Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 no.7 Texcoco Set./Nov. 2021 Epub 22-Mar-2022
https://doi.org/10.29312/remexca.v12i7.2760
Articles
The aeration rate in the aerobic degradation of the organic fraction of municipal solid waste
1División Químico Biológicas-Universidad Tecnológica de Tecámac. Tecámac, Estado de México, México. CP. 55740. (gcarrilloS@uttecamac.edu.mx).
2Laboratorio de investigación en procesos avanzados de tratamiento de aguas-Instituto de Ingeniería-Unidad Académica Juriquilla-Universidad Nacional Autónoma de México. Blvd. Juriquilla 3001, Querétaro, México. (macmarin@live.com.mx).
3Departamento de Biotecnología-Universidad Autónoma Metropolitana-Unidad Iztapalapa. México. CP. 09340. (saucedo@xanum.uam.mx).
4Departamento de Ingeniería Ambiental-Universidad Demócrito de Tracia. Vasilissis Sofias 12, Xanthi 671 00, Grecia. (dkomilis@env.duth.gr).
Microbial and enzymatic kinetics are important factors during the aerobic degradation of the organic fraction of municipal solid waste, these depend mainly on the incubation temperature and aeration rates. The objective of this research was to evaluate the process of aerobic degradation, by multiple variables and their combination to understand the interactions between aeration rates in aerobic degradation and their responses. Aeration rates were set at 0.032, 0.064, 0.125, 0.251 and 0.392 L of moist air kg-1 min-1 at 35 °C with inoculum. Microbial activity was evaluated indirectly by means of respirometry, that is, CO2 production and O2 consumption. Extracellular enzymatic activities (ie., pectinases, cellulases, xylanases and proteases) were quantified by releasing the reducing sugars. The different tests were carried out at the Metropolitan Autonomous University, Iztapalapa Unit in September 2019. Finding a strong positive relationship between xylanase and pectinase enzymatic activity and dry weight loss, along with increased cellulase and xylanase activities at higher aeration rates.
Keywords: enzymatic activities; respirometry; aeration rate
La cinética microbiana y enzimática son factores importantes durante la degradación aerobia de la fracción orgánica de los residuos sólidos urbanos, estas dependen principalmente de la temperatura de incubación y las tasas de aireación. El objetivo de esta investigación fue evaluar el proceso de degradación aerobia, por múltiples variables y su combinación para comprender las interacciones entre las tasas de aireación en la degradación aerobia y sus respuestas. Las tasas de aireación se fijaron en 0.032, 0.064, 0.125, 0.251 y 0.392 L de aire húmedo kg-1 min-1 a 35 °C con inóculo. La actividad microbiana se evaluó de forma indirecta por medio de la respirometria; es decir, la generación de CO2 y el consumo de O2. Las actividades enzimáticas extracelulares (es decir, pectinasas, celulasas, xilanasas y proteasas) se cuantificaron mediante la liberación de los azúcares reductores. Los diferentes ensayos se realizaron en la Universidad Autónoma Metropolitana Unidad Iztapalapa en septiembre de 2019. Encontrando una fuerte relación positiva entre la actividad enzimática xilanasa y pectinasa con la pérdida de peso en seco, junto con el aumento de las actividades celulasas y xilanasas a mayores tasas de aireación.
Palabras clave: actividades enzimáticas; respirometría; tasa de aireación
Introduction
Oxygen is essential for microbial activity during aerobic degradation (Guo et al., 2012). A minimum oxygen concentration of 5% (v/v) in the center of the compost pile is necessary to ensure aerobic conditions (Rasapoor et al., 2009). The optimal aeration rate depends on the composition of the raw materials and ventilation methods (Komilis et al., 2011). If aeration is expressed as flow intensity, that is, air flow per unit mass of a substrate, a variable independent of the scale is obtained (Rodríguez-Fernández et al., 2012). This, in fermentation in solid medium, is generally expressed as the average airflow rate (L) per kilogram of a substrate per time (min) (v kg-1 m-1 or vkgm, as used throughout the writing).
The temperature and moisture gradients of the solid mass must be controlled to achieve a satisfactory process (Martins et al., 2011). The temperature range between 35-40 °C favors the development of a diverse microbial consortium (Saludes et al., 2007). The increase in temperature in the compost pile is related to the increase in microbial activity (Raut et al., 2008; Li et al., 2013). The quality (stability and maturity) of the compost is determined by multiple variables or their combination (Bernal et al., 2009).
