Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 no.8 Texcoco Nov./Dez. 2021 Epub 02-Maio-2022
https://doi.org/10.29312/remexca.v12i8.3066
Articles
Biofortification with selenium improves bioactive compounds and antioxidant activity in jalapeño pepper
1Posgrado en Agua y Suelo-Tecnológico Nacional de México-Instituto Tecnológico de Torreón. Carretera Torreón-San Pedro km 7.5, Torreón, Coahuila, México. CP. 27170. (jazmontse@hotmail.com; joorvi66@hotmail.com).
2Facultad de Agricultura y Zootecnia-Universidad Juárez del Estado de Durango. Carretera Gómez Palacio-Tlahualilo km 32, Venecia, Gómez Palacio, Durango, México. CP. 35000 (u.gonzalez@ujed.mx).
3Campo Experimental Valle del Fuerte-INIFAP. Carretera internacional México-Nogales km 1609, Juan José Ríos, Sinaloa, México. CP. 81110. (eblnat68@gmail.com).
4Instituto de Ciencias Agrícolas de la Universidad Autónoma de Baja California. Carretera a Delta s/n, ejido Nuevo León, Mexicali, Baja California, México. CP. 21705. (fidel.nunez@uabc.edu.mx).
Selenium (Se) is an essential oligo-element for human health and in plants is considered a beneficial element, as it is a growth promoter and a trigger for the antioxidant response in plants. Biofortification with Se aims to obtain foods rich in this oligo-element, of high nutritional quality that help combat the problems of malnutrition in the population. The present work aims to evaluate the ability of selenate (Na2SeO4) on yield, biosynthesis of bioactive compounds and their accumulation in chili fruits. For this, five treatments were applied via nutrient solution: 0, 1.5, 3, 4.5 and 6 mg L-1. At harvest, nutraceutical quality, accumulation of Se in fruits, as well as crop yield were quantified. Biofortification with Se positively modified the biosynthesis of bioactive compounds and their concentration in fruit, without a decrease in yield. The incorporation of Se in the nutrient solution is an option to obtain functional foods with nutraceutical quality and with the possibility of improving public health after consumption.
Keywords: Capsicum annuum L.; bioactive compounds; functional food.
El selenio (Se) es un oligoelemento esencial para la salud humana y en las plantas es considerado un elemento benéfico, al ser un promotor del crecimiento y un detonador de la respuesta antioxidante en las plantas. La biofortificación con Se, tiene como objetivo obtener alimentos ricos en este oligoelemento, de alta calidad nutricional que ayuden a combatir los problemas de desnutrición en la población. El presente trabajo tiene como objetivo evaluar la capacidad del selenato (Na2SeO4) sobre el rendimiento, biosíntesis de compuestos bioactivos y su acumulación en frutos de chile. Para ello cinco tratamientos fueron aplicados vía solución nutritiva: 0, 1.5, 3, 4.5 y 6 mg L-1. En la cosecha, se cuantificó la calidad nutracéutica, la acumulación de Se en frutos, así como el rendimiento del cultivo. La biofortificación con Se modificó positivamente la biosíntesis de compuestos bioactivos y su concentración en fruto, sin disminución en el rendimiento. La incorporación de Se en la solución nutritiva es una opción para obtener alimentos funcionales con una calidad nutracéutica y con la posibilidad de mejorar la salud pública tras su consumo.
Palabras clave: Capsicum annuum L.; alimento funcional; compuestos bioactivos
Introduction
Selenium (Se) is an important oligo-element in human nutrition, is essential to form proteins and a cofactor of antioxidant enzymes such as glutathione peroxidase (GPX), which protects the human body by catalyzing the reduction of reactive oxygen species (ROS) (Schiavon et al., 2020). In addition, in mammals, Se forms at least 25 selenoproteins that carry out antioxidant, catalytic, anti-inflammatory, antiviral and antitumor functions (Avery and Hoffmann, 2018).
