Phosphorus (P) is an essential nutrient for plant growth and food production, because it is a primary component of the systems responsible for the storage and transfer of energy, it is a basic compound in the structures of macromolecules such as nucleic acids and phospholipids, so it can be said that its role is generalized in all plant physiological processes (Fernández, 2007), in addition, this element constitutes about 0.2% of plant dry matter (Aziz et al., 2013).
P is involved in a series of metabolic and structural functions; it is part of energy molecules such as adenosine diphosphate (ADP), adenosine triphosphate (ATP) or guanosine triphosphate (GTP), which can regulate plant enzymatic activity, and participates in the formation of DNA blocks and cell membranes (Hawkesford et al., 2012; Vinas et al., 2020). P accelerates maturation and promotes seed production, it is even an important part of numerous fundamental processes of plant metabolism, such as glycolysis, biosynthesis of carbohydrates and lipids, synthesis of chlorophylls and carotenoids and metabolism of organic acids (Estrada-Ortiz et al., 2011).
P deficiencies result in a preferential allocation of carbohydrates to the roots, resulting in a disparate increase in root growth in relation to the stem. This also results in the modification of photosynthesis and sugar metabolism, so that vegetative and reproductive growth and development is delayed (Aziz et al., 2013). In nature, P is not found as a free element, but exists in combination with other elements such as oxygen (O) or hydrogen (H), this occurs as a completely oxidized form called phosphate anion ( PO 4 3- ), or with one less oxygen called phosphite anion ( PO 3 3- ;) (Bozzo et al., 2004; Thao and Yamakawa, 2009). Under neutral pH conditions, the phosphate ion is present as phosphoric acid ( H 3 PO 4 ) and dioxophosphoric acid ( H 3 PO 2 ); the former being the form in which phosphate is normally transported in plant cells (McDonald et al., 2001; Mixquititla-Casbis and Villegas-Torres, 2016).
What are phosphites?
According to Havlin and Schlegel (2021), phosphites (Phis) are a reduced form of phosphates (Pis), derived from phosphorous acid (H 3 PO 3 - ), which regularly combine with nonmetallic cations such as potassium, sodium, calcium or ammonium. The terms ‘phosphite’ or ‘phosphonate’ are used in the literature to refer to salts derived from phosphorous acid (Thao and Yamakawa, 2009; Yáñez-Juárez et al., 2018). The chemical difference between phosphate ( H 2 PO 4 − ; Pi) and phosphite ( H 2 PO 3 − ; Phi) is an oxygen atom which is replaced by a hydrogen atom (Figure 1) (Lovatt and Mikkelsen, 2006, Yáñez-Juárez et al., 2018, Fathi et al., 2021).
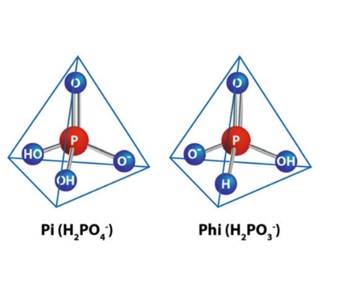
Figure 1 Structure of the phosphate (Pi) and phosphite (Phi) groups (Gómez-Merino and Trejo-Téllez, 2015).
Due to their structural similarity, phosphites are considered as analogs of phosphates; at present, the use of phosphites is widely accepted for their action in the control of phytoparasites and as a biostimulant in plants, however, their use as a source of phosphorus in plant nutrition is still debated (Gómez-Merino and Trejo-Téllez, 2015). Phosphites are marketed as foliar fertilizers and fungicides, these are within toxicity category III, which implies that their use in agriculture is not very dangerous for man, animals and the environment (Barpen, 2004). On the market, there are available formulations of the product in association with other nutrients such as potassium (K), calcium (Ca), boron (B), zinc (Zn) or manganese (Mn) (Mixquititla-Casbis and Villegas-Torres, 2016). Phosphorous acid ( H 3 PO 3 - ) and its salts contain higher concentrations of P (39%) in relation to traditional phosphate fertilizers (32% P) (Lovatt and Mikkelsen, 2006).