Several authors have attempted to develop mathematical, stochastic and statistical models to understand the multiple relationships and interactions between operational conditions and composting responses (Li et al., 2013). Multivariate statistical methods have been used to process data from the anaerobic digestion process, in which attempts are made to correlate biochemical composition with methane yields (Bayard et al., 2015; Liu et al., 2015; El Achkar et al., 2017). The combined use of principal component analysis (PCA), cluster analysis, and biodegradability testing can help correlate nutrient levels and microbial activity in anaerobic digestion (Gil et al., 2018).
During composting processes, PCA has been widely applied to classify the generation of bad odors and their correlation with different organic waste and their composition (Toledo et al., 2018). PCA algorithms employ physicochemical parameters in both aerobic and anaerobic processes. The composting process can be characterized well by extracellular enzymatic activity (Tiquia et al., 2002). Based on the above, the objective of this work was to evaluate the process of aerobic degradation based on various variables to model the relationships and interactions between the aeration rates during composting and the response variables such as: oxygen (O2) consumption, carbon dioxide (CO2) production, the consumption of reducing sugars and the xylanases, cellulases, proteases and pectinases enzymatic activities.
Materials and methods
Substrates and inoculum
The organic fraction of municipal solid waste (OFMSW) was obtained from food processing establishments in Iztapalapa, in Mexico City (Mexico). The stable compost of the mixed municipal solid waste composting plant of the Bordo Poniente of Mexico City (CPBP) was used as a source of microbial consortia (Mc) as an inoculum. The taking of the compost of the CPBP was carried out in accordance with the standard ASTM D-34 (2016).
Experimental strategy
The degradation of OFMSW was carried out in four tubular bioreactors (TB) with capacities of 0.98, 1.91 and 3.84 L. The TBs were packed with a wet-based mixture of: OFMSW 83%, microbial consortium 8%, sawdust 4%, paper 3% and pruning waste 2% to achieve an initial C/N ratio of 30 (Martínez-Valdez et al., 2015). The initial moisture was 70% (Liang et al., 2003). During aerobic degradation, aeration rates increased by a proportion of 0.5 in the different experiments (0.032, 0.064, 0.125, 0.251 and 0.392 vkgm). The respirometry system was operated according to Saucedo-Castañeda et al. (2013); Torres-Mancera et al. (2018). The calibration of the respirometry equipment was done with standard mixtures of CO2 y O2.
Identification of microorganisms
All TBs were inoculated (CPBP compost) and tests were performed at 35 °C due to the increased microbial activity (shown by respirometry) at that temperature (Martínez-Valdez et al., 2015). Once the process was completed, the TBs were unpacked, the material was weighed on an analytical balance (Ohaus Galaxy® 200) and the moisture, pH and enzymatic activity were determined. All samples were obtained at the time of maximum CO2 generation rate, as this indicated the highest microbial activity.
The analysis of the microbial population was performed using the technique of denaturing gradient gel electrophoresis (DGGE): samples were taken under all conditions and centrifuged at 8 049.6 g. The precipitate (1 g) was taken, and the DNA extraction and purification was carried out using the PowerSoil® DNA isolation kit (MoBio). Once the extraction and purification were done a 1% agarose gel was run to corroborate the success of the DNA extraction. Subsequently, a polymerase chain reaction (PCR) was performed to obtain in vitro copies of a fragment of the sequence of the 16S rRNA gene from each of the samples, which was carried out in a thermocycler (Multigene, Labnet International, Inc.).
The reaction mixture, with a final volume of 50 μl, contains 10 X of buffer, 1.5 mmol of MgCl2, 200 μm of each dNTP (Promega Corporation, USA), 10 pM of each primer, 1.5 U of GoTaq® Flexi DNA Polymerase (Promega Corporation, USA) and 1-20 ng μl-1 of DNA template. The amplification conditions were as follows: 2 min at 95 ºC of initial denaturation, 25 cycles consisting of 30 s at 95 ºC, 30 seconds at 50 ºC and 1 min at 72 ºC, followed by a final extension of 5 min at 72 ºC. The amplicons obtained were stored at 4 ºC after running by electrophoresis (100 V, 30 min) in a 1% agarose gel. After amplification of the 16S rRNA gene sequence, a new nested amplification was made using the previously obtained amplicon as a DNA template.