A deficiency in this element causes health problems, including Keshan disease (an endemic cardiomyopathy), Kashin-Beck disease (endemic deforming osteoarthropathy) (Ulloa et al., 2021), acceleration of carcinogenic processes in the prostate (Sonkusre, 2020), fertility problems and weakening of the immune system, defense system against viral infectious diseases such as influenza, human immunodeficiency virus, muscular dystrophy and cystic fibrosis (Khurana et al., 2019). According to the World Health Organization, consumption of Se in the human diet should fluctuate between 55 and 200 μg day-1 in adults (Górska et al., 2021). The most common way by which the human organism acquires Se is through the consumption of foods, such as meat or fish (Sariñana-Navarrete et al., 2021), which contribute a high percentage of Se to the required daily intake.
In the world, there are about one billion people with Se deficiencies, mainly due to the consumption of plant-based diets (Blażewicz et al., 2020), which contain low concentrations of Se, since this element is present in soil in small amounts (López et al., 2021). On the other hand, this element is not considered essential for plants, but it could be considered a beneficial element because, in low concentrations, Se increases yield, antioxidant content and its concentration in the edible part (Preciado-Rangel et al., 2021).
One strategy to increase the content of Se in foods of plant origin is through biofortification, which consists of enhancing the bioactivity and content of Se in the edible parts of plants (Gaucin-Delgado et al., 2020). Foliar fertilization is the most practical way to incorporate Se into the food chain (Lyons, 2018). On the other hand, jalapeño pepper (Capsicum annuum L.), one of the most cultivated plants in the world, due to its importance in human nutrition as in the pharmaceutical industry (Espinosa-Palomeque et al., 2020), the fruits are a source of vitamins (A, E and C), carotenoids, capsaicinoids and phenolic compounds with antioxidant properties for the human diet (Natividad-Torres et al., 2021).
The application of micronutrients through the biofortification of crops is a very useful tool not only to increase the quantity of essential micronutrients but also to improve the biosynthesis of bioactive compounds (Gaucin-Delgado et al., 2020). The objective of this work was to determine the effect of foliar biofortification with Selenium on the yield, nutraceutical quality and antioxidant capacity of jalapeño pepper.
Materials and methods
Plant material and growing conditions
The study was conducted in a greenhouse with an automatic cooling system located at the facilities of the Universidad Autónoma Agraria Antonio Narro (UAAAN, for its acronym in Spanish) in the City of Torreón, Coahuila, Mexico (25° 33’ 26” north latitude,103° 22’ 31” west longitude, at an altitude of 1 230 m). The study crop was the jalapeño pepper cv. Hijo de Mitla, it germinated in polystyrene trays with 200 holes filled with peat (Premier®, Mexico) as a substrate. These plant trays were covered with black plastic for 72 h and watered every 24 h. The transplantation was carried out 45 days after sowing the seed, when the plants had an average height of 150 mm, each plant per pot. The pots were black polyethylene bags of 500 thick and 18 L capacity, filled with different proportions of sand: perlite (80:20 vv). The river sand was washed and disinfected with a solution of 5% sodium hypochlorite. The bags were placed in double row at 30 cm from center to center between bags and 1.6 m between rows, to obtain a plant density of 4.2 plants m-2.
The water requirements of the crop were provided by manual irrigation to provide three irrigations per day, and each plant received 0.6 L in each irrigation, from transplantation to the beginning of flowering and 2.5 to 3.5 L from flowering to harvest. Pollination was carried out with an electric brush daily, from the beginning of flowering to setting. The minimum and maximum temperature inside the greenhouse fluctuated between 17.7 and 31.6 °C, respectively, while the minimum and maximum relative humidity ranged between 30 and 70%.
Treatments and experimental design
A completely randomized experimental design was used, applying five doses: 0, 1.5, 3, 4.5 and 6 mg L-1 of Na2SeO3 (Sigma Aldrich) (León-Morales et al., 2019). Treatments with Se were applied every 15 days, with a total of six applications through foliar fertilization. It was watered with nutrient solution (Steiner, 1984), pH and electrical conductivity were maintained at 5.5 and 2 dS m-1 respectively. The yield, the nutraceutical quality of the fruit and the accumulation of selenium in fruits were determined. Ten plants per treatment were used.
Crop yield
In each experimental unit, yield was estimated by considering the number and weight of the individual fruit per plant, recording individual fruit traits such as weight with a scale (Ohaus 3729®, Mexico).
