Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.13 no.1 Texcoco ene./feb. 2022 Epub 02-Mayo-2022
https://doi.org/10.29312/remexca.v13i1.2790
Articles
Creole maize from South-West of São Paulo, Brazil: diversity and seed quality
1Department of Agroindustrial Technology and Social Economy Rural (DTAiSeR-Ar)-Federal University of São Carlos (UFSCar). Anhanguera Road km 174, Araras, São Paulo, Brazil. CEP. 13604-900. (lah-cfl@hotmail.com; victor-ufscar@outlook.com; janice@ufscar.br).
2Agronomic Institute of the Secretariat of Agriculture of the State of São Paulo. Sebastião Ferraz de Camargo Penteado Road km 232, Caitê, Capão Bonito, São Paulo, Brazil. CP. 62. CEP. 18300-970. (cfachini@iac.sp.gov.br).
The maintenance of creole seeds promotes preservation of agrobiodiversity and family autonomy. For this reason, seeds quality is essential in the context of creole seeds because directly impacts the improvement of the production field and, consequently, its continuous existence. The study evaluated the diversity and the seed quality of creole maize seeds in two harvests in the South-West of São Paulo, Brazil, an important maize production site in Brazil. Seeds from both harvests (2019 and 2020) were evaluated regarding the physical (physical aspects, one test seed mass, test of infestation), physiological (water content, germination test, seedling emergence in soil, emergence speed index and cold test) and health potential (blotter test). Among 20 lots collected, the seeds were classified into five varieties according to family famers perception. It was observed a variation in terms of physical, physiological and health quality between the seed lots. The lots harvested in 2020 had the highest values of size, 1000 seed mass, germination and vigor. The blotter test identified for both harvests, mainly in 2019, high incidence of Aspergillus sp. and Penicillium sp., considered as storage fungi. Therefore, the variation in seed quality between the harvest refers mainly to the characteristics of the storage process performed. More studies on better strategies for creole maize seed storage are necessary to guarantee seed quality, since low seed quality is a risk for losing these materials.
Keywords Zea mays; physical analysis; physiological potential; creole variety
El mantenimiento de semillas criollas promueve la preservación de la agrobiodiversidad y la autonomía familiar. Por este motivo, la calidad de las semillas es fundamental, en el contexto de las semillas criollas, porque impacta directamente en la permanencia de esas semillas. El estudio evaluó la diversidad y la calidad de las semillas de maíces criollos, en dos cosechas en el suroeste de São Paulo, Brasil. Las semillas de ambas cosechas (2019 y 2020) se evaluaron en los aspectos físicos (masa de las semillas y prueba de infestación), fisiológicos (prueba de germinación, emergencia de plántula en suelo, índice de velocidad de emergencia y prueba de frío) y salud potencial (prueba de papel secante). Entre los 20 lotes recolectados, las semillas se clasificaron en cinco variedades según la percepción de los agricultores familiares. Se observó una variación de calidad física, fisiológica y sanitaria entre los lotes de semillas. Los lotes cosechados en 2020 tuvieron los valores más elevados en cuanto a tamaño, masa de 1 000 semillas, germinación y vigor. La prueba secante identificó para ambas cosechas, principalmente en 2019, una alta incidencia de Aspergillus sp. y Penicillium sp., considerados hongos de almacenamiento. Consecuentemente, la variación en la calidad de las semillas se debe a las características del proceso de almacenamiento. No obstante, resultan necesarios más estudios sobre estrategias de almacenamiento de semillas de maíces criollos para garantizar la calidad de las semillas, ya que la baja calidad de estas representa un riesgo de pérdida de estos materiales.
Palabras clave Zea mays; análisis físico; potencial fisiológico; variedad criolla
Introduction
The maize (Zea mays) production is mainly employed for animal feed and in the food industry. Even among the production demands of commercial cultivars of the market of commodities, because of globalization, creole maize has been maintained by the production and consumption in family farming, responsible for the preservation, maintenance and propagation of creole seeds linked to the traditions, histories, culture and feeding (Antonello et al., 2014).
Creole seeds have peculiar characteristics of great importance for the preservation of biodiversity, maintenance of genetic variability, adaptation to the production conditions, which gives higher resistance to the occurrence of pathogens and superior tolerance to climatic variations (Catão et al., 2013). Furthermore, the maintenance of these materials promotes farmer autonomy, allowing income generation and reducing production cost, since they have their own material for sowing.
Seed quality directly impacts the improvement of the production field and, consequently, its continuous existence. Therefore, the understanding of seed quality becomes crucial both for productivity improvement (Finch-Savage and Bassel, 2016) and for the maintenance of the agricultural diversity in the context of family farming. The seed quality concept refers to the determination of physical, physiological, genetic and health attributes in order to find the best materials for plant propagation.