The primers 1070F (5’ ATG GCT GTC GTC AGC T 3’) and 1392R+GC (5’ CGC CCG CCG CGC CGC CCC GCC GCC GCC GCC GCC CCG CCC CCC ACG GGC GGR GRG TAC 3’) were used using the same amplification conditions by changing the alignment temperature to 55°C. The samples were purified with a commercial kit (Wizard® SV Gel and PCR Clean-up System, Promega Corporation) following the protocol described by the supplier. The quantification of DNA nucleic acids was measured with the NanoDrop ND1000 spectrophotometer (Thermo Scientific). There was 1 μl with 500 ng of DNA from each sample added to 5 μl of loading buffer (0.25% bromophenol blue; 0.25% xylene cyanol; 30% glycerol, in water).
Amplicon profiles were analyzed with the universal mutation detection system DCode (Bio-Rad Laboratories, Hercules, CA) using acrylamide gels of 16 cm x 16 cm, using a 7% acrylamide gel with a denaturation gradient of 30-60%, with a ratio of (37.5:1), which were filtered at 0.45 μm. To do this, two solutions were prepared for the elaboration of the degradation gel from 30 to 60%. The 30% solution was prepared using 12.5 ml of 40% acrylamide/Bis, 2 ml of TAE 50X, 12 ml of formamide and 12.6 g of urea Molecular Biology grade (Sigma-Aldrich) and 60.9 ml of milli Q water per 100 ml. The 60% solution contained 12.5 ml 40% acrylamide/Bis, 2 ml of TAE 50X, 24 ml of formamide, 25.2 g of urea and 36.3 ml of milli Q water per 100 ml.
Both solutions were filtered through a 0.45 μm filter and degassed. The formation of the gel was carried out by the BioRad 475 gradient former, which was loaded with 20 ml of denaturing solution, 70 μl of 10% persulfate and 10 μl of TEMED. Gel electrophoresis was performed for 16 hours in TAE buffer (0.5 X, pH 8) at 60 °C using a PowerPac universal power supply (Bio-Rad), with an initial voltage of 200 V for 10 min, then the voltage was reduced at 85 V for 16 h (Neilson et al., 2013). The gel was stained according to the technique described by Radojkovic and Kušic (2000). The staining procedure consisted of an initial pre-stain fixation for 3 min in 10% ethanol, 0.5% acetic acid.
Staining for 5 min in fixation solution plus 0.2% silver nitrate, the washing of the gel in water for 2 min, and development for about 5 min in 30% NaOH and 0.1% formaldehyde in Milli-Q water. Once the gel was run, the identified bands were cut, and a 5 min extraction was performed. With water and stir. A 30% agarose gel (80 V, 45 min) was applied to corroborate the presence of DNA and then sequencing was performed by Macrogen.
Evolutionary history was deduced using the maximum likelihood method based on the Kimura 2-parameters model (Kimura, 1980), previously selected 8 by a Bayesian information criterion. The tree with the highest probability of trunk (-1499) is shown. The percentage of trees in which the associated taxa were grouped is shown next to the branches.
The initial trees for the heuristic search were obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with the superior log likelihood value. The tree is drawn to scale, with the lengths of the branches measured in the number of substitutions per site. The analysis used 32 nucleotide sequences. All positions containing gaps and missing data were removed. There were 239 positions in the final dataset. Evolutionary analyses were performed in MEGA7 (Kumar et al., 2016).
Measurement of enzymatic activity
The activities of the extracellular enzymes, pectinase, cellulase and xylanase, were quantified by the release of reducing sugars. Reactions were carried out in a citrate buffer (50 mmol, pH 5.5). Pectinase activity was measured using 1% birch pectin incubated at 37 °C for 30 minutes (Zhang et al., 2000). Cellulase activity was quantified using 2% carboxymethylcellulose incubated at 50°C for 30 minutes (Ghose, 1987).
Xylanase activity was measured using 1% xylan from birch wood incubated at 50 °C for 15 minutes (Loera and Córdova, 2003). All reaction volumes were: 750 ml of substrate with 250 ml of enzymatic extract, mixed in an orbital stirring of 250 rpm. The solutions were measured at a wavelength of 540 nm with a Beckman® DU 640 spectrophotometers.