Nutraceutical quality
Preparation extract: for the determination of nutraceutical quality (phenolic compounds, flavonoids and antioxidant capacity), samples of 2 g of fresh fruit were mixed with 10 ml of ethanol in plastic tubes that were closed with screw cap. A ‘Stuart’-type stirrer was used to keep the mixture stirred for 24 h. After that, the tubes were centrifuged at 3 000 rpm for 5 minutes (Cardeño, 2007). The supernatants were extracted for physical-chemical analysis.
Total phenolic content
It was determined by a modification of the Folin-Ciocalteau method (Singleton, 1999). Fifty microliters of ethanolic extract were taken, diluted in 3 ml of mQ water, 250 μl of Folin-Ciocalteau (1N) was added, it was stirred and left to react for 3 min. Subsequently, 750 μl of Na2CO3 (20%) and 950 μl of mQ water were added. The solution was left to stand for 2 and the samples were measured in a UV-Vis spectrophotometer (CGoldenwall, wavelength range 340-1 000 nm and a spectral bandwidth: 5 to 760 nm). The standard solution was prepared with gallic acid. The results were expressed in mg 100 g-1 of fresh weight.
Total flavonoids
They were determined by spectrophotometry (Lamaison, 1990). Two hundred fifty microliters of ethanolic extract were taken and mixed with 1.25 ml of mQ water and 75 μl of NaNO2 (5%), it was left 5 min and 150 μl of AlCl3 (10%) was added. Subsequently, 500 μl of NaOH (1 M) and 275 μl of mQ water were added. It was vigorously stirred, and the samples were quantified in a UV-Vis spectrophotometer (CGoldenwall, wavelength range 340-1 000 nm and spectral bandwidth: 5 to 510 nm). The standard was prepared with quercetin dissolved in absolute ethanol (y= 0.0122x-0.0067; r2= 0.965) The results were expressed in mg 100 g-1 of fresh weight.
Total antioxidant capacity
It was measured by the DPPH + method in vitro (Brand-Williams, 1995). A solution of DPPH + (Aldrich) in ethanol was prepared, at a concentration of 0.025 mg ml-1. Fifty microliters of the ethanolic extract were mixed with 1.95 μl of DPPH + solution, after 30 min the samples were quantified in a UV-Vis spectrophotometer (CGoldenwall, wavelength range at 340-1 000 nm and a spectral bandwidth: 5 to 517 nm). The results were expressed in equivalent to μM equiv Trolox 100 g m-1 fresh weight.
Capsaicin
Capsaicin content was measured by an adaptation of the method proposed by Cisneros-Pineda et al. (2007). The absorbance of the filtered extract was then obtained in a UV-Vis spectrophotometer (Goldenwall, wavelength range at 340-1000 nm) previously calibrated with acetonitrile as a blank at a wavelength of 273 nm. The capsaicin content was calculated by means of a standard curve using capsaicin (Sigma, St. Louis, Missouri, USA) as standard and the results are expressed in mg g-1 in fresh weight of capsaicin. The analyses were performed in triplicate.
Accumulation of selenium in the crop
The dried chili samples were ground in a porcelain mortar and digested with nitric and perchloric acid (3:1), using a hot plate at 100 °C. The solution was filtered and boiled to obtain 100 ml of working solution with deionized water. The concentration of selenium in tomato fruits was determined by atomic absorption spectrophotometry (Hsieh and Ganther, 1975), the results were expressed in μg kg-1 of dry weight of fruits.
Statistical analysis
The normality and homogeneity of the variances of the data obtained were verified using the Kolmogorov-Smirnov and Bartlett tests, respectively. Subsequently, simple classification analysis of variance and multiple comparison of means were performed using Tukey’s test at a probability of 5% (p≤ 0.05), with the help of the statistical analysis package SAS v 9.0 (SAS Institute, 2004).
Results and discussion
Yield
The addition of Se did not significantly affect yield, (Figure 1)because Se is not considered an essential element for plant metabolism, supplementation with Se would not be expected to cause changes in crop growth and yield (Hernández-Hernández et al., 2019); however, it has been reported that applying high doses of Se reduces the yield of crops, because it causes oxidative stress in the plant, which can increase the production of free radicals by defending against the toxic effect caused by ROS (Hibaturrahman et al., 2020), on the contrary, using low doses increases yield (Rady et al., 2020) and prevents oxidation by regulating the uptake and redistribution of essential elements in antioxidant systems or maintaining the ion balance and structural integrity of the cell (Pannico et al., 2019), allowing the plant to adapt metabolically as physiologically as a response to the attack of oxidative stress caused by the increase of free radicals under stress conditions (Hasanuzzaman et al., 2020).