The tests for the determination of these attributes are usually performed for seed commercialization aiming at controlling and standardizing the materials that will be commercialized (Brasil, 2009). Nevertheless, for creole seeds, the proposal of quality evaluation does not refer to the commercialization potential, but to guaranteeing seeds able to generate high percentage of seedlings, promoting an appropriate stand and assuring high productivities. The physical quality refers to the integrity of seeds and seed lots, considering the physical purity and physical damages caused at different production stages (De Medeiros et al., 2019).
The physiological quality, which includes attributes related to germination and vigor, demonstrates which seeds present viability and potential to generate seedlings in a faster and more uniform way even in adverse field conditions (Han et al., 2014). The health potential is related to the presence or absence of field and storage pathogens (Santos et al., 2014). Thus, the quality tests provide information to assist in lot differentiation and in the decision-making of the family farmers, such as which are the best storage strategies (Da Silva et al., 2019). Therefore, it is possible to guarantee a better quality control of these materials used by family farmers.
The South-West region of São Paulo in Brazil was chosen as the place for acquisition and analysis of the creole maize seeds, since it has agricultural visibility and strong presence of the family agriculture, which constitutes the scenario of creole maize seeds. This culture is highlighted by the economic, historic, cultural bias and is considered an indemnity icon because of the intimate relationship presented by the inhabitants of the region (Fachini and Mariuzzo, 2019). Therefore, this research aimed evaluate the diversity of creole maize seed production in the South-West of São Paulo, Brazil, and evaluating their physical, physiological and health quality.
Materials and methods
The creole maize seeds collected for this study were from family farmers properties of the South-West of São Paulo, Brasil, in the municipalities related to the project ‘Roteiro do Milho’ (Fachini and Mariuzzo, 2019), namely Capão Bonito, Guapiara, Itapeva, Ribeirão Branco and Ribeirão Grande. For the harvest of 2019, the lots were stored differently for each farmer, except lot APA 2, whose seeds were collected directly from the field. For the harvest of 2020, the analysis was performed immediately after harvest, without storage. The access to the materials was authorized by SISBIO (nº 70283-1) and had prior consent, signed by all farmers participating in this research, (CAAE nº 22150919.7.0000.5504) which contribute with some essential information as variety classification and storage conditions for each lot.
After the materials were collected, the lots were placed in plastic packaging and the seeds were maintained under laboratory condition, without control of temperature and relative humidity of the air, for approximately two weeks. Before the analyses, the seeds were photographed to obtain information regarding the physical characterization, with evaluation of color, size and shape. Seed size was determined by a scale of checkerboard pattern, with dimensions of 0.5 centimeter on a blue paper. After conditioning they were evaluated regarding the physical, physiological and health potential by the tests described below.
One thousand seed mass: performed in four repetitions of 100 seeds per lot, in which the seeds were weighed on an analytical scale of four decimal digits (0.0001g), with the results extrapolated to one thousand seeds, adjusting the water content to 13% (Brasil, 2009).
Test of infestation: four repetitions of 25 seeds per sample were soaked in a paper substrate moistened with water at the proportion of 2.5 times the paper mass, for 3 h, in a controlled environment of BOD at 25 ºC. Each seed was cut, and which presented tunnels or larvae were considered infested (Brasil, 2009).
Water content: it was conducted in a oven at 105 ±3 ºC for 24 h (Brasil, 2009).
Germination and first count of germination: four repetitions of 50 seeds were distributed in two paper sheets of the germitest type, moistened with water in a volume equivalent to 2.5 times the paper dry mass, and subsequently they were covered with an additional sheet for the manufacture of the rolls. The rolls were maintained in germination chamber, of the BOD type, at the temperature of 25 °C and photoperiod of 8 h for seven days. The counts of normal seedlings were performed on the fourth day, determining the first germination count, and on the seventh day, to determine the total germination (Brasil, 2009).
Seedling emergence in soil: the soil was sieved and stored in plastic boxes (40x22x7 cm) and moistened with 60% of the water retention capacity. The test was conducted considering four repetitions with 50 seeds for each lot, which were sown at 1 cm of depth, maintained in open environment, without control of temperature and relative humidity of the air, for 14 days, computing the percentage of emerged seedlings.
Emergence speed index-ESI: it was performed together with the test of seedling emergence in soil, computing the number of emerged seedlings for each of the lots in the period of 14 interspersed days. The index was calculated by the formula proposed by Maguire (1962), considered as the ratio between the number of emerged seedlings each day on the day of evaluation. ESI= E1 /N1 + E2 /N2 + E3 /N3 + ...+ En /Nn. Where: E1, E2, E3, ..., En = number of seedlings emerged on the day of observation and N1, N2, N3, ..., Nn = number of days after sowing.
Cold test in roll with soil: performed with four repetitions of 50 seeds for each lot in which the seeds were placed in a paper roll of the germitest type, composed of three sheets and moistened with a water volume equivalent to 2.5 times its dry mass, adding a volume of 0.2 L roll-1 of sieved soil. The rolls were initially maintained in cold chamber at the temperature of 10 ºC for a period of seven days. After this period, the rolls were transferred to a germination chamber, of the BOD type, at 25 °C with photoperiod of 8 h for additional seven days (De Almeida Silva et al., 2008). After this period, the rolls were evaluated, computing the percentage of normal seedlings.