Phis cannot be converted into Pis within the plant, due to the absence of the ptxD gene, which is present in many soil bacteria (Wu et al., 2018); therefore, they do not participate in biochemical pathways, so that negative effects of these on plant metabolism are observed (Varadarajan et al.,2002). However, Phis make the use of specific fungicides more effective and increase the defenses of plants against the possibility of infection by some pathogen (Bettiol, 2006; Lovatt and Mikkelsen, 2006).
Phosphites in the control of phytopathogens
Phosphites have been widely studied as an alternative for the control of phytoparasitic organisms. Their efficacy has been proven against protozoa, oomycetes, fungi, bacteria and nematodes. The efficacy levels of phosphites in the control of phytoparasitic organisms vary depending on the ion bound to the phosphite (potassium phosphite, calcium phosphite, among others), the method of application (via root or foliarly), the phytopathogenic organism and the host plant (Monsalve et al., 2012; Yáñez-Juárez et al., 2018).
The effectiveness of phosphites in the control of phytopathogens occurs directly or indirectly (Figure 2). In the first, the phosphite ion, when in contact with phytopathogenic organisms, affects their growth and reproduction, by influencing the expression of genes that encode the synthesis of compounds indispensable in the structure and physiology of the cell. For example, in oomycetes, the application of phosphites inhibits the oxidative phosphorylation of metabolism, in the mycelium, they inhibit growth and change the composition of the surface, increase the activity of the pentose phosphate pathway and inhibit enzymes allosterically regulated by phosphate (Yáñez-Juárez et al., 2018; García-Velasco et al., 2020).
The indirect pathway is related to the increase in the resistance of the plant. Phosphite has been considered as a biostimulator of systemic acquired resistance (SAR), when entering the cells of plant tissue, it activates biochemical and structural defense mechanisms (such as the production of polysaccharides, phytoalexins or pathogenesis-related proteins ‘PR’) that restrict the penetration and survival of pathogens (Monsalve et al., 2012; Yáñez-Juárez et al., 2018).
Wong et al. (2009) compared the effect of the phosphite ion and the phosphate ion on the growth of Phytophtora cinnamomi in vitro and verified the susceptibility of microorganisms to the phosphite ion, but not to the phosphate ion. Mogollón and Castaño (2012) demonstrated the reduction of the number and size of colonies and percentage of germination of Mycosphaerella fijiensis in Agar V8 culture medium by applying a concentration of 27 ml L-1 of potassium phosphite. For their part, Hofgaard et al. (2010) obtained decreases of 60, 80 and 90% in the mycelium growth of Fusarium culmorum, Fusarium graminearum and Microdochium majus respectively, with 10 μl ml-1 of potassium phosphite.
King et al. (2010) demonstrated that there was a potassium phosphite-induced modification in the expression of genes that encode the synthesis of proteins that constitute the cell wall and cytoskeleton of Phytophtora cinnamomi, which caused breaks in the cell wall and distortions in the hyphae. Amiri and Bompiex (2011) concluded that there was a germination percentage of 53 and 0% of Penicillium expansum conidia in malt dextrose agar medium, when 2 mg L-1 of Phi was applied and heating the medium to 20 and 50 °C, respectively. On the other hand, Cerioni et al. (2013) showed that concentrations of 229, 334, 360, 469, 498 and 580 mg L-1 of Phi inhibited the germination of Penicillium digitatum conidia in potato dextrose agar (PDA) culture media.
Araujo et al. (2010) evaluated the efficacy of potassium phosphite in the control of Colletotrichum gloeosporoides, which causes glomerella leaf spot disease in Malus domestica, using in vivo and in vitro experiments. They showed that the addition of phosphites interferes with mycelial development, which reduced the severity of the disease in leaves up to 62% in in vivo experiments, while in vitro, the diameter of the colony decreased up to 94%.
As for the indirect pathway, phosphites can activate the natural defense mechanisms of plants by producing phytoalexins, PR (pathogenesis-related) proteins and structural polysaccharides. This activation can occur due to the high mobility of phosphites, which are rapidly absorbed via root or foliarly (Daniel and Guest, 2005; Ribeiro-Chagas et al., 2020).
