Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 no.8 Texcoco nov./dic. 2021 Epub 02-Mayo-2022
https://doi.org/10.29312/remexca.v12i8.2718
Articles
Mites associated with maize in Mexico
1Facultad de Ingeniería y Ciencias-Universidad Autónoma de Tamaulipas-Centro Universitario Victoria. Cd. Victoria, Tamaulipas, México. CP. 87149. (mapatcg@gmail.com; akserranod@outlook.com).
2 Estancia posdoctoral-CONACYT.
For Mexico, maize (Zea mays L.) is one of the crops with the greatest cultural and economic importance. One of the problems that decrease its production is the presence of primary pests, which when controlled with chemical insecticides contribute to the appearance of others considered secondary, such as mites. In the cultivation of maize, nine species of mites belonging to the families Acaridae and Tetranychidae and one unidentified species of Iolinidae have been recorded. The species with a wide distribution record belong to the genera Oligonychus and Tetranychus, belonging to the family Tetranychidae, mainly of phytophagous habits. In infested plants, a whitish or tanned appearance of the foliage is observed. Economically important populations appear during June, July and August, particularly if the weather is hot and dry. The greatest impact on yield occurs when mites damage the leaves at or above the level of the cob. The stages of the crop where more damage is quantified are at the beginning of flowering and grain filling, since the presence of high populations of mites in these stages causes stunted cobs with small grains to occur due to the dehydration they cause. Cultural practices, crop improvement, natural enemies and acaricides can be used as management strategies. Therefore, this review of species of mites that are considered as a pest in one of the most important crops in Mexico aims to present different biological and ecological aspects important in the mite-maize interaction.
Keywords: Acaridae; economic importance; foliage; Iolinidae; Tetranychidae
Para México, el maíz (Zea mays L.) es uno de los cultivos con mayor importancia cultural y económica. Uno de los problemas que disminuyen su producción es la presencia de plagas primarias, que al ser controladas con insecticidas químicos contribuyen a la aparición de otras consideradas secundarias, como los ácaros. En el cultivo de maíz se han registrado nueve especies de ácaros que pertenecen a las familias Acaridae y Tetranychidae y una especie sin identificar de Iolinidae. Las especies con un amplio registro de distribución pertenecen a los géneros Oligonychus y Tetranychus, pertenecientes a la familia Tetranychidae, principalmente de hábitos fitófagos. En plantas infestadas se observa un aspecto blancuzco o bronceado del follaje. Las poblaciones económicamente importantes aparecen durante junio, julio y agosto, particularmente si el clima es cálido y seco. La mayor afectación al rendimiento se presenta cuando los ácaros dañan las hojas en o arriba del nivel de la mazorca. Las etapas del cultivo donde se cuantifican más daños son al inicio de la floración y llenado de grano, ya que la presencia de altas poblaciones de ácaros en estas etapas provoca que se produzcan mazorcas raquíticas con granos pequeños debido a la deshidratación que causan. Como estrategias de manejo se pueden utilizar las prácticas culturales, mejoramiento del cultivo, enemigos naturales y como último recurso los acaricidas. Por lo anterior, esta revisión de especies de ácaros que son considerados como plaga en uno de los cultivos más importantes de México, tiene como objetivo exponer diferentes aspectos biológicos y ecológicos importantes en la interacción ácaro-maíz.
Palabras clave: Acaridae; follaje; importancia económica; Iolinidae; Tetranychidae
The cultivation of maize (Zea mays L.) had its origin in Mexico, from where it spread north to Canada and south to Argentina. The oldest evidence of its existence dates back about seven thousand years and was found by archaeologists in the Tehuacán Valley, state of Puebla in Mexico. Maize is considered the most traditional and important crop in the economy and culture of the Mesoamerican peoples, since it creates sources of employment and is the primary and irreplaceable food of most Mexican families (González, 2004; DGSV-CNRF, 2020).
From the moment of sowing, maize is exposed to the attack of numerous pests. Environmental conditions, land preparation, crop rotation, and weed control are among the various factors that influence the emergence of pests and diseases in the cultivation. In Mexico, 75 species of pests associated with maize have been recorded, classified according to the damage they cause, so we find pests in root, foliage, cob, and grain (Sifuentes, 1985; Ortega, 1987). Some of them were considered as secondary pests, however, they have come to be classified as primary because they have caused significant economic losses in the cultivation, this undoubtedly as a result of intensive management and poor agricultural practices (CESAVEM, 2015).
Mites were considered secondary pests despite their association with crops since the beginning of agriculture approximately 12 000 years ago in historical records (Badii and Abreu, 2006), because they were regulated by their natural enemies. However, the irrational use of broad-spectrum organic synthetic pesticides used since World War II intervened negatively and caused an imbalance in the natural balance between predator and prey populations (Badii et al., 2010).
Pest mites cause severe damage to cultivated plants by feeding on the different organs of plants, establishing and increasing their populations in their hosts. The presence of these organisms has a negative impact on production and yield. For their control, different acaricides have been applied, which cause serious resistance problems, especially in species of the genus Tetranychus (Stavrinides et al., 2010), due to their continuous use in addition to eliminating the beneficial fauna in the agroecosystem, which has generated that different control strategies based mainly on an Integrated Pest Management system are sought (Lomelí and Rodríguez, 2010; Rodríguez, 2012).