Figure 1 Effect of Se on the yield of chili fruits. Data are shown as mean ± standard deviation (SD) (n= 50). Columns with different letters are significantly different according to Tukey’s test (p< 0.05).
The plant presents differential response to Se depending on the dose used, it has both a positive and negative impact on plants, since in low concentrations, Se accumulates as a stimulant by promoting growth, plant physiology, increasing yield (Gaucin-Delgado et al., 2020) and in high doses, negative effects are exerted on the growth and physiology of the plant (Silva et al., 2020) and even cell death (Shahid et al., 2018).
Bioactive compounds
The biosynthesis of phenolic compounds, flavonoids and antioxidant capacity was positively influenced by the application of Se (Figure 2a-2c), obtaining the highest values with 6 mg L-1. Various studies have shown that the application of Se can increase the production of bioactive compounds (Motesharezadeh et al., 2020). Results like those of the present study were obtained in Capsicum annuum L., where the application of Se increased several bioactive compounds such as phenols, flavonoids and antioxidant capacity (Natividad-Torres et al., 2021).

Figure 2 Effect of Se on the content of phenolics (a) total flavonoids (b); antioxidant capacity (c); and capsaicin (d) in chili fruits. Data are shown as mean ± standard deviation (SD) (n= 50). Columns with different letters are significantly different according to Tukey’s test (p< 0.05).
This bioactive capacity is possibly attributed to the synergistic action that Se has, as it can act as a vital element by altering various physiological and biochemical processes (D’Amato et al., 2018), in addition, Se directly affects the antioxidant defense system by increasing the potential of plants to overcome the conditions of biotic and abiotic stress (Hachmann et al., 2019). The results obtained in our study are supported by Hibaturrahman et al. (2020), who found that supplementation in nutrient solution with Na2SeO4 increases antioxidant activity, thus protecting plants from oxidative stress.
The production of foods rich in bioactive compounds directly influences the cellular and physiological activities (Sabatino et al., 2019), obtaining, after their intake, a beneficial contribution to human health (Shahid et al., 2018) due to their various characteristics, protection in coronary diseases (Groth et al., 2020) and after avoiding cellular aging (Hernández-Hernández et al., 2019), in addition to being used as an alternative for cancer prevention (Vinceti et al., 2018).
Capsaicin
Capsaicin is an oleoresin, an active component of hot peppers (Friedman et al., 2019). Capsaicin was positively affected by the doses of Se evaluated (Figure 2d). The concentration of capsaicin was in accordance with the doses of Se used, the increase in the pungency of the chili improves its quality, since this characteristic is appreciated by consumers (Uarrota et al., 2021). The results indicate a response of the capsaicin content in the fruits of jalapeño pepper to Se, although the mechanism of action in this process has not been defined. Biosynthesis is strongly influenced by genotype-environment interactions in Capsicum fruits (Naves et al., 2019), phenylpropanoids (Aza-González et al., 2011) and ABC transporters, specifically the ABCC and ABCG subfamilies, which may be playing important roles in the transport of secondary metabolites such as capsaicin and dihydrocapsaicin to placental vacuoles, affecting their content in fruits (Lopez-Ortiz et al., 2019).
Probably to the interaction of Se in the plant by causing stressful effects (Avery and Hoffmann, 2018) that could be related to an increase in the PAL activity and capsaicin synthase, increasing the accumulation of capsaicinoids in chili (Ulloa et al., 2021), by negatively regulating peroxidase activity at appropriate levels (Hernández‐Pérez et al., 2020).
Although biosynthesis is a controlled genetic trait, the environment also plays an important role depending on the genotype (Scossa et al., 2019), in addition, this feature is also regulated from the point of view of development and the environment (Naves et al., 2019). On the other hand, capsaicin provides the spicy oral sensation in most chili peppers (Scossa et al., 2019); however, its most important biological properties are its ability to act as antioxidants to reduce oxidative stress that leads to the prevention of several degenerative diseases (Friedman et al., 2019).