Wu et al. (2018) demonstrated significant changes in the metabolomics of potato leaves (Solanum tuberosum L.) when they came into contact with applications of Phi, they found changes in the concentration of products intermediary of sugar metabolism, as well as the citric acid cycle. The groups of several sugars were smaller, probably due to the interference of Phi in phosphorylation reactions that could influence the phosphorylated monosaccharide group, which ultimately affect free sugar levels and starch accumulation. In addition, Phi applications are associated with higher levels of the components of the phenylpropanoid pathway, such as chlorogenic acid, caffeic acid, and salicylic acid, compounds that are essential components in plant defense mechanisms (Figure 3).

Figure 3 Main metabolites identified in the metabolomics of potato leaf samples and alteration in their abundance in plants treated with Phi. Highlighted in yellow boxes are metabolites that show an increase of more than 1.3 times in abundance in plants treated with Phi, while in blue boxes are those that show a decrease of more than 1.3 times in abundance. Metabolites in white boxes were identified but their abundance did not change in plants treated with Phi compared to controls (Wu et al., 2018).
However, comparative reports of control efficacy between phosphites and conventional fungicides indicate that the former are less effective and cannot replace them completely, but their integration as part of an integrated management program allows reducing the use of fungicides and reducing the possibility of generating resistance by organisms (Liljeroth et al., 2016). Silva et al. (2013) found a significant decrease in the area under the progress curve of the disease caused by Peronospora manshurica with applications of acibenzolar S-methyl and phosphites in soybean (Glycine max).
Da Silva Neves and Blum (2014) reported that applications of mixtures of the fungicides pyraclostrobin + epoxiconazole, thiophanate methyl + flutriafol and tebuconazole, along with applications of potassium phosphite reduce the severity of Phakopsora pachyrhizi in soybean. Ribero-Chagas et al. (2020) mention that with applications of phosphites with cyproconazole, it is possible to control the incidence of Curvularia sp., in the hybrid 30F53YH of corn (Zea mays L.).
Jackson et al. (2000) reported a restriction in the development of lesions caused by P. cinnamomi in Eucalyptus marginata tissues, due to a significant increase in the enzymes 4-coumarate coenzyme A ligase and cinnamyl alcohol dehydrogenase and soluble phenols. Olivieri et al. (2012) reported increases in pectin content in tissues of the periderm and cortex of potato tubers treated with potassium phosphite, which improved resistance to microbiological attacks. On the other hand, Eshraghi et al. (2011) showed that Arabidopsis thaliana seedlings inoculated with P. cinnamomi treated with potassium phosphite show an increase in the production of callose and hydrogen peroxide (H2O2) in infected cells.
Use of phosphites as a source of phosphorus
The conventional form of absorption of P by plants is phosphates. Currently, there are several chemical products for agricultural use, which have been presented as fertilizers for supplementation of P, mainly via foliar, which use as a primary source of this element reduced forms such as K or Ca phosphites among others; however, studies on this fertilizing effect point to low or no action (Bertsch et al., 2009). Thao et al. (2008) concluded that potassium phosphite applied via root or foliarly does not provide nutrition to spinach plants (Spinacia oleracea L.), on the contrary, it severely inhibited root growth in plants with phosphate deficiency, even with low levels of foliar applications of phosphites.
Thao and Yamakawa (2009) are blunt in mentioning that phosphites are not fertilizers and have no beneficial effect on the growth of healthy plants, so they should not be marketed as such. Thao et al. (2009) studied the effect of phosphites in relation to phosphates on the growth and quality of lettuce (Lactuca sativa L.) and demonstrated that the addition of phosphites did not improve plant growth or quality. Bertsch et al. (2009) studied the ability of phosphites to meet the needs of P in lettuce (Lactuca sativa L.), tomato (Solanum lycopersicum) and banana (Musa × paradisiaca) and demonstrated that P in the form of phosphite is not usable by the plant to meet its P needs and tends to cause harm, and that under conditions of P deficiency, the phosphites via root did not contribute to the growth of the crops, but intensified the deterioration of the foliage and the root.