Importance of phytophagous mites
For mites that feed on plants, the relative stability of their environment and the durability of their food source has led them to certain common patterns of lifestyles. They are usually multivoltine (with several generations a year), with a high reproductive rate and in general do not practice phoresy as a means of transport but move mainly aided by wind currents (Krantz and Lindquist, 1979; Otero, 2012).
Most phytophagous mites belong to the order Trombidiformes, suborder Prostigmata, have a mouth apparatus (Gnathosoma) adapted to feed on plants, where the bases of the chelicerae have fused, and the mobile finger has become a stylet adapted to puncture the epidermis of plants (Figure 1). Mites of the genus Tetranychus break the epidermal tissue, remove the cellular content, destroying the palisade and spongy parenchyma cells, this destroys the chloroplasts, which translates into a decrease in the photosynthetic rate, stomatal conductance and perspiration, affecting growth, development and production (Jeppson et al., 1975; Walter and Proctor, 2013). The severity of damage from phytophagous mites depends on the habits of the mite and the physiological conditions in which the host plant is. Certain changes in the biological cycle are also observed, which are clearly a tendency to adapt to the host plant (Walter and Proctor, 2013; Almaguel and de la Torre, 2014).
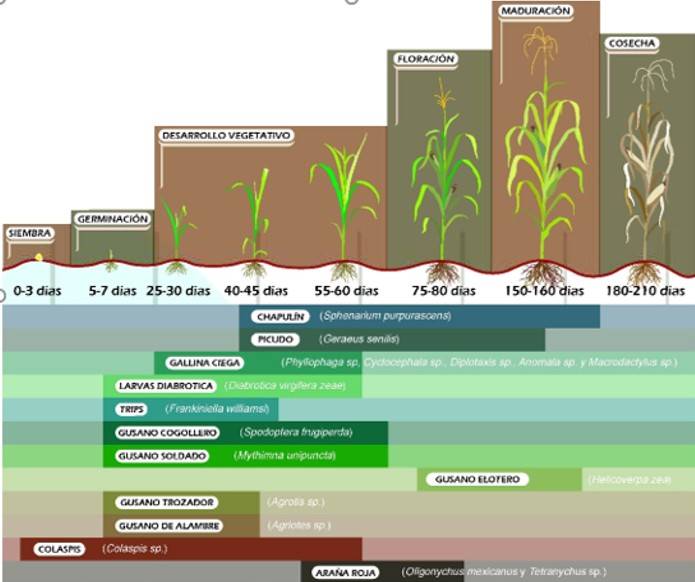
Several factors affect pest mite population dynamics from one year to the next, including temperature, humidity, precipitation, soil type, pesticide application, host proximity and natural enemies. In general, the appearance of pests in the cultivation of maize is related to the phenology of the plant (Figure 2), high temperatures along with drought stress, elements that accompany high populations of mites. Sandy soil types can also contribute to high populations of mites, as in these soils, plants often suffer water stress in western states, even under irrigation (CESAVEG, 2012).
Acarofauna associated with maize
Three families of economic importance have been recorded for Mexico: Acaridae, Iolinidae and Tetranychidae (Table 1).
Table 1 Species of mites associated with maize in Mexico.
| Family Genus and species | State/region | Site | Reference | |||
| No data | Foliage | Root | Stored grains | |||
| Acaridae | ||||||
| Sancassania berlesei (Michael, 1903) ** | Mexico City | X | 11 | |||
| S. mycophaga (Mégnin, 1874) ** | Campeche, Mexico City, Morelos | X | 11 | |||
| Tyrophagus putrescentiae Scharnk, 1871* | Mexico City, Guanajuato, Morelos, Puebla | X | X | 11 | ||
| Iolinidae | ||||||
| Pronematus sp. | Mexico City, Morelos | X | 11, 12 | |||
| Tetranychidae | ||||||
| Bryobia praetiosa (Koch, 1836) | No data | X | 10, 14 | |||
| Oligonychus spp.* | Aguascalientes, Mexico City, Coahuila, State of Mexico, Jalisco, Morelos, Puebla, Tlaxcala, southern Mexico | X | 1-9, 11, 13-15 | |||
| O. mexicanus (McGregor and Ortega, 1953) * | Aguascalientes, Coahuila, State of Mexico | X | 1, 8, 11, 14 | |||
| O. pratensis (Banks, 1912) * | Coahuila, Morelos, Puebla | X | 4, 7, 13-15 | |||
| O. stickneyi (McGregor, 1919) * | Mexico City, Morelos, Tlaxcala | X | 2, 3, 7, 8, 11, 14 | |||
| O. zeae (McGregor, 1955) * | Southern Mexico | X | 5, 14 | |||
| Tetranychus spp.* | Coahuila | X | 8-10, 12 | |||
| T. urticae Koch, 1835* | Coahuila, Sinaloa, Tamaulipas⁑, Veracruz | X | 8, 10, 12, 16 | |||
| Total species | 1 | 6 | 3 | 1 | ||
*= primary pest; **= secondary pest; ⁑= new record, present work. 1) McGregor and Ortega (1953); 2) Pritchard and Baker (1955); 3) Beer and Lang 1958; 4) Baker and Pritchard (1962); 5) Estébanes-González and Baker (1968); 6) Tuttle, Baker and Abbatiello (1974); 7) Tuttle, Baker et al. (1976); 8) McGregor and Gutiérrez (1983); 9) Ortega (1987); 10) Estébanes and Rodríguez-Navarro (1991); 11) Rodríguez-Navarro (1999); 12) Hoffmann and López-Campos (2000); 13) Reséndiz and Aguillón-Trejo (2009); 14) Estébanes (2010); 15) Reséndiz-García and García-Severiano (2016); and 16) Rodríguez-Escobar et al. (2021).