Protease activity was determined using a modified method from Alef and Nannipieri (1995). An aliquot of 1 ml of enzymatic extract was added to 5 ml of 2% casein solution and incubated at 50 °C under stirring for 2 h. Enzymatic activities were expressed as U per gram of dry matter, where 1 unit (U) is the amount of enzyme that catalyzes the generation of 1 μmol of product per minute under enzymatic reaction conditions.
Results and discussion
Effect of aeration during composting
To analyze the effect of the aeration rate (vkgm), the CO2 content (v/v) produced under all conditions was evaluated (Figure 1). CO2 production was 31, 20, 14, 9, and 2% at 0.032, 0.064, 0.125, 0.251, and 0.392 vkgm, respectively. A principal component analysis (PCA) is shown in Figure 2a. The PCA showed that a main group containing cumulative CR, instantaneous CR, pectinase and CO2 as grouped variables, which are associated with each other, and another main group contained reducing sugars, xylanase and cellulase (Figure 2a). In addition, it was observed that vkgm and protease are negatively correlated with pectinase, CO2 and CR.
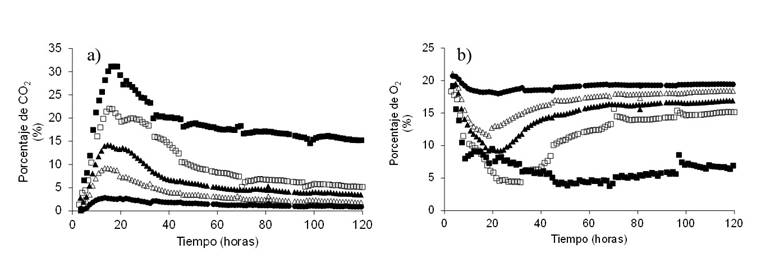
Figure 1 Concentration of a) CO2; and b) O2 at the gaseous outlet during the aerobic degradation of OFMSW at different aeration rates (vkgm) ■ 0.032, □ 0.064, ▲ 0.125, ∆ 0.201 and ● 0.392).
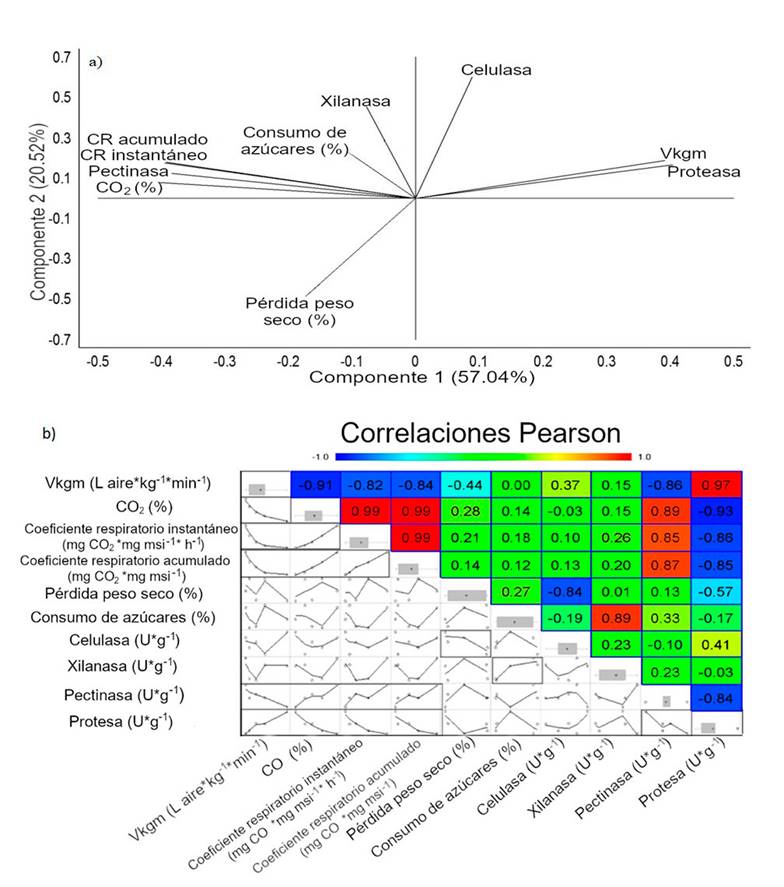
Figure 2 a) principal component analysis (PCA); and b) correlation matrix and Pearson’s correlation coefficients between aeration rate, physicochemical, respirometric and enzymatic responses determined.