These results differ from those of studies that describe that the use of phosphites in agriculture as a source of phosphate fertilization can occur if the phosphites come into contact with microorganisms that have the ability to oxidize them to phosphates (McDonald et al., 2001; Manna et al., 2016). Several species of Bacillus can oxidize phosphite and hypophosphite (H2PO2) to phosphate. In addition, several common laboratory strains, including Escherichia coli, have been reported to be able to oxidize phosphites. Microbial oxidation of phosphite is possible thanks to the action of the enzyme phosphite dehydrogenase (PTDH) (Figure 4) (Relyea and Van der Donk, 2005).

Figure 4 Equation of oxidation of phosphite to phosphate by the PTDH enzyme (Relyea and Van der Donk, 2005).
Lovatt (1999) reported increases in orange yield (Citrus × sinensis) when performing foliar applications of phosphite, based on the number of fruits harvested per tree. Similarly, Albrigo (1999) found positive effects on the number of flowers, yield and total soluble solids in orange fruits after a foliar spray. Bertsch et al. (2009) indicate that phosphite + phosphate concentrations promoted greater total absorption of P in lettuce, tomato and banana; however, they did not identify the chemical form in which P is found within the plant.
Estrada-Ortiz et al. (2011) showed that, in the fruiting stage, the addition of 30% of the total P as phosphite stimulated the metabolism of strawberry (Fragaria × ananassa Duch.), increasing the concentrations of chlorophylls a, b and total, amino acids and proteins. Similarly, Estrada et al. (2012) showed, in lettuce cultivation, that phosphite applications of up to 0.5 meq L-1 of nutrient solution favored the accumulation of N, P and K in the roots and stimulated the accumulation of chlorophylls a, b and total in leaves, without affecting the dry matter gain of the plant.
Lovatt and Mikkelsen (2006) stated that the negative effects on plant growth are the result of inappropriate use of phosphites as a primary source of P or with applications in excessive amounts. Since phosphite is chemically different from phosphate, these differences must be taken into consideration to avoid phytotoxic effects.
Conclusions
The use of phosphites as fungicides or biostimulators of the systemic acquired resistance of plants is widely accepted, to avoid the incidence of phytoparasitic organisms. The direct mode of action helps especially against oomycetes, in addition to fungi, bacteria, protozoa and nematodes. On the other hand, the application of phosphites in crops promotes an improvement in the defense system of plants and the amounts of structural polysaccharides, PR proteins and phytoalexins increase. Although phosphites by themselves are less effective in the control of phytoparasites, they can be included in the integrated control of diseases, to further reduce the incidence in crops.
The fertilizing capacity of phosphites is still debated, although these can be absorbed via root or foliarly, and can be transported via xylem or phloem, they cannot be used as a direct form of phosphate nutrition and therefore, they cannot be direct substitutes for phosphate fertilizers. However, the ability of soil microorganisms to oxidize phosphites to phosphates opens up a possibility that these can be applied as a source of fertilization complementary to phosphate fertilizers. It should also be considered that there are studies that support the effectiveness of phosphites as a partial source of fertilization. It is necessary to continue research on the efficacy of applying phosphites as a source of complementary fertilization in intensive agriculture.
Acknowledgements
The authors thank the National Council for Science and Technology (CONACYT, for its acronym in Spanish) for the scholarship provided to the first author to carry out the Doctorate in Agricultural Sciences and Natural Resources.