Five species of phytophagous mites are common in the cultivation of maize, most are found in foliage: Oligonychus mexicanus, O. pratensis, O. stickneyi, O. zeae and Tetranychus urticae (Figure 3) (Rodríguez-Navarro, 1999; Reséndiz and Aguillón-Trejo, 2009), the species of the genus Oligonychus have a greater number of distribution records in the states of the Mexican Republic (Table 1).

Figure 3 Tetranychid mites in maize. A) Tetranychus urticae, female and B) Oligonychus sp., nymph. Photographs provided by Ronald Ochoa, Systematic Entomology Laboratory, USDA.
It is worth mentioning that the free access database Spider mites web was reviewed: (http://www1.montpellier.inra.fr/CBGP/spmweb/index.php) in which eight more species of the genus Tetranychus are listed [T. desertorum Banks (1900), T. gloveri Banks (1900), T. kanzawai Kishida (1927); T. ludeni Zacher (1913), T. marianae McGregor (1950), T. pacificus McGregor (1919), T. turkestani, Ugarov and Nikolskii (1937) and T. yusti McGregor (1955)] and a species of the genus Oligonychus [O. afrasiaticus (McGregor, 1939)] associated with maize in Mexico, but when reviewing the original articles and comparing the distribution records, these species do not correspond to the afore mentioned plant host, so these records are not valid. In this work, Tetranychus urticae associated with the cultivation of maize in the state of Tamaulipas (Güémez) was recorded for the first time.
Mites can damage maize from the seedling stage to maturity (CESAVEG, 2012). However, the appearance of phytophagous mites is mainly concentrated in the last stage of vegetative development and in almost the entire stage of flowering. O. pratensis is predominant at the beginning of the growing season, while T. urticae and O. mexicanus extend throughout the growing and flowering season. It is important to establish that the species indicated as common in the cultivation of maize, feed mainly on species of grasses and differ in their susceptibility and resistance to insecticides, a situation that has caused difficulties in their management and control (CESAVEM, 2015).
It is worth mentioning that T. urticae has more than 900 hosts worldwide, some examples are cotton, beans, strawberry, citrus, carnation, almond, rose and walnut, also, in greenhouses, it attacks cucumber, tomato, eggplant and chili pepper, so it is undoubtedly one of the most important species in agriculture worldwide (Badii et al., 2010; Migeon and Dorkeld, 2020).
Symptoms and damage from mites
On the foliage infested by these phytophagous mites, a whitish or tanned appearance was observed. Slightly infested leaves show transparent pale spots or eruption (Figure 4A). Tetranychid colonies usually occur on the underside of the leaves, producing long spots that extend from the central vein to the margins, or appear scattered on the leaf blade, with little web production (Salas, 1978). When the colonies are large and abundant, the foliage is seen to be covered with the silky tissue on which the mites walk (Figure 4B) (García-Martell et al., 1981). Consequently, the plant has less chlorophyll at its disposal for growth and loses physiological balance; in severe infestations, the plants become unproductive.

Figure 4 A) symptoms and damage from mites on maize leaves; and B) colonies of Tetranychus urticae in foliage.
Economically important populations appear during June, July and August; particularly if the weather is hot, windy and dry. The effects on yield are most severe when mites damage the leaves at or above the level of the cob. Severe infestations resemble drought stress as damage progresses from the base of the plant upwards (CESAVEG, 2012). The increase in tetranychid populations can lead to considerable economic losses; mainly when the plant is at the beginning of flowering and grain filling.
Under these conditions, the dehydration caused to the foliage by the feeding of the mite combines with heat and causes a stunted development of the cob with small grains and a lower weight, with estimated losses of up to 50% (Ortega, 1987; Metwally et al., 2014).
Regarding astigmatid mites (Acaridae), many species infest the underground parts (roots) and occasionally the leaves of various plants (Otero, 2012), mainly attack parts that previously present mechanical damage or have been attacked by fungi. It has been a topic of discussion whether the observed damage is actually caused by these mites, pathogenic or saprophagous fungi, as well as the possible role of these mites in the epidemiology of fungal diseases of the attacked plants (Díaz et al., 2000). The quality and yield of ensiled maize may also decrease due to the feeding of mites, mainly due to stored grain mites of this family (Rodríguez-Navarro, 1999).
Mites of the family Iolinidae have a wide variety of eating habits, ranging from predation and mycophagy to parasitism (Walter et al., 2009). Many iolinids live in plants, but are not supposed to feed on them, but are predators of small arthropods (Van de Velde et al., 2021) or feed on fungi or detritus found on leaves or stems. Others are considered phytophagous, but in all cases the observations are discordant and there is no agreement as to what their food really is (Lindquist, 1998).
Biology and behavior
Family Acaridae
The family Acaridae is included in the cohort Astigmatina of the order Sarcoptiformes (Lindquist et al., 2009). It is an enormously diversified group that includes parasitic, saprophagous, granivorous, mycophagous species, among others. As a relevant feature, most of these mites have short, chelated, two-articles chelicerae, with the basal article very globose and with strong muscles that allow them to feed on solid particles, in free-living species, the type of feeding can be considered chewer (Norton, 1998).