Selenium content in the crop
The addition of 6 mg L-1 increased the content of Se in chili fruits, there is a positive correlation between Selenium in fruits and its availability (r2= 0.98) (Figure 3). The absorption of Se depends on the age of the plant, plant species and the chemical form of the element being applied, its concentration and the method of application (Yin et al., 2019). Vegetables accumulate higher amounts of Se (Dai et al., 2019).
Previous studies show that biofortification significantly increases the quantity of essential elements in the edible part of plants (Silva et al., 2020), which may increase in crops biofortified with Se up to 30% with respect to untreated crops (Zhu et al., 2017). Se in plants is metabolized along with sulfur within plant tissues, being transformed into selenoproteins that allow it to be stored as Se-Met (Zhang et al., 2019), accelerating transport, accumulation, volatilization and tolerance to Se (Raina et al., 2020).
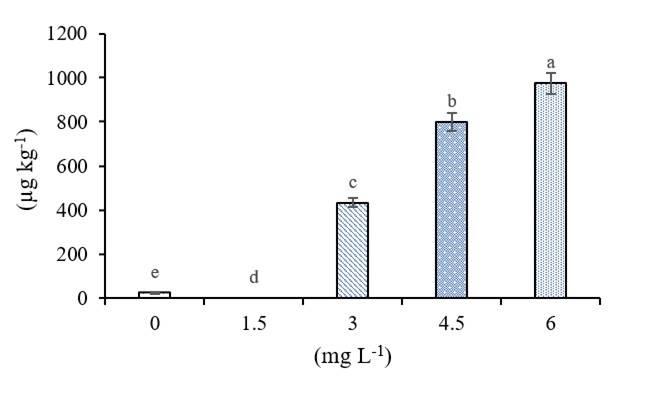
Figure 3 Concentration of Se in chili fruits. Data are shown as mean ± standard deviation (SD) (n= 50). Columns with different letters are significantly different according to Tukey’s test (p< 0.05).
It acts as a potent antioxidant, by allowing lower levels of lipid peroxidation and increased activity of antioxidant enzymes, as well as better resistance to oxidative stress (Skrypnik et al., 2019). According to the dietary guide for Americans, most of the required nutrients should be obtained with food intake (Padilla-Samaniego et al., 2020). In this sense, the consumption of 0.74 g of jalapeño pepper biofortified with selenium supplies the daily requirements of this oligo-element, which is 55 mg per day in adults (Chomchan et al., 2017; León-Morales et al., 2019).
Conclusions
Agronomic biofortification with selenium improves nutraceutical quality and Se concentration, without affecting the yield of the jalapeño pepper crop, the use of Se is an alternative to obtain functional foods and increase the accumulation of oligo- element in jalapeño pepper fruits, with the possibility of protecting human health with its consumption.
Literatura citada
Avery, J. C. and Hoffmann, P. R. 2018. Selenium, selenoproteins, and immunity. Nutrients. 10(9):1203-1223. [ Links ]
Aza-González, C.; Núñez-Palenius, H. G. and Ochoa-Alejo, N. 2011. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Reports. 30(5):695-706. [ Links ]
Błażewicz, A.; Szymańska, I.; Dolliver, W.; Suchocki, P.; Turło, J.; Makarewicz, A. and Skórzyńska-Dziduszko, K. 2020. Are obese patients with autism spectrum disorder more likely to be selenium deficient?. Research findings on pre-and post-pubertal children. Nutrients . 12(11):3581. [ Links ]
Brand-Williams, W.; Cuvelier, M. E. and Berset, C. 1995. Use of a free radical method to evaluate antioxidant activity. LWT-Food Science Technology. 28(1):25-30 [ Links ]
Cardeño, Á.V.; Molina, M. C.; Miranda, I.; García, G. T.; Morales, J. M. and Stashenko, E. E. 2007. Actividad antioxidante y contenido total de fenoles de los extractos etanólicos de Salvia aratocensis, Salvia Sochensis, Bidens reptons y Montanoa ovalifolia. Scientia et Technica.13(33):205-207. [ Links ]
Chomchan, R.; Siripongvutikorn, S. and Puttarak, P. J. 2017. Selenium bio-fortification: an alternative to improve phytochemicals and bioactivities of plant foods. Functional Foods in Health Disease. 7(3):263-279. [ Links ]