The analysis showed that a 3-component model explains 96.34% of the variability and a 2-component model explains 76%. A dispersion matrix and Pearson’s correlation coefficients between each pair of variables are included in Figure 2b. It was observed that the aeration rate (vkgm) has a significant negative correlation with respect to pectinase activity (-0.86) and with respirometric activity responses such as CO2 content (-0.91), instantaneous CR (-0.82) and cumulative CR (-0.84). on the other hand, vkgm has a significant positive correlation with protease activity (0.97).
In addition, the high activity of proteases indicates that soluble proteins are degraded by proteases, and it could be a consequence of pectinase activity being reduced as a result. Protease activity has a significant positive correlation with cellulase activity (0.41). These observations were also reported by Goyal et al. (2005); He et al., (2013), who observed an increase in protease and cellulase activity during the active phase of composting. Kayikçioğlu et al. (2011) reported that the maximum protease activity coincided with the maximum microbial activity.
The PCA revealed that pectinase has a significant positive correlation with the respirometric indices, namely CO2 (0.89), instantaneous CR (0.85) and cumulative CR (0.87) and a negative correlation with vkgm (-0.86). Meanwhile, proteases have a significant negative correlation with respirometric indices such as CO2 (-0.93), instantaneous CR (-0.86) and cumulative CR (-0.85) and a significant positive correlation with vkgm (0.97).
The above confirms that protease is correlated with aerobic conditions, while pectinase is only present in anaerobic conditions. Puyuelo et al. (2011); Xu et al. (2012) have pointed out that the increase in enzymatic activity depends on the microbial consortia present and the concentration of microorganisms. Therefore, the change in protease and pectinase activity could indicate changes in microbial consortia during the process.
Microorganisms present in different aeration conditions
The identification of bacteria (Figure 3) Citrobacter freundi, Klebsiella oxytoca can be found. Klebsiella oxytoca has been reported as a high producer of cellulose enzymes (Golias et al., 2002). In low aeration rates (0.032, 0.064 and 0.125 vkgm), the presence of Enterobacteria has been revealed, which is related to low protease and high pectinase activities (i.e., the prevalence of semi-anaerobic environments). These bacteria are chemoorganotrophic with both respiratory and fermentative metabolism. Most are negative oxidases, except for Plesiomonas. Most reduce nitrate to nitrite, except for fermentations of Saccharobacter and some strains of Erwinia and Yersinia (Brenner and Farmer, 2005).
Some strains, such as E. cloacae, E. hafniae or E. aerogenes are producers of lipase. On the other hand, Buttiauxella sp. is known as a phytase producer and a facultative anaerobe of the Enterobacteriacea family (Mitra et al., 2010). In the condition with the highest rate of aeration tested (0.392 and 0.251 vkgm), Acinetobacter bacteria were the most representative, being strictly aerobic with positive catalase and negative oxidase activity. Most strains can grow in a simple minimum medium with a single carbon source and an incubation temperature between 20-30 ºC.
Lewin et al. (2016); Wang et al. (2016) mentioned that Actinobacter contributes to the global carbon cycle through the decomposition of plant biomass. Its enzymes may contribute to the industrial-scale decomposition of cellulosic plant biomass into simple sugars that could be converted into biofuels. Actinobacter hosts a substantially broad catalog of cellobiohydrolase, β-glucosidase, acetyl xylan esterase, arabinofuranosidase, pectin lyase, and ligninase genes.