The biological cycle of the acarids includes the typical phases of the development of mites: egg, larva, protonymph, deutonymph, tritonymph and adult, but in this group, the deutonymph differs considerably, since it is a heteromorphic phase (of resistance), more sclerosed than the rest of the other phases, with reduced, not functional gnathosoma, so it does not feed and is generally phoretic (it uses other animals of greater size to move from a place to another) (O’Connor, 2009).
One of the species of economic importance in this family is T. putrescentiae, which develops its biological cycle in 8.46 days, at a temperature of 32 oC and a relative humidity of 60-90% (Hughes, 1976; Zhang and Fan, 2005), in semitropical and tropical regions. This species can be considered as a secondary pest in a crop, but in stored grains, it can be a primary pest (Rodríguez and Estébanez, 1998). In the maize crop, Sancassania berlesei and S. mycophaga are found in roots, where the humidity is relatively higher than in another part of the plant, there, it can feed on roots that have previously been damaged by fungi and bacteria, although sometimes it can also feed on the nematodes present there (Bilgrami, 2008; Aguilar-Marcelino, 2015).
Family Iolinidae
In the family Iolinidae, the chelicerae are fused at their base or are separated, but do not have a wide movement from each other. They are chelated but the fixed finger is usually much shorter than the mobile finger, so they do not form a true pincer and the mobile finger acts on its own, like an awl (Walter et al., 2009).
In the case of Pronematus sp., it has been reported as a predator of other mites and small arthropods, so it is not ruled out that its presence is because it feeds on tetranychid populations that can be very abundant in the foliage (Gerson et al., 2003). Metwally et al. (2014) mention P. ubiquitus McGregor as an active predator on maize foliage for four months, interacting with other predators of the family Phytoseiidae.
Family Tetranychidae
Tetranychid mites are characterized by having chelicerae of two articles, which form a structure called stylophore, where the basal articles are fused together and the distal articles are very long and curved (measuring 130 mµ from the curved part to the tip) and are projected together with the stylophore to pierce the plants that serve as food (Jeppson et al., 1975; De Moraes and Flechtmann, 2008).
Due to their large size, chelicerae can reach layers of the palisade parenchyma when mites feed on the upper side, or spongy parenchyma when mites feed on the underside (De Moraes and Flechtmann, 2008). Perforated cells die and can cause the death of adjacent cells by being isolated from others. As a result of high infestations of these mites, chlorotic, necrotic spots form or the plants take on a tanned appearance, such as if burnt. During the warmer hours of the day, they look withered, and the accumulated damage can cause defoliation or death of the plants (Almaguel and Estrada, 2012).
Reproduction is a key aspect in the economic importance of tetranychids, these stand out for their rapid development, high fecundity and the fact that many species produce abundant web, which serves as protection and means of transport (Saito, 2010). Reproduction in most tetranychids is haplo-diploid, females are produced through sexual reproduction and males by arrenotoca parthenogenesis. This allows that, if a female arrives alone to populate a new site, being able to produce males allows to quickly increase the population. Therefore, the type of reproduction, the intense selection pressure caused by chemical control and acaricides on the two-spotted mite (T. urticae) potentiate the development of genetic resistance to acaricides in a comparatively short time (Villegas-Elizalde et al., 2010; Mitina et al., 2021).
Management strategies
The problems caused by mites require better management practices, which can range from cultural practices, crop improvement, use of natural enemies (mainly predators) and as a last resort the use of acaricides (Almaguel and Estrada, 2012).
A preventive treatment is crop management appropriate to the conditions of each maize variety. As tetranychid mites survive best in elevated temperatures (> 30 ºC) and a dry environment, it is recommended to keep the soil moist to keep the temperature as low as possible and a certain degree of humidity (Badii et al., 2010). Carrying out an irrigation helps to reduce the population of mites that appears as a problem since the flowering of the crop (CESAVEG, 2012).
The opportune moment to perform the control is when the first webs in the mature leaves of the bottom of the maize are detected. It is very appropriate that the irrigations are not lengthened, for example, the first supplemental irrigation should be given between 45-50 days after sowing (during the end oif the initial development stage and beginning of vegetative development), the third supplemental irrigation between 80-85 days (during the beginning of flowering) (CESAVEG, 2012).
Another method of control is to till the land. This method helps to reduce the population of wintering females in the soil, as well as remove weeds, since these act as alternate hosts that provide food and shelter for some pests (Bolaños-Espinoza et al., 2001), in addition to tillage, it is advisable to destroy alternative hosts and residues or stubble, as well as sanitation or hygiene of the crop (CESAVEG, 2012).
A tool for the control of phytophagous mites in this crop is the use of resistant plant varieties (Tadmor et al., 1990), which has been reported for many crops (Archer et al., 1990; Flexner et al., 1991).
The resistance of the host plant should be an integral component in the control of arthropod pests due to its durability and safety characteristics (Álvarez-Gil, 2015). Maize’s resistant to mite infestations can decrease the need for chemical application for their control, since the rate of increase of mite populations can be reduced or the ability of the plant to tolerate the attack of these can be increased and with it, more options of integrated pest management (IPM) in the crop can be implemented (Archer et al., 1990; Ruckert et al., 2021).