D’Amato, R.; Fontanella, M. C.; Falcinelli, B.; Beone, G. M.; Bravi, E.; Marconi, O.; Benincasa, P. and Businelli, D. J. 2018. Selenium biofortification in rice (Oryza sativa L.) sprouting: effects on Se yield and nutritional traits with focus on phenolic acid profile. J. of Agric. Food Chem. 66(16):4082-4090. [ Links ]
Dai, H.; Wei, S.; Skuza, L. and Jia, G. 2019. Selenium spiked in soil promoted zinc accumulation of Chinese cabbage and improved its antioxidant system and lipid peroxidation. Ecotoxicol. Environ. Safety. 180(19):179-184. [ Links ]
Espinosa-Palomeque, B.; Cano-Ríos, P.; Salas-Pérez, L.; González-Rodríguez, G.; Reyes-González, A.; Ayala-Garay, A. V. and Preciado-Rangel, P. J. 2020. Vermicompost on the production and nutraceutical quality of jalapeño pepper fruits (Capsicum annuum L.). Terra Latinoam. 38(4):795-803. [ Links ]
Friedman, J. R.; Richbart, S. D.; Merritt, J. C.; Brown, K. C.; Denning, K. L.; Tirona, M. T.; Valentovic, M. A.; Miles, S. L. and Dasgupta, P. 2019. Capsaicinoids: multiple effects on angiogenesis, invasion and metastasis in human cancers. Biomedicine Pharmacotherapy. 118(109317):2-9. [ Links ]
Gaucin-Delgado, J. M.; Hernandez-Montiel, L. G.; Sanchez-Chavez, E.; Ortega-Ortiz, H.; Fortis-Hernandez, M.; Reyes-Pérez, J. J. and Preciado-Rangel, P. 2020. Agronomic biofortification with selenium improves the yield and nutraceutical quality in tomato under soilless conditions. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 48(3):1221-1232. [ Links ]
Górska, S.; Maksymiuk, A. and Turło, J. 2021. Selenium-containing polysaccharides structural diversity, biosynthesis, chemical modifications and biological activity. Appl. Sci. 11(8):3717. [ Links ]
Groth, S.; Budke, C.; Neugart, S.; Ackermann, S.; Kappenstein, F. S.; Daum, D. and Rohn, S. 2020. Influence of a selenium biofortification on antioxidant properties and phenolic compounds of apples (Malus domestica). Antioxidants. 9(2):187-209. [ Links ]
Hachmann, T. L.; Rezende, R.; Matumoto-Pintro, P. T.; Saath, R.; Anjo, F. A. and Menezes, C. S. L. 2019. Yield, antioxidant activity and shelf-life of cauliflower inflorescences under drought stress and foliar spraying of selenium. Ciência e Agrotecnologia. 43 [ Links ]
Hasanuzzaman, M.; Bhuyan, M. B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K. and Fujita, M. 2020. Selenium in plants: boon or bane? Environ. Exp. Bot.178(10):104170. [ Links ]
Hernández-Hernández, M.; León-Morales, J.; López-Bibiano, Y.; Saldaña-Sánchez, W. D. and García-Morales, S. 2019. Efecto comparativo del selenito y selenato en el crecimiento y contenido de pigmentos fotosintéticos en plantas de pimiento (Capsicum annuum L.) Biotecnología y Sustentabilidad. 3(2):12-12. [ Links ]
Hernández‐Pérez, T.; Gómez‐García, M. D. R.; Valverde, M. E. and Paredes‐López, O. J. 2020. Capsicum annuum (hot pepper): An ancient Latin‐American crop with outstanding bioactive compounds and nutraceutical potential. A review. Comprehensive Reviews in Food Science Food Safety. 19(6):2972-2993. [ Links ]
Hibaturrahman, S. N.; Koyama, H.; Kameo, S.; Waspodo, P.; Wardana, A. A. and Surono, I. S. 2020. Effect of cocoyam modified starch (Xanthosoma sagittifolium), beetroot juice, cocoyam modified starch adsorbing beetroot on plasma selenium and glutathione peroxidase of pre-diabetic rat. IOP Conference Series: Earth and Environmental Science. IOP Publishing. 426(1):012184. [ Links ]
Hsieh, H. S. and Ganther, H. E. 1975. Acid-volatile selenium formation catalyzed by glutathione reductase. Biochemistry. 14(8):1632-1636. [ Links ]