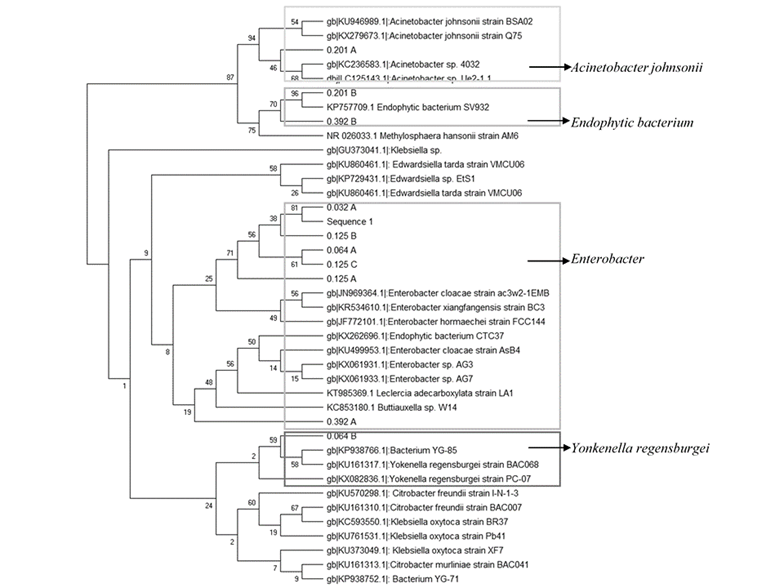
Figure 3 Molecular phylogenetic analysis by the maximum probability method. The experimental conditions used were: Inoculation by composting of CPBP, temperature set at 35 °C, initial moisture 70% (wb), pH 6, various aeration rates, namely 0.032, 0.064, 0.125, 0.251 and 0.392 vkgm. All samples were obtained at the time of maximum CO2 generation rate.
Conclusions
The results revealed a strong positive correlation between xylanase, pectinase and dry weight loss, while all enzymatic activities increased at higher aeration rates. In this sense, the high consumption of reducing sugars was correlated only with the activity of xylanase and pectinase, since cellulase and protease had a lower activity. DGGE revealed that at the highest aeration rates (0.392 and 0.251 vkgm), the presence of Actinobacter was dominant. Therefore, it can be inferred that under a system in aerobic conditions, the presence of Actinobacter favors the degradation of organic matter.
Literatura citada
Alef, K. and Nannipieri, P. 1995. Chapter 7-Enzyme activities. In: Methods in Applied Soil Microbiology and Biochemistry. Kassem, A.; Paolo, N. (eds). 1st Edition. Academic Press. London. 311- 373 pp. [ Links ]
ASTM (American Society for Testing and Materials) D5231-92. 2016. Standard Test Method for Determination of the Composition of Unprocessed Municipal Solid Waste. ASTM International. West Conshohocken. Pensilvania. USA. 6 p. https://doi.org/10.1520/D5231-92R16 . [ Links ]
Bayard, R.; Gonzalez-Ramirez, L.; Guendouz, J.; Benbelkacem, H.; Buffière, P. and Gourdon, R. 2015. Statistical analysis to correlate bio-physical and chemical characteristics of organic wastes and digestates to their anaerobic biodegradability. Waste and biomass valorization. 6 (5), 759-769. https://doi.org/10.1007/s12649-015-9411-2. [ Links ]
Bernal, M. P.; Alburquerque, J. A. and Moral, R. 2009. Composting of animal manures and chemical criteria for compost maturity assessment. A review Bioresource Technology. 100(22):5444-5453. https://doi.org/10.1016/j.biortech.2008.11.027. [ Links ]
Brenner, D. J. and Farmer III, J. J. 2005. Family I. Enterobacteriaceae. In: Bergey’s Manual of Systematic Bacteriology. Brenner D. J.; Krieg N. R.; Staley J. T. ; Garrity G. M.; Boone, D. R.; Vos, P.; Goodfellow, M.; Rainey, F. A. and Schleifer, K. H. (eds). 2nd ed. Springer. New York, USA. 587-607 pp. https://doi.org/10.1002/9781118960608.fbm00222. [ Links ]
El Achkar, J. H.; Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R. G. and Lanoisellé, J. L. 2017. Anaerobic digestion of nine varieties of grape pomace: correlation between biochemical composition and methane production. Biomass Bioenergy. 107:335-344. https://doi.org/10.1016/j.biombioe.2017.10.030. [ Links ]
Ghose, T. K. 1987. Measurement of cellulase activities. Pure Appl. Chem. 59(2):257-268. https://doi.org/10.1351/pac198759020257. [ Links ]
Gil, A.; Toledo, M.; Siles, J. A. and Martín, M. A. 2018. Multivariate analysis and biodegradability test to evaluate different organic wastes for biological treatments: anaerobic co-digestion and co-composting. Waste Management. 78:819-828. https://doi.org/10.1016/j.wasman. 2018.06.052. [ Links ]
Golias, H.; Dumsday, G. J.; Stanley, G. A. and Pamment, N. B. 2002. Evaluation of a recombinant Klebsiella oxytoca strain for ethanol production from cellulose by simultaneous saccharification and fermentation: comparison with native cellobiose-utilising yeast strains and performance in co-culture with thermotolerant yeast and zymomonas mobilis. J. Biotechnol. 96(2):155-168. https://doi.org/10.1016/S0168-1656(02)00026-3 . [ Links ]