Blotter test: to analysis the health potential, the test was performed in four repetitions of 25 seeds for each lot. The seeds were maintained on two sheets of blotting paper, moistened with distilled water, for a period of seven days in a controlled environment in BOD at 20 ±2 ºC and photoperiod of 8 h. After seven days, under enough luminosity for the observations of the fractional components, with the help of magnifying glasses with resolution of 10 to 40x, the seeds were examined and computed regarding the presence of typical signs of the main phytopathogenic fungi linked to the maize seeds (Brasil, 2009).
Statistical analysis
The experiment was conducted in a completely randomized design considering the isolated analysis for each of the harvests (2019 and 2020). The data were analyzed by analysis of variance (Anova) and when there was significant effect, the means were compared by the Tukey’s test at 5% of probability.
Results and discussion
Among these materials produced (15 lots from 2019 and 5 lots from 2020), the seeds were classified by the producers into five varieties, namely: yellow with white straw (ex: Figure 1 PED), yellow with purple straw (ex: Figure 1 LED 1), red with purple straw (ex: Figure 1 IVO 2), striped with brown straw (ex: Figure 1 IVO 1) and colored, with the variety yellow with purple straw being produced by the highest number of farmers (eight farmers).

Figure 1 Physical characterization of creole maize seeds produced by family farmers in South-West of São Paulo in the 2019 crop. A square of squared paper measures 0.5 x 0.5 cm.
It is important to highlight that the collection of the seed lots for the physical, physiological and sanitary assessments was not limited to only one variety. Some farmers, such as for instance; LED provided five seed lots considered by him as the same variety (yellow with purple straw). What differs these lots are conditions such as period and place of sowing and period of harvest and way of storages. In general, the seeds presented uniform sizes varying between rounded and flat shapes, of yellow, orange, red color, or striped in red and yellow and with shiny aspect (Figures 1 and 2).
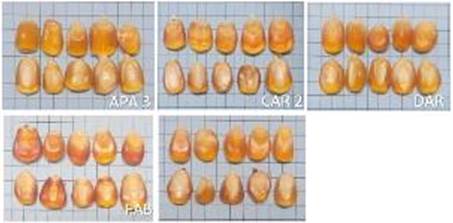
Figure 2 Physical characterization of creole maize seeds produced by family farmers in South-West of São Paulo in the 2020 crop. A square of squared paper measures 0.5 x 0.5 cm.
The mean seed size varied between 0-15 mm in length and 6-10 mm in width, and in the harvest of 2019 all LED lots were composed of the biggest seeds and those from PED were considered with the smallest dimension (Figure 1). Regarding the harvest of 2020, the seeds from lots of APA 3 and DAR were identified as the biggest (Figure 2). The seed size and mass are influenced by several cellular processes, including genetic and environmental factors (Zhang et al., 2016).
Usually, for the commercialization of maize seeds, there is a classification during processing regarding shape and size to standardize and facilitate sowing. For the family farmers, it was reported that, before storage, the seeds in the middle of the ear are separated, thus selecting the flat and the biggest ones to sowing.
The water content of the seeds influences directly in the aspects related to storage and physiological and sanitary quality. In the harvest of 2019, before stored, the seeds were dried with lower water contents, varied between 10.6 and 18.2 and in the harvest of 2020, they varied between 15.7 and 21.4 (Table 1).
Table 1 Water content (WC), one thousand seed mass (TM) and seed infestation (SF) of creole seed lots of corn from South-West of São Paulo in the 2019 and 2020 harvests. Cells with (-) indicate test not performed due to unavailability of seed.
| Lot | WC (%) | TM (g) | SF (%) |
|---|---|---|---|
| Harvest 2019 | |||
| APA 1 | 10.6 | 394.18 bc* | - |
| APA 2 | 13.4 | 351.32 de | 6 cde |
| CAR 1 | 18.2 | 407.7 a | 33 a |
| CID 1 | 11.1 | 392.31 bc | 0 e |
| IVO 1 | 15 | 357.37cd | 3 cde |
| IVO 2 | 15.6 | 381 b | 11 bc |
| IVO 3 | 14.5 | 372.35 bc | 11 bc |
| LED 1 | 18.3 | 368.26 b | 16 b |
| LED 3 | 14 | 376.41 bc | 2 de |
| LED 4 | - | 348.35 e | 10 bcd |
| LED 5 | 16.3 | 366.76 bc | 4 cde |
| LED 6 | 15.7 | 365.21 bc | 1 e |
| MAR 1 | - | 425.48 a | - |
| NIV 1 | - | 353.65 de | - |
| PED 1 | 13.1 | - | 1 e |
| CV (%) | - | 2.21 | 48.32 |
| Harvest 2020 | |||
| APA 3 | 18.2 | 402.57 b | 0 c |
| CAR 2 | 21.4 | 438.84 a | 0 c |
| DAR 1 | 15.7 | 387.4 c | 1 b |
| FAB 1 | 20.5 | 317.79 d | 2 a |
| NIV 2 | 20.6 | 282.51 e | 1 b |
| CV (%) | - | 3.26 | 1.32 |
*= averages followed by the same letter in each harvest, for each test, do not differ by Tukey’s test in 5% probability; VC= variation coefficient.