REFERENCES
Albrigo, L. G. 1999. Effects of foliar applications of urea or nutriphite on flowering and yields of Valencia orange tres. Proc. Fla. State Hort. Soc. 112(1):1-4. [ Links ]
Amiri, A. and Bompeix, G. 2011. Control of penicillium expansum with potassium phosphite and heat treatment. Crop Protec. 30(2):222-227. https://doi.org/10.1016/j.cropro.2010. 10.010. [ Links ]
Araujo, L.; Valdebenito-Sanhueza, R. M. and Stadnik, M. J. 2010. Avaliação de formulações de fosfito de potássio sobre Colletotrichum gloeosporioides in vitro e no controle pósinfeccional da mancha foliar de glomerella em macieira. Tropical Plant Pathol. 35(1):54-59. http://www.scielo.br/pdf/ tpp/v35n1/a10v35n1. [ Links ]
Aziz, T.; Sabir, M.; Farooq, M.; Maqsood, M. and Ahmad, H. 2013. Phosphorous deficiency in plants: responses, adaptive mechanisms, and signaling. Plant signaling understanding the molecular crosstalk. Springer, New Delhi. 133-148 pp. Doi: 10.1007/978-81.322-1542-4-7. [ Links ]
Barpen. 2004. Ficha técnica Agrifos® 400 SL. http://www.ghcia.com.co/plm/source/productos/2418-13-206.htm . [ Links ]
Bertsch, F.; Ramírez, F. y Henríquez, C. 2009. Evaluación del fosfito como fuente fertilizante de fósforo vía radical y foliar. Agron. Costarricense. 33(2):249-265. https://www.redalyc.org/articulo.oa?id=436/43613279009 . [ Links ]
Bettiol, W. 2006. Productos alternativos para el manejo de enfermedades en cultivos comerciales. Fitosanidad. 10(2):85-98. [ Links ]
Bozzo, G. G.; Singh, V. K. and Plaxton, W. C. 2004. Phosphate of phosphate addition promotes the proteolytic turnover of phosphate-starvation inducible tomato purple acid phosphatase isozymes. FEBS letters. 573(1):51-54. [ Links ]
Cerioni, L.; Rapisarda, V. A.; Doctor, J.; Fikkert, S.; Ruiz, T.; Fassel, R. and Smilanick, J. L. 2013. Use of phosphite salts in laboratory and semicommercial tests to control citrus postharvest decay. Plant Dis. 97(2):201-212. http://dx.doi.org/10.1094/PDIS-03-12-0299-RE. [ Links ]
Da-Silva, N. J and Blum, L. E. 2014. Influência de fungicidas e fosfito de potássio no controle da ferrugem asiática e na produtividade da soja. Rev. Caatinga. 27(1):75-82. https://www.redalyc.org/articulo.oa?id=2371/237130153009. [ Links ]
Daniel, R. and Guest, D. 2005. Defense responses induced by potassium phosphonate in Phytophthora palmivora challenged Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 67(3-5):194-201. [ Links ]
Eshraghi, L.; Anderson, J.; Aryamanesh, N.; Shearer, B.; McComb, J.; Hardy, G. E. S. and Brien, P. A. 2011. Phosphite primed defense responses and enhanced expression of defense genes in Arabidopsis thaliana infected with phytophthora cinnamomi. Plant Athology. 60(6):1086-1095. https://doi.org/10.1111/j.1365-3059.2011.02471.x. [ Links ]
Estrada-Ortiz, E.; Trejo, L. I.; Gómez, F. C.; Núñez, R. y Sandoval, M. 2011. Respuestas bioquímicas en fresa al suministro de fósforo en forma de fosfito. Rev. Chapingo Ser. Hortic. 17(3):129-138. [ Links ]
Estrada, O. E.; Gómez, F. C.; Silva, H. V.; Castillo, A. M. y Avitia, E. 2012. Respuestas de lechuga a la aplicación de fosfito en la solución nutritive. In: Congreso Nacional de Ciencias Agronómicas. Colegio de Postgraduados en Ciencias Agrícolas. Texcoco, Estado de México. [ Links ]
Fathi, Z.; Zamani, K. and Malboobi, M. 2021. Phosphite, biotechnology, modern agriculture. Crop Biotechnol. 10(32):55-70. Doi: 10.30473/cb.2021.57825.1833. [ Links ]