Goyal, S.; Dhull, S. K. and Kapoor, K. K. 2005. Chemical and biological changes during composting of different organic wastes and assessment of compost maturity. Bior. Technol. 96(14):1584-1591. https://doi.org/10.1016/j.biortech.2004.12.012. [ Links ]
Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y. and Shen, Y. 2012. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bior. Technol. 112:171-178. https://doi.org/10.1016/j.biortech.2012.02.099. [ Links ]
He, Y.; Xie, K.; Xu, P.; Huang, X.; Gu, W.; Zhang, F. and Tang, S. 2013. Evolution of microbial community diversity and enzymatic activity during composting. Res. Microbiol. 164(2):189-198. https://doi.org/10.1016/j.resmic.2012.11.001. [ Links ]
Kayikçioğlu, H. H. and Okur, N. 2011. Evolution of enzyme activities during composting of tobacco waste. Waste Manag. Res. 29(11):1124-1133. https://doi.org/10.1177/0734242X10392813 . [ Links ]
Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Ev. 16(2):111-120. https://doi.org/10.1007/BF01731581. [ Links ]
Komilis, D.; Kontou, I. and Ntougias, S. 2011 . A modified static respiration assay and its relationship with an enzymatic test to assess compost stability and maturity. Bior. Technol. 102(10):5863-5872. https://doi.org/10.1016/j.biortech.2011.02.021. [ Links ]
Kumar, S.; Stecher, G. and Tamura, K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7):1870-1874. https://doi.org/10.1093/molbev/msw054 . [ Links ]
Lewin, G. R.; Carlos, C.; Chevrette, M. G.; Horn, H. A.; McDonald, B. R.; Stankey, R. J. and Currie, C. R. 2016. Evolution and ecology of actinobacteria and their bioenergy applications. Annual Review MicrobioL. 70:235-254. https://doi.org/10.1146/annurev-micro-102215-095748. [ Links ]
Li, Z.; Lu, H.; Ren, L. and He, L. 2013. Experimental and modeling approaches for food waste composting: a review. Chemosphere. 93(7):1247-1257. https://doi.org/10.1016/j.chemosphere.2013.06.064. [ Links ]
Liang, C.; Das, K. C. and McClendon, R. W. 2003. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend . Bior. Technol. 86(2):131-137. https://doi.org/10.1016/S0960-8524(02)00153-0 . [ Links ]
Liu, X.; Bayard, R.; Benbelkacem, H.; Buffière, P. and Gourdon, R. 2015. Evaluation of the correlations between biodegradability of lignocellulosic feedstocks in anaerobic digestion process and their biochemical characteristics. Biom. Bioen. 81:534-543. https://doi.org/10.1016/j.biombioe.2015.06.021. [ Links ]
Loera, O. and Córdoba, J. 2003. Improvement of xylanase production by a parasexual cross between Aspergillus niger strains. Braz. Archiv. Biol. Technol. 46(2):177-181. https://doi.org/10.1590/S1516-89132003000200006. [ Links ]
Martínez-Valdez, F. J.; Martínez-Ramírez, C.; Martínez-Montiel, L.; Favela-Torres, E.; Soto-Cruz, N. O.; Ramírez-Vives, F. and Saucedo-Castañeda, G. 2015. Rapid mineralisation of the organic fraction of municipal solid waste. Bior. Technol. 180:112-118. https://doi.org/10.1016/j.biortech.2014.12.083. [ Links ]
Martins, S.; Mussatto, S. I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C. N. and Teixeira, J. A. 2011 . Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 29(3):365-373. https://doi.org/10.1016/j.biotechadv.2011 .01.008. [ Links ]
Mitra, S.; Khare, S. K. and Singh, R. 2010. Alkaline lipase production from Enterobacter aerogenes by solid-state fermentation of agro-industrial wastes. International Journal of Environment and Waste Management , 5 (3-4), 410-418. https://doi.org/10.1504/IJEWM.2010.032017. [ Links ]