Chemical control
In general, tetranychid mites are a pest that reproduces quite quickly and are even resistant to some agrochemicals used for their control. The development of resistance to acaricides by T. urticae is widely documented worldwide, where the cases cited exceed 200, including recently appearing acaricides, such as abamectin (Flores et al., 2007). Among the active substances cited to which T. urticae has shown resistance are: fenazaquin and tebufenpyrad (Gorman et al., 2001); pyridaben, fenpyroximate, hexitiazox and clofentezin (Nauen et al., 2001); chlorfenapyr (Van Leeuwen et al., 2006); dicofol and fenbutaestan (García-Marí, 2005); sistox (2,4,5-T) and parathion (Sukhoruchenko and Dolzhenko, 2008); and tetradiphon (García-Marí et al., 1988). In Mexico, some organosynthetic insecticides of various chemical groups and the biorational type are authorized for the control of Tetranychus urticae in maize, such as: abamectin, oxidemeton, propargite, etoxazole, acequinocyl, spiromesifen (COFEPRIS, 2020; DGSV-CNRF, 2020).
One method to effectively counteract the presence of mites is the combination of insecticides with fatty acids or mineral oils in total applications. Applications with sulfur (powder) is an alternative, although if dry weather persists, several should be made. When the crop is in an advanced state, chemical control becomes difficult (CESAVEG, 2012).
Biological control
This method has been practiced for a long time and consists of using and/or letting the natural enemies of a pest act, in order to keep its population fluctuations below the economic thresholds (Badii et al., 2010). There are a large number of biological control agents that have been found for T. urticae, with Phytoseiulus persimilis Athias-Henriot and Amblyseius californicus McGregor (Phytoseiidae) being among the most used as a control measure in greenhouse and field conditions (CABI, 2020).
Phytoseiulus persimilis was the first biological control agent used in greenhouses and is still very effective today (Rodríguez et al., 2003; Zhang, 2003). However, under dry and warm conditions, it has difficulty keeping tetranychid colonies under control. Under such conditions, the mite A. californicus can be used, as it is more tolerant of higher temperatures, lower relative humidity and pesticides than P. persimilis (Malais and Ravensberg, 2006). Among the predator species available in the Mexican market for the control of Tetranychus urticae and other species of pest mites are P. persimilis, A. californicus and Neoseiulus cucumeris (Oudemans), as well as the cecidomid Feltiella acarisuga (Vallot) (Koppert Mexico, 2020).
Conclusions
Worldwide, tetranychid mites are the best known, studied and combated for the damage they cause to agricultural crops. Among the species mentioned in this study, those belonging to the family Tetranychidae are those that are considered of economic importance for the cultivation of maize. It is important to mention that, although there are 9 species of mites recorded for maize in Mexico, currently, the populations of phytophagous mites do not represent a problem of economic interest for the crop due to the implementation of Integrated Pest Management strategies and programs that are carried out in different regions of the country.
Acknowledgements
The authors thank CONACYT for the financing of the postdoctoral stay of the first author and doctoral studies of the second author. To the postgraduate degree in Agricultural Systems and Environment of the Faculty of Engineering and Sciences, Autonomous University of Tamaulipas. This work is derived from the project UATINVES20-18: biology and biological control of the fall armyworm Spodoptera frugiperda J.E. Smith (Lepidoptera: Noctuidae) in select localities of Tamaulipas, Mexico
REFERENCES
Aguilar-Marcelino, L.; Quintero-Martínez, M. T.; Mendoza-de-Gives, P.; Bautista-Garfias, C. R.; López-Arellano, M. E. y Reyes-Guerrero, D. E. 2015. Hábitos de alimentación de Sancassania mycophaga (=Caloglyphus mycophagus) (Acari: Acaridae) sobre los nematodos Haemonchus contortus (L3) y Panagrellus redivivus. Entomol. Mex. 2(1):200-205. [ Links ]
Almaguel, L. y De la Torre, S. P. 2014. Manual de acarología agrícola. Instituto de investigaciones de sanidad vegetal (INISAV). Cuba. 343 p. [ Links ]
Almaguel, L. y Estrada, V. E. G. 2012. Tetranychidae. Ácaros de importancia agrícola. Estrada-Venegas, E. G.; Acuña-Soto, J. A.; Chaires-Grijalva, M. P y A. Equihua-Martínez (Ed.). Sociedad Mexicana de Entomología y Colegio de Postgraduados. Texcoco, México. 122-157 pp. [ Links ]