The maize seed, considered orthodox, presents higher tolerance to desiccation with limit of water content between 5 and 15%, which allows its germination after dry storage in the long term (Waterworth et al., 2015). Therefore, lower water contents are recommended to assure the seed lot quality for a longer period. For maize seeds, water contents considered ideal during storage vary between 7 and 11%. The harvest of 2020 obtained higher water contents than those recommended, a mean of 13%. Nonetheless, this has not become a problem, since the analysis occurred after the harvest, without storage, a step in which the seeds with high water content increase their deterioration process.
The results of one thousand seed mass - TM (Table 1) demonstrated great variability of the materials with distinct origins. Lots of CAR 1 and MAR 1, of the harvest of 2019, obtained the highest values relative to the mass of 1 000 seeds, whereas lots APA 2, LED 4 and NIV 1 are those with lowest mass. For the seeds from the harvest of 2020, which were not dried before being evaluated, high values of TM were observed for lots of CAR 2 and APA 3. There was no direct relationship of this variable with the variety or color of the creole maize seed produced. The difference on TM is probably due to a genetic characteristic and to the production system of each material.
It was possible to verify high values of infestation in the harvest of 2019. Infestation is evaluated by the presence of damages, such as tunnels, or insects’ larvae and adults in the seeds, which may appear mainly during storage. The adult insect observed during the test was the maize weevil, Sitophilus zeamais Motschulsky, 1855 (Coleoptera: Curculionidae).
According to Tefera et al. (2016), pests such as larger grain borer and maize weevil can cause between 14 and 36% of maize grain loss. In the harvest of 2020, the percentage of infestation was low, with lot FAB presenting the highest percentage of infestation with only 2%, followed by lots of DAR and NIV 2, both with 1%. The lowest values are related to the evaluation of the seeds immediately after harvest, evidencing that the problem of infestation occurs mainly in the storage period.
The highest germination percentages, in the harvest of 2019, occurred in lots of APA 2 and PED, both above 90% (Table 2). Although these two lots have the similarity of being classified as yellow creole maize with white straw, APA 2 did not undergo storage and PED is different from the others, since its storage was performed using polyethylene terephthalate (PET) packaging and refrigeration in fridge (temperature close to 10 °C). The understanding of the main characteristics of the storage is mandatory for the preservation, with the water content of the seeds, temperature and relative humidity of the storage environment as the key factors for the maintenance of the physiological potential until the use of the seeds (Da Silva et al., 2019).
Lots of IVO 1, IVO 3 and LED 4 had intermediary values for germination, varying between 70 and 80%, and presenting common storage, sometimes in plastic gallons of 5L and other times, in plastic boxes with straw and ear. Low germination occurred in lots of APA 1, CID 1, MAR 1 and NIV 1, all in inappropriate storage conditions, such as in cardboard box (CID 1 and MAR 1), in the period of approximately six months at room temperature or in polyethylene plastic bags at room temperature (NIV 1). If the storage conditions after harvest are not appropriate, this results in a lower germination rate (Wang et al., 2020), which was observed for most of the lots from the harvest of 2019.
Considering the vigor tests, for the harvest of 2019, it was verified that all certified the superiority of lots of APA 2 and PED (Table 2). For the first count of germination, only IVO 1 was classified as presenting intermediary vigor, immediately after the group with the best quality (APA 2 and PED). Lots of IVO 2, IVO 3, LED 3 and LED 4 were ranked right after, being considered of intermediary quality. The test of emergence for the harvest of 2019 demonstrated the behavior of the lots similar to that observed in germination, with the highest values in APA 2 (73.1%), IVO 3 (73.1%) and PED (66.5%). In the others, emergence was smaller, reaching zero in variety CID. It is worth highlighting that MAR 1 and NIV 1 were not evaluated because of the lack of seeds available. The fast and uniform germination, and seedling emergence, can guarantee high and stable production of the production fields (Li et al., 2016).