Khurana, A.; Tekula, S.; Saifi, M. A.; Venkatesh, P. and Godugu, C. 2019. Therapeutic applications of selenium nanoparticles. Biomedicine Pharmacotherapy . 111(12):802-812. [ Links ]
Lamaison, J. and Carnet, A. 1990. The levels of the main flavonoids completing of the flower of Craaegeus monogyna Jawq and Crataegeus laevigata (poiret) as a function of the vegetation. Pharm Acta Helv. 65(1):315-320. [ Links ]
León-Morales, J.; Panamá-Raymundo, W.; Langarica-Velázquez, E. and García-Morales, S. 2019. Selenio y vanadio en la germinación y el crecimiento de plántulas de chile (Capsicum annuum L.) y rábano (Raphanus sativus). Rev. Bio Ciencias. 6(10): e425-e441. [ Links ]
Lopez-Ortiz, C.; Dutta, S. K.; Natarajan, P.; Peña-Garcia, Y.; Abburi, V.; Saminathan, T.; Nimmakayala, P. and Reddy, U. K. 2019. Genome-wide identification and gene expression pattern of ABC transporter gene family in Capsicum spp. PloS One. 14(4): e0215901- e0215924. [ Links ]
López, E. A. T.; Sandoval-Rangel, A.; Mendoza, A. B.; Ortiz, H. O.; Pliego, G. C. and de la Fuente, M. C. 2021. Nanopartículas de selenio absorbidas en hidrogeles de quitosán-polivinil alcohol en la producción de pepino injertado. Rev. Mex. Cienc. Agríc. 6(26):159-169. [ Links ]
Lyons, G. 2018. Biofortification of cereals with foliar selenium and iodine could reduce hypothyroidism. Front. Plant Sci. 9(730):2-8. [ Links ]
Motesharezadeh, B.; Ghorbani, S. and Alikhani, H. A. 2020. The effect of selenium biofortification in alfalfa (Medicago sativa). J. Plant Nutr. 43(2):240-250. [ Links ]
Natividad-Torres, E. A.; Guevara-Aguilar, A.; Sánchez, E.; Sida-Arreola, J. P.; Muñoz-Márquez, E. and Chávez-Mendoza, C. J. 2021. Effect of the processing on the antioxidant capacity and bioactive compounds content of jalapeno pepper for chipotle and commercial sauces. Acta Agríc. Pec. 7(1):E00710007-E00710009. [ Links ]
Naves, E. R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W. L.; Nunes-Nesi, A.; Peres, L. E. and Zsögön, A. 2019. Capsaicinoids: pungency beyond Capsicum. Trends Plant Sci. 24(2):109-120. [ Links ]
Padilla-Samaniego, M. V.; Naranjo-Rodriguez, C. E.; Ramírez-Anormaliza, R. I.; Lozada-Meza, M. L.; Solís-Manzano, A. M. and Calderón-Vallejo, C. V. 2020. Tamaño y porciones del consumo de alimentos de la población: disponibilidad de información actualizada. Rev. Eugenio Espejo.14(2):30-50. [ Links ]
Pannico, A.; El-Nakhel, C.; Kyriacou, M. C.; Giordano, M.; Stazi, S. R.; De Pascale, S. and Rouphael, Y. 2019. Combating micronutrient deficiency and enhancing food functional quality through selenium fortification of select lettuce genotypes grown in a closed soilless system. Front. Plant Sci. 10(19):1495-1534. [ Links ]
Preciado-Rangel, P.; Hernández-Montiel, L. G.; Valdez-Cepeda, R. D.; Cruz-Lázaro, E. D. L.; Lara-Capistrán, L.; Morales-Morales, B. and Gaucin-Delgado, J. M. 2021. Biofortification with selenium increases bioactive compounds and antioxidant capacity in tomato fruits. Terra Latinoam. 39(e979):1-10. [ Links ]
Rady, M. M.; Belal, H. E.; Gadallah, F. M. and Semida, W. M. 2020. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Scientia Hortic. 9(34):113-128. [ Links ]
Raina, M.; Sharma, A.; Nazir, M.; Kumari, P.; Rustagi, A.; Hami, A.; Bhau, B. S.; Zargar, S. M. and Kumar, D. 2020. Exploring the new dimensions of selenium research to understand the underlying mechanism of its uptake, translocation, and accumulation. Physiol. Plantarum. 171(4):882-895. [ Links ]
Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F. and De Pasquale, C. 2019. Effect of selenium enrichment and type of application on yield, functional quality and mineral composition of curly endive grown in a hydroponic system. Agronomy. 9(4):207-222. [ Links ]
Sariñana-Navarrete , M. D. Á.; Hernández-Montiel, L. G.; Sánchez-Chavez, E.; Reyes-Perez, J. J.; Murillo-Amador, B.; Reyes-González, A. and Preciado-Rangel, P. 2021. Foliar fertilization of sodium selenite and its effects on yield and nutraceutical quality in grapevine. Rev. Fac. Agron. 38(4):806-824. [ Links ]
Schiavon, M.; Nardi, S.; Dalla, V. F. and Ertani, A. 2020. Selenium biofortification in the 21st century: status and challenges for healthy human nutrition. Plant Soil. 453(1):1-26. [ Links ]
Scossa, F.; Roda, F.; Tohge, T.; Georgiev, M. I. and Fernie, A. R. 2019. The hot and the colorful: understanding the metabolism, genetics and evolution of consumer preferred metabolic traits in pepper and related species. Critical Rev. Plant Sci. 38(6):339-381. [ Links ]
Shahid, M.; Niazi, N. K.; Khalid, S.; Murtaza, B.; Bibi, I. and Rashid, M. I. 2018. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 234(17):915-934. [ Links ]
Singleton, V. L.; Orthofer, R. and Lamuela-Raventós, R. M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 299(14):152-178. [ Links ]
Silva, D. F.; Cipriano, P. E.; de Souza, R. R.; Javier, M. S.; Faquin, V.; de Souza Silva, M. L. and Guilherme, L. R. G. 2020. Biofortification with selenium and implications in the absorption of macronutrients in Raphanus sativus L. J. Food Comp. Analysis. 86(18):103382-103409. [ Links ]
Skrypnik, L.; Kurkova, T. and Chupakhina, G. 2019. Accumulation of selenium in rye plants (Secale cereale L.) at different stages of development and grain quality due to selenate soil supplementation. Appl. Ecol. Environ. Res. 17(2):2385-2421. [ Links ]
Sonkusre, P. 2020. Specificity of biogenic selenium nanoparticles for prostate cancer therapy with reduced risk of toxicity: an in vitro and in vivo study. Front. Oncol. 9(1):1541-1559. [ Links ]
Steiner, A. A. 1984. The universal nutrient solution. International Congress on Soilless ISOSC. 1:25. [ Links ]
Uarrota, V. G.; Maraschin, M.; de Bairros, Â. D. F. M. and Pedreschi, R. 2021. Factors affecting the capsaicinoid profile of hot peppers and biological activity of their non-pungent analogs (Capsinoids) present in sweet peppers. Crit. Rev. Food Sci. Nutr. 61(4):649-665. [ Links ]
Ulloa, J.; Muñoz, J. S. C.; Barrios, A. V. F.; Van Uden, E. and Tafur, S. 2021. Importancia y beneficios del consumo de huevo de gallina enriquecido con selenio: revisión narrativa. Rev. Nutrición Clínica y Metabolismo. 4(3):124-129. [ Links ]
Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M. P.; Horneber, M.; D’Amico, R. and Crespi, C. M. 2018. Selenium for preventing cancer. Cochrane Database of Systematic Reviews. 1(1):1465-1858. [ Links ]
Yin, H.; Qi, Z.; Li, M.; Ahammed, G. J.; Chu, X. and Zhou, J. J. 2019. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Safety . 169(19):911-917. [ Links ]
Zhang, L.; Hu, B.; Deng, K.; Gao, X.; Sun, G.; Zhang, Z.; Li, P.; Wang, W.; Li, H. and Zhang, Z. J. 2019. NRT1. 1B improves selenium concentrations in rice grains by facilitating selenomethinone translocation. Plant Biotechnol. J. 17(16): 1058-1068. [ Links ]
Zhu, Z.; Chen, Y.; Shi, G. and Zhang, X. 2017. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 219(1):179-184. [ Links ]
Received: June 01, 2021; Accepted: September 01, 2021











 texto em
texto em 