Archer, T. L.; Onken, A. B.; Bynum, E. D. and Peterson, G. C. 1990. Banks grass mite (Oligonychus pratensis) abundance on sorghum cultivars with different levels of nitrogen use andmetabolism efficiency. Exp. Appl. Acarol. 9(3):177-182. [ Links ]
Badii, M. H. y Abreu, J. L. 2006. Control biológico una forma sustentable de control de plagas. Daena Inter. J. Good Consci. 1(1):82-89. [ Links ]
Badii, M. H.; Landeros, J. y Cerna, E. 2010. Regulación poblacional de ácaros plaga de impacto agrícola (population regulation of pest mites of agricultural significance). Daena: Inter. J Good Consc. 5(1):270-302. [ Links ]
Baker, E. W. y Pritchard, A. E. 1962. Arañas rojas de América Central (Acarina: Tetranychidae). Rev. de la Sociedad Mexicana de Historia Natural. 23(1):309-340. [ Links ]
Beer, R. E. and Lang, D. S. 1958. The Tetranychidae of Mexico. University of Kansas Scientifical Bulletin. 38(15):1231-1259. [ Links ]
Bilgrami, A. L. 2008. Biological control potential of predatory nematodes. In: Ciancio, A. Mukerji (Ed.). Integrated management and biocontrol of vegetable and grain crops nematodes. 3-28 pp. [ Links ]
Bolaños-Espinoza, A.; Bravo-Mojica, H.; Equihua-Martínez, A.; Trinidad-Santos, A.; Ramírez-Valverde, G. and Domínguez-Valenzuela, J. A. 2001. Densidad y daños de plagas del maíz, bajo labranza convencional y de conservación. Acta Zoológica Mexicana. 83(1):127-141. [ Links ]
CABI. 2020. Tetranychus urticae (two-spotted spider mite). https://www.cabi.org/isc/datasheet /53366 . Noviembre de 2021. [ Links ]
CESAVEG, 2012. Manual de plagas y enfermedades en el maíz. Campaña manejo fitosanitario del maíz SENASICA. Comité Estatal de Sanidad Vegetal, Guanajuato. (http://www.cesaveg.org.mx/html/folletos/folletos-11/folleto-maiz-11.pdf. [ Links ]
CESAVEM, 2015. Plagas rizófagas del maíz. Campaña manejo fitosanitario del maíz. SENASICA. Comité Estatal de Sanidad Vegetal, Estado de México. Página electrónica. http://www.cesavem.mx/img/fitosanitariodelmaiz/maiz.pdf. [ Links ]
COFEPRIS. 2020. Consulta de registros sanitarios de plaguicidas, nutrientes vegetales y LMR. http://siipris03.cofepris.gob.mx/Resoluciones/Consultas/ConWebRegPlaguicida.asp. [ Links ]
De Moraes, G. J. y Flechtmann, C. H. W. 2008. Manual de acarología: acarología básica e ácaros de plantas cultivadas no Brasil. Ribeirão Preto. Holos. 288 p. [ Links ]
DGSV-CNRF. 2020. Araña roja de dos manchas Tetranychus urticae (Koch.) (Arachnida: Acari: Tetranychidae). SADER-SENASICA. Dirección General de Sanidad Vegetal. Centro Nacional de Referencia Fitosanitaria. Ficha técnica. Tecámac, Estado de México. 22 p. [ Links ]
Díaz, A.; Okabe, K.; Eckenrode, C. J.; Villani, M. G. and OConnor, B. M. 2000. Biology, ecology, and management of the bulb mites of the genus Rhizoglyphus (Acari: Acaridae). Exp. Appl. Acarol. 24(2):85-113. [ Links ]
Estébanes, G. M. L. 2010. Ácaros fitófagos en hortalizas. En: memorias del primer simposio internacional de acarología en México. Sánchez, G. M. C.; Álvarez, S. E.; Vega, O. H. E. y García, F. J. (Ed.). Universidad Autónoma Chapingo, México. 21-24 pp. [ Links ]
Estébanes, G. M. L. y Baker, E. W.1968. Arañas rojas de México (Acarina: Tetranychidae). Anales de la Escuela Nacional de Ciencias Biológicas. 15(1):61-133. [ Links ]
Estébanes, G. M. L. y Rodríguez, N. S. 1991. Observaciones sobre algunos ácaros de las familias Tetranychidae, Eriophyidae, Acaridae y Tarsonemidae (Acari), en Hortalizas de México. Folia Entomológica Mexicana. 83(1):199-212. [ Links ]
Flexner, J. L.; Westigard, P. H.; Gonzáles, P. and Hilton, R. 1991. The effect of groundcover and herbicide treatment in twospotted spider mite density and dispersal in Southern Oregon pear orchards. Entomologia Experimentalis et Applicata. 60(2):111-123. [ Links ]
Flores, A. F.; Silva, G. A.; Tapia, M. V. y Casals, P. B. 2007. Susceptibilidad de Tetranychus urticae Koch (Acari: Tetranychidae) colectada en Primula obconica Hance y Convolvulus arvensis L. a acaricidas. Agric. Téc. 67(2):219-224. [ Links ]
García-Marí, F. 2005. Resistencia de Tetranychus urticae y Panonychus citri a acaricidas en el cultivo de los cítricos. Phitoma. 173(1):71-78. [ Links ]
García-Marí, F.; Roca, D.; Fonbuena, P.; Ferragut, F. y Costa-Comelles, J. 1988. Acción de los acaricidas tetradifón y dicofol sobre huevos y adultos de Panonychus citri (McGregor) y Tetranychus urticae Koch (Acari. Tetranychidae), en cítricos. Boletín de Sanidad Vegetal. Plagas.14(1):163-169. [ Links ]
García-Martell, C. 1981. Lista de insectos y ácaros perjudiciales a los cultivos en México. 2a (Ed.). Fitófilo. 86(1):1-196. [ Links ]