Table 2 Germination (G), first count of germination (FG), emergence of seedlings (ES), emergence speed index (ESI) and cold test (CT) of lots of Creole maize seeds from South-West of São Paulo in the 2019 and 2020 harvests. Cells with (-) indicate a test not performed due to the unavailability of seeds.
| Lot | G (%) | FG (%) | ES (%) | ESI | CT(%) |
|---|---|---|---|---|---|
| Harvest 2019 | |||||
| APA 1 | 2.4 f * | 0 g | - | - | - |
| APA 2 | 98.5 a | 83 ab | 73.1 a | 12.33 ab | 93.8 a |
| CAR 1 | 59 d | 30 de | 56.9 abc | 4.62 cd | 26 cd |
| CID 1 | 0 f | 0 g | 0 e | 0 d | 0 e |
| IVO 1 | 80.5 b | 74 b | 47.5 bcd | 7.69 cd | 59.5 b |
| IVO 2 | 62 cd | 50.5 c | 53.1 abcd | 8.44 bcd | 55 b |
| IVO 3 | 74.5 b | 61 c | 73.1 a | 12.68 ab | 62.5 b |
| LED 1 | 36 e | 32.5 d | 39.4 cd | 5.19 cd | 33 c |
| LED 3 | 57 d | 56 c | 44.4 bcd | 7.77 cd | - |
| LED 4 | 70 bc | 54.5 c | 48.8 abcd | 8.38 bcd | 18.8 cd |
| LED 5 | 35 e | 8 fg | 30.6 d | 5.2 cd | 21.5 cd |
| LED 6 | 34.5 e | 19 ef | 38.8 cd | 5.71 cd | 18.5 d |
| MAR 1 | 0 f | 0 g | - | - | - |
| NIV 1 | 0 f | 0 g | - | - | - |
| PED | 93 a | 86.5 a | 66.5 ab | 12.81 a | 88.5 a |
| CV (%) | 9.11 | 12.22 | 21.16 | 46.71 | 14.74 |
| Harvest 2020 | |||||
| APA 3 | 90.5 a | 66.5 a | 100 a | 13.87 a | 93.5 a |
| CAR 2 | 98 a | 69.5 a | 100 a | 12.76 ab | 100 a |
| DAR 1 | 88 a | 51 a | 100 a | 10.93 c | 98.5 a |
| FAB 1 | 96 a | 51.5 a | 100 a | 12.07 bc | 97.5 a |
| NIV 2 | 84 a | 59.5 a | 100 a | 11.57 bc | 96.5 a |
| CV (%) | 23.67 | 21.99 | 0 | 5.71 | 3.64 |
*= averages followed by the same letter in each harvest, for each test, do not differ by Tukey’s test in 5% probability.
In the evaluation of the emergence speed index (EIP), for the harvest of 2019, the emphasis was for lots PED, APA 2 and IVO 3 (Table 3), demonstrating the seeds have higher speed in relation to the emergence of their seedlings, indicating higher vigor (Mavi et al., 2010). In the others, the values were significantly inferior, reaching zero again, for CID 1, in which there was no emergence. Germination and seedling emergence are two independent and vulnerable stages in the life cycle of a plant, determined by both seed quality and the genetic variation and environmental factors (Zhang et al., 2019).
Table 3 Incidence of Acremonium sp., Fusarium sp., Aspergillus sp. and Penicillium sp. fungi in lots of creole maize seeds from South-West of São Paulo in the 2019 and 2020 harvests.
| Code | Acremonium sp. | Fusarium sp. | Aspergillus sp. | Penicillium sp. |
|---|---|---|---|---|
| Harvest 2019 | ||||
| APA 1 | 7 bc* | 13 cde | 31 c | 100 a |
| APA 2 | 4 bc | 0 e | 0 f | 74 b |
| CAR 1 | 12 ab | 0 e | 40 c | 57 c |
| CID | 2 bc | 5 de | 12 de | 100 a |
| IVO 1 | 2 bc | 28 cd | 57 b | 97 a |
| IVO 2 | 1 bc | 28 cd | 79 a | 93 ab |
| IVO 3 | 5 bc | 3 e | 0 f | 98 a |
| LED 1 | 17 a | 54 ab | 2 ef | 98 a |
| LED 3 | 4 bc | 14 cde | 0 f | 98 a |
| LED 4 | 5 bc | 23 cde | 5 ef | 91 ab |
| LED 5 | 8 b | 47 bc | 5 ef | 89 ab |
| LED 6 | 7 bc | 58 ab | 0 f | 88 ab |
| MAR | 8 b | 47 bc | 5 ef | 89 ab |
| NIV 1 | 0 c | 64 a | 18 d | 100 a |
| PED | 1 bc | 3 e | 65 b | 67 b |
| CV (%) | 61.83 | 29.78 | 23.37 | 126.91 |
| Harvest 2020 | ||||
| APA 3 | 0 b | 0 c | 1 b | 81 ab |
| CAR 2 | 0 b | 0 c | 2 b | 61 c |
| DAR | 0 b | 2 b | 1 b | 94 a |
| FAB | 4 a | 13 a | 40 a | 71 bc |
| NIV 2 | 3 a | 4 b | 3 b | 72 bc |
| CV (%) | 40.32 | 22.78 | 76.21 | 12.39 |
*= averages followed by the same letter in each harvest, for each fungus, do not differ by Tukey's test in 5% probability.