Fernández, M. T. 2007. Fósforo: amigo o enemigo. ICIDCA. Sobre los derivados de la caña de azúcar. 41(2):51-57. https://www.redalyc.org/articulo.oa?id=223114970009. [ Links ]
García-Velasco, R.; Mora-Herrera, M. E.; Mejía-Carranza, J.; Aguilar-Medel, S. y González-Millán, M. 2020. Fosfitos de potasio en el manejo de peronospora sparsa berkeley y calidad floral del cultivo de rosa cv Samouraï. Acta Agrícola y Pecuaria. 7(1):1-10. https://doi.org/10.30973/aap/2021.7.0071004. [ Links ]
Gómez-Merino, F. C. and Trejo-Téllez, L. I. 2015. Biostimulant activity of phosphite in horticulture. Scientia Hortic. 196(1):82-90. https://doi.org/10.1016/j.scienta.2015.09.035. [ Links ]
Havlin, J. L. and Schlegel, A. J. 2021. Review of phosphite as a plant nutrient and fungicide soil systems. 5(3):52-71. https://doi.org/10.3390/soilsystems5030052. [ Links ]
Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I. S. and White, P. 2012. Chapter 6 functions of macronutrients. In: marschner, P. (Ed.), marschner’s mineral nutrition of higher plants, third edition. Academic press, San Diego. 135-189 pp. https://doi.org/10.1016/B978-0-12-384905-2.00006-6. [ Links ]
Hofgaard, I. S.; Ergon, A.; Henriksen, B. and Tronsmo, A. M. 2010. The effect of potential resistance inducers on development of microdochium majus and Fusarium culmorum in winter wheat. Eur. J. Plant Pathol. 128(2):269-281. https://doi.org/10.1007/s10658-010-9662-5. [ Links ]
Jackson, T. J.; Burgess, T.; Colquhoun, I. and Hardy, G. 2000. Action of the fungicide phosphite on Eucalyptus marginate inoculated with Phytophthora cinnamomi. Plant Patholol. 49(1):147-154. https://doi.org/10.1046/j.1365-3059.2000.00422.x. [ Links ]
King, M.; Reeve, W.; Van-Hoek, M. B.; Williams, N.; McComb, J.; Brien, P. A. and Hardy, G. E. 2010. Defining the phosphite-regulated transcriptome of the plant pathogen phytophthora cinnamomi. Mol. Genet Genomics. 284(6):425-435. https://doi.org/10.1007/s00438-010-0579-7. [ Links ]
Liljeroth, E.; Lankinen, A.; Wiik, L.; Burra, D. D.; Alexandersson, E. and Andreasson, E. 2016. Potassium phosphite combined with reduced doses of fungicides provides efficient protection against potato late blight in large-scale field trials. Crop Protec. 86(1):42-55. [ Links ]
Lovatt, C. J. 1999. Timing citrus and avocado foliar nutrient applications to increase fruit set and size. HortTech. 9(4):607-612. [ Links ]
Lovatt, C. and Mikkelsen, R. 2006. Phosphite fertilizers: what are they? can you use them? what can they do? Better Crops. 90(4):11-13. [ Links ]
Manna, M.; Achary, V. M. M.; Islam T. M; Agrawal, P. Q. and Reddy, M. K. 2016. The development of a phosphite-mediated fertilization and weed control system for rice. Scientific Reports. 6(1):1-13. https://doi.org/ 10.1038/srep24941. [ Links ]
McDonald, A. E.; Grant, B. R. and Plaxton W. C. 2001. Phosphite (phosphorous acid): its relevance in the environment and agriculture, and influence on the plant phosphate starvation response. J. Plant Nutr. 24(10):1505-1519. Doi: 10.1081/PLN-100106017. [ Links ]
Mixquititla-Casbis, G. y Villegas-Torres, O. G. 2016. Importancia de los fosfatos y fosfitos en la nutrición de cultivos. Acta Agrícola y Pecuaria . 2(3):55-61. [ Links ]
Mogollón, A. M. and Castaño, J. 2012. Evaluación in vitro de inductores de resistencia sobre Mycosphaerella fijiensis Morelet. Rev. Facultad Nacional de Agronomía. 65(1):6327-6336. [ Links ]