Gerson, U.; Smiley, R. L. and Ochoa, R. 2003. Tydeidae. In: Mites (Acari) for pest control. Tydeidae. Blackwell science Ltd. USA. 258-262 pp. [ Links ]
González, A. 2004. Maíz, transgénicos y pueblos indígenas de México. Rev. Semillas. 22:28-32. [ Links ]
Gorman, K. F.; Hewitt, D. I. and Devine, G. J. 2001. New developments in insecticide resistance in the glasshouse white fly (Trialeurodes vaporariorum) and the two-spotted spider mite (Tetranychus urticae) in the UK. Pest. Mang. Sci. 58(2):123-130. [ Links ]
Hoffmann, A. y López-Campos, G. 2000. Biodiversidad de los ácaros en México. Universidad Nacional Autónoma de México (UNAM)- Consejo Nacional de la Biodiversidad (CONABIO). México, DF. 230 p. [ Links ]
Hughes, A. M. 1976. The mites of stored food and houses. Tech. Bull. Min. Agr. Fisheries and Foods. London. Núm. 9. 402 p. [ Links ]
Jeppson, R. L.; Keifer, H. H. and Baker, E. W. 1975. Mites injurious to economic plants. Berkeley, University of California Press. 614 p. [ Links ]
Koppert México. 2020. Partners with Nature. https://www.koppert.mx . November 2021. [ Links ]
Krantz, G. W. and Lindquist, E. E. 1979. Evolution of phytophagous mites (Acari). Annual Review Entomol. 24(1):121-158. [ Links ]
Lindquist, E. E. 1998. Evolution of phytophagy in trombidiform mites. Exp. Appl. Acarol. 22(1):81-100. [ Links ]
Lindquist, E. E.; Krantz, G. W. and Walter, D. E. 2009. Classification. In: a manual of acarology. Krantz, G. W. and Walter, D. E. (Ed.). Texas Tech University Press, Lubbock, Texas. 97-103 pp. [ Links ]
Lomelí, F. R. y Rodríguez, N. S. 2010. La Importancia de los ácaros en el control biológico. In: memorias del primer simposio internacional de acarología en México. Sánchez, G. M. C.; Álvarez, S. E.; Vega, O. H. E. y García, F. J. (Ed.). Universidad Autónoma Chapingo (UACH). Texcoco, Estado de México. 68-76 pp. [ Links ]
Malais, M. y Ravensberg, W. J. 2006. Conocer y reconocer la biología de las plagas de invernadero y sus enemigos naturales. Editorial Koppert Biological Systems, Países Bajos. 21-38 pp. [ Links ]
McGregor, E. A. and Ortega, A. A. 1953. Una nueva araña roja de México, Paratetranychus mexicanus sp. n. (Acarina, Tetranychidae). Folleto Tecnológico de la Secretaría de Agricultura y Ganadería (SAGAR). México, DF. 10(1):1-7. [ Links ]
McGregor, R. y Gutiérrez, O. 1983. Guía de insectos nocivos para la agricultura en México. Alhambra Mexicana. México. 166 p. [ Links ]
Metwally, A. M.; El-Naggar, M. E.; Abou-El-Sooud, A. B. and Amer, A. I. 2014. Ecological and biological studies on Tetranychus urticae Koch on maize single hybrids at Gharbia Governorate. Annal. Agric. Sci. Moshtohor. 52(3):357-368. [ Links ]
Migeon, A. and Dorkeld, F. 2020. Spider mites. a comprehensive database for the Tetranychidae. http://www1.montpellier.inra.fr/CBGP/spmweb. [ Links ]
Mitina, G.; Tulaeva, I. A.; Malysh, S. M. and Tokarev, Y. 2021 Molecular genetic analysis of resistance-associated mutations in the experimental lines of spider mite Tetranychus urticae Koch, selected for resistance to bifenthrin and abamectin. Inter. J. Acarol. 47(8) doi: 10.1080/01647954.2021.1990406. [ Links ]
Nauen, R.; Stumpf, N.; Elbert, A.; Zebitz, C. P. W. and Kraus, W. 2001. Acaricide toxicity and resistence in larvae of different strains of Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae). Pest Manag. Sci. 57(3):253-261. [ Links ]
Norton, R. A. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exper. Appl. Acarol. 22(21):559-594. https://doi.org/10.1023/A:1006135509248. [ Links ]
O’Connor, B. M. 2009. Astigmatid mites (Acari: Sarcoptiformes) of forensic interest. Exp. Appl. Acarol. 49(1-2):125-133. https://doi.org/10.1007/s10493-009-9270-2. [ Links ]
Ortega, A. C. 1987. Insectos nocivos del maíz: una guía para su identificación en el campo. Centro Internacional para el Mejoramiento del Maíz y Trigo (CIMMYT). El Batán, Texcoco, Estado de México. 106 p. [ Links ]
Otero, C. G. 2012. Bioecología de las principales familias y especies de ácaros fitófagos. In: ácaros de importancia agrícola. Estrada-Venegas, E. G.; Acuña-Soto, J. A.; Chaires-Grijalva, M. P y Equihua-Martínez, A. (Ed.). Sociedad Mexicana de Entomología y Colegio de Postgraduados. Texcoco, México. 109-121 pp. [ Links ]
Pritchard, A. E. and Baker, E. W. 1955. A revision of the spider mite family Tetranychidae. Memoirs Series, San Francisco, Pacific Coast Entomological Society. 2(1):1-472. [ Links ]
Reséndiz, G. B y Aguillón-Trejo, M. G. 2009. Identificación de los ácaros asociados al maíz (Zea mays L.) en la comarca lagunera. Agroproductividad. 2(3):32-34. [ Links ]