Using the cold test (Table 2) it was possible to observe, in the harvest of 2019, higher percentages for lots of APA 2 (93.8%) and PED (88.5%). The other lots stayed below with 62.5%. The cold test is part of the most used parameters in relation to the vigor of the maize seeds (Gu et al., 2017). Seeds of high vigor have obvious advantages in terms of germination and seedling emergence, even under field stress conditions (Zhang et al., 2019). It can be understood that the most vigorous lots are more able to withstand adverse conditions in the field, such as water stress or low temperatures.
Observing the data on germination and vigor together, in the harvest of 2019, the studied lots can be grouped into superior (APA 2, PED, IVO 1), intermediary (IVO 1, IVO 2, IVO 3, LED 3, LED 4) and inferior (APA1, CID 1, LED1, LED 5, LED 6, MAR 1, NIV 1) regarding the physiological potential. It is possible to highlight that the superior lots presented common aspects in some essential factors to obtain better results. The similar storage conditions, such as the use of polyethylene (PET) material and a lower index of infestation are examples of this.
For the harvest of 2020, all lots evaluated were classified as presenting high physiological potential, with germination above 84%, seedling emergence of 100% and result of the cold test above 93% (Table 2). Only the emergence speed index allowed a ranking among the lots; nonetheless, since vigor must be assessed considering a set of tests, and not an isolated result (Marcos Filho, 2015), this ranking was not considered. In this harvest, the high physiological potential of the materials is related to the absence of storage. This indicates that the field conditions in which the seeds were produced are appropriate and favorable to produce seeds with quality. Therefore, the storage conditions can be indicated as being the priority factor in the reduction of the physiological potential in creole maize seeds in the South-West of São Paulo.
In the Blotter test (Table 3), the incidence of fungi from the field (Acremonium sp. and Fusarium sp.) and storage (Aspergillus sp. and Penicillium sp.) was verified with the storage fungi observed in higher amount. A similar behavior was observed by Ferreira et al. (2013), who worked with maize seeds and concluded that the field fungi associated with the seeds decreased during the storage period, whereas the storage fungi increased.
The fungi associated to the seeds can promote a drop in the physiological quality and a little uniform emergence, which leads to partial or total loss in productivity (Parsa et al., 2016). Stefanello et al. (2015) also identified reduction of the physiological quality of storage creole maize seeds due the deterioration caused by Aspergillus sp., Penicillium sp. and Fusarium sp.
In the harvest of 2019, lot LED 1 presented the highest percentage of the presence of the fungus Acremonium sp., with 17%, followed by lots LED 5 and MAR with 8%, and APA 1 and LED 6 with 7%. The plants attacked by A. strictum have their development paralyzed and the damages cause symptoms of root rot in the plants (Goswami et al., 2008).
The highest occurrence of Fusarium sp. was observed in lots NIV 1 (64%), LED 6 (58%) and LED 1 (54%). The other lots had inferior values, reaching close to zero for APA 3. This fungus is associated with maize in most of the stages of the growth cycle of this plant, it is a parasite, saprophyte, and causes severe rot in the plant stem (Lanubile et al., 2014). The detection of phytopathogenic fungi in seeds is very important, since they can cause reduction in germination and initial development of seedlings, besides providing an efficient propagation of disease to other cultures.
Aspergillus sp. and Penicillium sp., are considered storage fungi and cause the rot of grains and maize seeds, and they promote the loss of the germination power and the root of the mass of seeds because of the rise in the breathing rate. For Aspergillus sp., the highest occurrence was observed in lots of IVO 2 (79%), PED with (65%) and IVO 1 with (57%) (Table 3).
The highest percentage of occurrence among all fungi was verified for Penicillium sp., with 100% in lots of APA 1, CID 1 and NIV 1, and the smallest percentage was of (67%) of lot PED. These values suggest that the high percentage of the presence of storage fungi are caused by favorable conditions of humidity, temperature at which the seeds are maintained right after the harvest and during storage.
In the harvest of 2020, lot FAB presented the highest percentage of the presence of the fungus Acremonium sp., with 4%, followed by lot NIV 2 with 3% and the other lots had values in zero (Table 3). The highest occurrence of Fusarium sp. was observed in lots FAB (13%), NIV 2 with (4%) and DAR with (2%). The other lots, APA 3 and CAR 2, had values in zero. For Aspergillus sp., the highest occurrence was observed in lot FAB (40%), followed by NIV 2 with (3%), CAR 2 with (2%), APA 3 and DAR, both with (1%). The highest percentage of occurrence of all fungi was verified again for Penicillium sp., in lots of DAR with (94%), APA 3 with (81%), NIV 2 with (72%) and FAB with (71%).
It is possible to verify that the field fungi had the lowest occurrence, which evidences the good quality of the seed derived from the field, such as the result observed in APA 2 from the harvest of 2019. Conversely, the storage conditions are the main interferences in the low quality of the seeds, since the seeds from harvest 2020 were collected directly from the field, did not undergo the storage process, and obtained a high index of storage fungi from this step. According to Khosravi et al. (2007), fungi are one of the main causes of deterioration and loss of quality in maize seeds, together with the attack of insects. Since storage conditions are directly related to the incidence of fungi and to the physiological quality of the seeds, it is extremely important to intervene to guarantee the maintenance of quality seeds and originate fields to produce seeds with quality. This is possible, given the performance of lot PED, in which the farmer accomplished a better management of the creole seeds during storage, obtaining superior results in the tests in relation to the other materials analyzed.