Neilson, J. W.; Jordan, F. L. and Maier, R. M. 2013. Analysis of artifacts suggests EGGD should not be used for quantitative diversity analysis. J. Microbiol. Methods. 92(3):256-263.https://doi.org/10.1016/j.mimet.2012.12.021. [ Links ]
Puyuelo, B.; Ponsá, S.; Gea, T. and Sánchez, A. 2011 . Determining C/N ratios for typical organic wastes using biodegradable fractions. Chemosphere. 85(4):653-659. https://doi.org/10. 1016/j.chemosphere.2011.07.014. [ Links ]
Radojkovic, D. and Kušic, J. 2000. Silver staining of denaturing gradient gel electrophoresis gels. Clinical Chem. 46(6):883-884. [ Links ]
Rasapoor, M.; Nasrabadi, T.; Kamali, M. and Hoveidi, H. 2009. The effects of aeration rate on generated compost quality, using aerated static pile method. Waste Management . 29(2):570-573. https://doi.org/10.1016/j.wasman.2008.04.012. [ Links ]
Raut, M. P.; Prince-William, S. P; Bhattacharyya, J. K.; Chakrabarti, T. and Devotta, S. 2008. Microbial dynamics and enzyme activities during rapid composting of municipal solid waste-a compost maturity analysis perspective. Bior. Technol. 99(14):6512-6519. https://doi.org/10.1016/j.biortech.2007.11.030. [ Links ]
Rodríguez-Fernández, D. E.; Rodríguez-León, J. A.; de-Carvalho, J. C.; Karp, S. G.; Sturm, W.; Parada, J. L. and Soccol, C. R. 2012. Influence of airflow intensity on phytase production by solid-state fermentation. Bior. Technol. 118:603-606. https://doi.org/10.1016/j.biortech. 2012.05.032. [ Links ]
Saludes, R. B.; Iwabuchi, K.; Kayanuma, A. and Shiga, T. 2007. Composting of dairy cattle manure using a thermophilic-mesophilic sequence. Biosystems Engineering. 98(2):198-205. https://doi.org/10.1016/j.biosystemseng.2007.07.003. [ Links ]
Saucedo-Castañeda, G.; Favela-Torres, E.; Viniegra-González, G.; Torres-Mancera, M. T.; Figueroa-Montero, A. and Rosales-Zamora, G. 2013. Respirometry system with remote management for the on-line monitoring of the concentration of CO2 and O2 and flow of the exhausting gases in biological processes. Mexican patent 336733 granted January 22 th, 2016. [ Links ]
Tiquia, S. M. 2002. Evolution of extracellular enzyme activities during manure composting. J. Appl. Microbiol. 92(4):764-775. https://doi.org/10.1046/j.1365-2672.2002.01582.x. [ Links ]
Toledo, M.; Siles, J. A.; Gutiérrez, M. C. and Martín, M. A. 2018. Monitoring of the composting process of different agroindustrial waste: Influence of the operational variables on the odorous impact. Waste Management . 76:266-274. https://doi.org/10.1016/j.wasman. 2018.03.042. [ Links ]
Torres-Mancera, M. T.; Figueroa-Montero, A.; Favela-Torres, E.; Rosales-Zamora, G.; Nampoothiri, K. M. and Saucedo-Castañeda, G. 2018. Online monitoring of solid-state fermentation using respirometry. In current developments in biotechnology and bioengineering. Elsevier. 97-108 pp. https://doi.org/10.1016/B978-0-444-63990-5.00006-2. [ Links ]
Wang, C.; Dong, D.; Wang, H.; Müller, K.; Qin, Y.; Wang, H. and Wu, W. 2016. Metagenomic analysis of microbial consortia enriched from compost: new insights into the role of Actinobacteria in lignocellulose decomposition. Biotechnol. Biofuels. 9(1):22-31. https://doi.org/10.1186/s13068-016-0440-2. [ Links ]
Xu, S. Y.; Karthikeyan, O. P.; Selvam, A. and Wong, J. W. C. 2012. Effect of inoculum to substrate ratio on the hydrolysis and acidification of food waste in leach bed reactor. Bior. Technol. 126:425-430. https://doi.org/10.1016/j.biortech.2011.12.059. [ Links ]
Zhang, J.; Henriksson, G. and Johansson, G. 2000. Polygalacturonase is the key component in enzymatic retting of flax. J. Biotechnol. 81(1):85-89. https://doi.org/10.1016/S0168-1656(00)00286-8 . [ Links ]
Received: June 01, 2021; Accepted: August 01, 2021











 texto em
texto em 