Monsalve, V.; Viteri, R.; Rubio, C. y Tovar, D. 2012. Efectos del fosfito de potasio en combinación con el fungicida metalaxyl mancozeb en el control de Mildeo velloso (peronospora destructor berk) en cebolla de bulbo (Allium cepa L.). Rev. Facultad Nacional de Agronomía Medellín . 65(1):6317-6325. http://www.redalyc.org/articulo.oa?id= 179924340003. [ Links ]
Olivieri, F. P.; Feldman, M. L.; Machinandiarena, M. F.; Lobato, M. C.; Caldiz, D. O.; Dalo, G. R. and Andreu, A. B. 2012. Phosphite applications induce molecular modifications in potato tuber periderm and cortex that enhance resistance to pathogens. Crop Protec. 32(1):1-6. https://doi.org/10.1016/j.cropro. 2011.08.025. [ Links ]
Relyea, H. A. and Van-Donk, W. A. 2005. Mechanism and applications of phosphite dehydrogenase. Bioorganic Chem. 33(3):171-189. Doi: 10.1016/j.bioorg.2005.01.003. [ Links ]
Ribeiro-Chagas, J.; Costa, R.; Dos-Santos, G.; Abadia, M. and Costa, E. 2020. Foliar fungal diseases control and productivity depending on the phosphite and fungicide application in two corn hybrids. Biot. Veg. Villa clara. 20(1):33-41. http://scielo.sld.cu/scielo.php? script=sci-arttext&pid=S207486472020000100033&lng=es&nrm=iso. [ Links ]
Silva, O.; Santos, A. A.; Deschamps, C.; Dalla-Pria, M. y May-Mio, L. 2013. Fontes de fosfito e acibenzolar-S-metílico associados a fungicidas para o controle de doenças foliares na cultura da soja. Tropical plant pathology. 38(1):72-77. [ Links ]
Thao, H. and Yamakawa, T. 2009. Phosphite (phosphorous acid): fungicide, fertilizer or bio-estimulator? Soil Sci. Plant Nutr. 55(2):228-234. Doi: 10.1111/j.1747-0765.2009.00365.x. [ Links ]
Thao, H.; Yamakawa T.; Myint, A. and Sarr, P. 2008. Effects of phosphite, a reduced form of phosphate, on the growth and phosphorus nutrition of spinach (Spinacia oleracea L.). Soil Sci. Plant Nutr. 54(5):761-768. [ Links ]
Thao, H.; Yamakawa T. and Shibata, K. 2009. Effect of phosphite-phosphate interaction on growth and quality of hydroponic lettuce (Lactuca sativa). Soil Sci. Plant Nutr Sci. 172(3):378-384. [ Links ]
Varadarajan, D. K; Karthikeyan, A. S.; Matilda, P. D. and Raghothama, K. G. 2002. Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol. 129(3):1232-1240. [ Links ]
Vinas, M; Mendez, J. C. and Jiménez, V. M. 2020. Effect of foliar applications of phosphites on growth, nutritional status and defense responses in tomato plants. Sci. Hortic. 265(1):109-200. Doi: 10.1016/j.scienta.2020.109200. [ Links ]
Wong, M. A.; McComb, B. J.; Hardy, B. G. E. J. and Brien, P. A. 2009. Phosphite induces expression of a putative proteophosphoglycan gene in phytophthora cinnamomi. Australasian Plant Pathol. 38(3):235-241. https://doi.org/10.1071/AP08101. [ Links ]
Wu, L.; Gao, X.; Xia, F.; Joshi, J.; Borza, T. and Wang-Pruski, G. 2018. Biostimulant and fungicidal effects of phosphite assessed by GC-TOF-MS analysis of potato leaf metabolome. Physiol. Mol. Plant Pathol . 106(1):49-56. https://doi.org/10.1016/j.pmpp. 2018.12.001. [ Links ]
Yáñez-Juárez, M. G.; López-Orona, C. A.; Ayala-Tafoya F.; Partida-Ruvalcaba, L.; Velázquez-Alcaraz T. J. and Medina-López R. 2018. Phosphites as alternative for the management of phytopathological problems. Rev. Mex. Fitopatol. 36(1):79-94. https://doi.org/10.18781/r.mex.fit.1710-7 . [ Links ]
Received: January 01, 2022; Accepted: February 01, 2022











 texto em
texto em 