Therefore, a quality material, regardless of the purpose of use of the creole maize, is essential in several aspects that are vital to agriculture, such as the maintenance of biodiversity, increase in productivity and acquisition of sanity in production fields. It is evident that the use of creole seeds is also coupled with lower production costs, which becomes a great alternative to enable the acquisition of inputs, promote agricultural and cultural heritage, generate income, promote food safety, besides contributing to the Brazilian genetic heritage. Thus, the guarantee of the quality if these creole seeds enhances the agricultural management and promotes the required autonomy for family agriculture in the South-West of São Paulo.
Conclusions
The creole maize seeds in the South-West of São Paulo present variation in terms of the physical, physiological, and sanitary quality, and this variation refers mainly to the characteristics of the storage process performed. It is necessary to deepen studies that aim at better storage techniques to maintain seed quality, in addition to promoting activities of rural extension that raise awareness on the farmers regarding seed quality. The implement of strategies of specialized technical assistance for creole materials will leverage the strengthening of family farmers related to the traditional systems and will trigger a better rural development of the South-West region of São Paulo.
The risk for losing creole maize seeds occurs because of factors such as low seed quality, contamination of the materials by fungi and attacks of insects. The lot that approached the best storage conditions was the one stored in hermetic packages (PET bottles) and in a condition of low temperature, which might ensure the continuous existence of the creole maize seeds of the South-West of São Paulo.
Acknowledgements
This work was developed with the support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Código de Financiamento 001. To the family farmers from the South-West of São Paulo, for the availability of time and of the creole maize seeds studied in this research.
REFERENCES
Antonello, L. M.; Muniz, M. F. B.; Brand, S. C.; Rodrigues, J.; Menezes, N. L. and Kulczynski, S. M. 2014. Significance and relations of differentially expressed genes in response to Aspergillus flavus infection in maize. Scientific Reports. 4(1):4815. Doi: 10.1038/srep04815. [ Links ]
Brasil. 2009. Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes. Brasília. 395 p. [ Links ]
Catão, H. C. R. M.; Magalhães, H. M.; Sales, N. L. P.; Brandão-Junior, D. S. and Rocha, F. S. 2013. Incidência e viabilidade de sementes crioulas de milho naturalmente infestadas com fungos em pré e pós-armazenamento. Ciência Rural. 43(5):764-770. Doi: https://doi.org/10.1590/S0103-84782013000500002. [ Links ]
Da Silva, G. H.; Toledo, M. Z.; Teixeira, R. N.; Rossi, R. F. and Nakagawa, J. 2019. Influence of the storage environment on the physiological quality of millet seeds (Pennisetum glaucum (L.) R. Br.). J. Seed Sci. 41(3):286-292. Doi: https://doi.org/10.1590/2317-1545v41n3208200. [ Links ]
De Almeida Silva, T. T.; Von Pinho, E. V. R.; Cardoso, D. L.; Ferreira, C. A.; Alvim, P. O. and Costa, A. A. F. 2008. Physiological quality of corn seeds in the presence of biostimulants. Ciência e Agrotecnologia. 32(3):840-846. [ Links ]
De Medeiros, A. D.; Zavala-León, M. J.; Silva, L. J.; Oliveira, A. M. S. and Dias, D. C. F. S. 2019. Relationship between internal morphology and physiological quality of pepper seeds during fruit maturation and storage. Agron. J. 112(1):25-35. [ Links ]
Fachini, C.; Mariuzzo, P. and Cerdan, L. M. I. 2019. O roteiro do milho: a construção do turismo gastronômico no Vale do Paranapanema-SP. Em: Lavandoski, J.; Brambilla, A. y Vanzella, E. (Org.). Alimentação e turismo: oferta e segmentos turísticos. 1a (Ed.). João Pessoa. Editora do CCTA. 1(1):251-278. [ Links ]
Ferreira, V. F.; Oliveira, J. A.; Ferreira, T. F.; Reis, L. V.; Andrade, V. and Neto, J. M. 2013. Quality of maize seeds harvested and husked at high moisture levels. J. Seed Sci . 35(3):276-277. Doi: https://doi.org/10.1590/S2317-15372013000300001. [ Links ]