Reséndiz-García, B. y García-Severiano, J. 2016. Identificación y biología del tetraníquido del maíz (Zea mays L.) en San Sebastián Cuacnopalan, Puebla. Entomol. Mex. 3(1):39-44. [ Links ]
Rodríguez, M.; Sánchez, M.; Navarro, M. y Aparicio, V. 2003. Los fitoseidos, depredadores efectivos de araña roja. Horticultura. 21(4):41-43. [ Links ]
Rodríguez, N. S. 1999. Ácaros. In: catálogo de insectos y ácaros plaga de los cultivos agrícolas de México. (Ed). Sociedad Mexicana de Entomología. Publicaciones Esp. 125-140 pp. [ Links ]
Rodríguez, N. S. 2012. Importancia agrícola y económica de los ácaros. In: ácaros de importancia agrícola . (Ed.). Sociedad Mexicana de Entomología y Colegio de Postgraduados. Texcoco, Estado de México. 98-108 pp. [ Links ]
Rodríguez, N. S. y Estébanes, G. M. L. 1998. Acarofauna asociada a algunas vegetales de importancia agrícola y económica en México. (Ed.). Ciencias Biológicas y de la Salud. Universidad Autónoma Metropolitana (UAM)- Xochimilco. 1ª (Ed). México. 105 p. ISBN: 970-654-415-1. [ Links ]
Rodríguez-Escobar, J. G.; Rodríguez-Falconi, R. y Cruz-Gutiérrez, R. 2021. Efecto de acaricidas sobre Tetranychus sp. en maíz (Zea mays) en el estado de Veracruz. Braz. J. Animal Environ. Res. 4(3):4504-4511. doi: 10.34188/bjaerv4n3-132. [ Links ]
Ruckert, A.; Golec, J. R.; Barnes, C. L. and Ramírez, R. A. 2021. Banks grass mite (Acari: Tetranychidae) suppression may add to the benefit of drought-tolerant corn hybrids exposed to water stress. J. Econ. Entomol. 114(1):187-196. https://doi.org/10.1093/jee/toaa269 . [ Links ]
Saito, Y. 2010. Plant mites and sociality. Diversity and evolution. Springer Tokyo. 187 p. https://doi.org/10.1007/978-4-431-99456-5. [ Links ]
Salas, F. A. L. 1978. Algunas notas sobre las arañitas rojas (Tetranychidae: Acari) halladas en Costa Rica. Agron. Costar. 2(1):47-59. [ Links ]
Sifuentes, J. A. 1985. Plagas del maíz en México. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Folleto técnico núm. 85. 49 p. [ Links ]
Stavrinides, M. C.; Van Nieuwenhuyse, P.; Van Leeuwen, T. and Mills, N. J. 2010. Development of acaricide resistance in Pacific spider mite (Tetranychus pacificus) from California vineyards. Exp. Appl. Acarol. 50(3):243-254. [ Links ]
Sukhoruchenko, G. I. and Dolzhenko, V. I. 2008. Problems of resistance development in arthropod pests of agricultural crops in Russia. EPPO Bulletin. 38(1):119-126. [ Links ]
Tadmor, Y.; Lewinsohn, E.; Abo-Moch, F.; Bar-Zur, A. and Mansur, F. 1999. Antibiosis of maize inbred lines to the carmine spider mite, Tetranychus cinnabarinus. Phytoparasitica. 27(1):1-7. [ Links ]
Tuttle, D. M.; Baker, E. W. and Abbatiello, M. 1976. Spider mites of Mexico (Acarina: Tetranychidae). Internat. J. Acarol. 2(2):1-102. [ Links ]
Tuttle, D. M.; Baker, E. W. and Abbatiello, M. 1974. Spider mites from northwestern and north central Mexico (Acarina: Tetranychidae). Smithsonian Contributions to Zoology. 171(1):1-18. [ Links ]
Van de Velde, V.; Duarte, M. V. A.; Benavente, A.; Vangansbeke, D.; Wäckers, F. and De Clercq, P. 2021. Quest for the Allmitey: Potential of Pronematus ubiquitus (Acari: Iolinidae) as a biocontrol agent against Tetranychus urticae and Tetranychus evansi (Acari: Tetranychidae) on tomato (Solanum lycopersicum L.). bioRxiv. 1-16 pp. https://doi.org/ 10.1101/2021.04.08.438973. [ Links ]
Van-Leeuwen, T.; Van-Pottelberge, S. and Tirry, L. 2006. Biochemical analysis of a chlorfenapyr-selected resistant strain of Tetranychus urticae Koch. Pest. Manag. Sci. 62(5):425-433. [ Links ]
Villegas-Elizalde, S. E.; Rodríguez-Maciel, J. C.; Anaya-Rosales, S.; Sánchez-Arroyo, H.; Hernández-Morales, J. y Bujanos-Muñiz, R. 2010. Resistencia a acaricidas en Tetranychus urticae (Koch.) asociada al cultivo de fresa en Zamora, Michoacán, México. Agrociencia. 44(1):75-81. [ Links ]
Walter, D. E. and Proctor, H. C. 2013. Mites: ecology, evolution and behaviour: life at a microscale. Springer Science Business Media Dordrecht. 281 p. doi: 10.1007/978-94-007-7164-2-8. [ Links ]
Walter, D. E.; Lindquist, E. E.; Smith, I. M.; Cook, D. R. and Krantz, G. W. 2009. Order Trombidiformes. (Ed.), a manual of acarology . 3th (Ed.). Texas Tech University Press, Lubbock. 233-420 pp. [ Links ]
Zhang, Z. 2003. Mites of Greenhouses identification. biology and control. Cabi Publishing. 240 p. [ Links ]
Zhang, Z. Q. and Fan, Q. H. 2005. Revision of Tyrophagus Oudemans (Acari: Acaridae) of New Zealand and Australia. Landcare research New Zealand Ltd. Summary Final Report Operational Research. 189 p. [ Links ]
Received: November 01, 2021; Accepted: December 01, 2021











 texto en
texto en