Finch-Savage, W. E. and Bassel, G. W. 2016. Seed vigor and crop establishment: extending performance beyond adaptation. J. Exp. Bot. 67(3):567-591. Doi: https://doi.org/10.1093/jxb/erv490. [ Links ]
Goswami, J.; Pandey, R. K.; Tewari, J. P. and Goswami, B. K. 2008. Management of root knot nematode on tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum. J. Environ. Sci. Health Part B. 43(3):237-240. Doi: https://doi.org/10.1080/03601230701771164. [ Links ]
Gu, R.; Li, L.; Liang, X.; Wang, Y.; Fan, T.; Wang, Y. and Wang, J. 2017. The ideal harvest time for seeds of hybrid maize (Zea mays L.) XY335 and ZD958 produced in multiple environments. Scientific reports. 7(1):1-9. Doi: https://doi.org/10.1038/s41598-017-16071-4. [ Links ]
Han, Z.; Ku, L.; Zang, Z.; Zang, J.; Gou, S.; Liu, H.; Zhao, R.; Ren, Z.; Zhang, L.; Su, H.; Dong, L. and Chen, Y. 2014. QTLs for seed vigor-related traits identified in maize seeds germinated under artificial aging conditions. PloS One. 9(3):1-13. Doi: https://doi.org/ 10.1371/journal.pone.0092535. [ Links ]
Khosravi, A. R.; Mansouri, M.; Bahonar, A. S. and Shokri, H. 2007.Mycoflora of maize harvested from Iran and imported maize. Pakistan J. Biol. Sci. PJBS. 10(24):4432-4437. Doi: 10.3923/pjbs.2007.4432.4437. [ Links ]
Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A. and Bellin, D. 2014. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genomics. 15(1):710-726. Doi: 10.1186/1471-2164-15-710. [ Links ]
Li, Z.; Wang, X.; Liao, T.; Feng, Q. and Zhang, D. 2016. A self-developed system for visual detection of vegetable seed vigor index. Int. J. Agric. Biol. 18(1):86-91. Doi: 10.17957/IJAB/15.0066. [ Links ]
Maguire, J. D. 1962. Speed of germination aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 2(2):176-77. Doi: https://doi.org/10.2135/cropsci1962.0011183X000200020033x. [ Links ]
Marcos-Filho, J. 2015. Fisiologia de sementes de plantas cultivadas. Londrina. Abrates. 459-497 pp. [ Links ]
Mavi, K.; Demir, I. and Matthews, S. 2010. Mean germination time estimates the relative emergence of seed lots of three cucurbit crops under stress conditions. Seed Sci. Technol. 38(1):14-25. Doi: https://doi.org/10.15258/sst.2010.38.1.02. [ Links ]
Parsa, S.; García-Lemos, A. M.; Castillo, K.; Ortiz, V.; López-Lavalle, B.; Braun, J. and Vega, F. E. 2016. Fungal endophytes in germinated seeds of the common bean, Phaseolus vulgaris. Fungal Biology. 120(5):783-790. Doi: https://doi.org/10.1016/j.funbio.2016.01.017. [ Links ]
Santos, G. R.; Tschoeke, P. H.; Silva, L. G.; Silveira, M. C. A. C.; Reis, H. B.; Brito, D. R. and Carlos, D. S. 2014. Sanitary analysis, transmission and pathogenicity of fungi associated with forage plant seeds in tropical regions of Brazil. J. Seed Sci . 36(1):54-62. Doi: https://doi.org/10.1590/S2317-15372014000100007. [ Links ]
Stefanello, R.; Muniz, M. F. B.; Nuner, U. R.; Dutra C. B. and Somavilla, I. 2015. Physiological and sanitary qualities of maize landrace seeds stored under two conditions. Ciência Agrotecnologica. 39(4):339-347. Doi: https://doi.org/10.1590/S1413-70542015000 400004. [ Links ]
Tefera, T.; Mugo, S. and Beyene, Y. 2016. Developing and deploying insect resistant maize varieties to reduce pre-and post-harvest food losses in Africa. Food Security. 8(1):211-220. Doi: 10.1007/s12571-015-0537-7. [ Links ]
Wang, Y.; Peng, Y.; Zhuang, Q. and Zhao, X. 2020. Feasibility analysis of NIR for detecting sweet corn seeds vigor. J. Cereal Sci. 93(2):1-12. Doi: https://doi.org/10.1016/j.jcs.2020.102977. [ Links ]
Waterworth, W. M.; Bray, C. M. and West, C. E. 2015. The importance of safeguarding genome integrity in germination and seed longevity. J. Exp. Bot . 66(12):3549-3558. Doi: https://doi.org/10.1093/jxb/erv080. [ Links ]
Zhang, C.; Luo, T.; Liu, J.; Xian, M.; Yuan, J.; Hu, L. and Xu, Z. 2019. Evaluation of the low-temperature tolerance of rapeseed genotypes at the germination and seedling emergence stages. Crop Sci. 59(4):1709-1717. Doi: https://doi.org/10.2135/cropsci2019.03.0160. [ Links ]
Zhang, X.; Hirsch, C. N.; Sekhon, S. R.; Leon, N. and Kaeppler, S. M. 2016. Evidence for maternal control of seed size in maize from phenotypic and transcriptional analysis. J. Exp. Bot . 67(6):1907-1917. Doi: https://doi.org/10.1093/jxb/erw006. [ Links ]
Received: October 2021; Accepted: January 2022











 texto en
texto en 


