Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 no.5 Texcoco Jun./Ago. 2021 Epub 14-Mar-2022
https://doi.org/10.29312/remexca.v12i5.2700
Articles
Low frequency ultrasound as an enhancer for the germination process of Stizolobium pruriens
1Department of Plant Health, Rural Engineering and Soil DEFERS-São Paulo State University ‘Júlio de Mesquita Filho’ UNESP. Ilha Solteira SP, Brazil. ZC. 15385-000. (adrielle.prates@unesp.br; gabrielaoliverio.bio@gmail.com; katia.maltoni@unesp.br)
2Department of Exact Sciences-University of São Paulo USP. PO Box 9, Piracicaba SP, Brazil. ZC. 13418-900. (beatrizgl@usp.br).
3Department of Exact Sciences-University of São Paulo USP, PO Box 9, Piracicaba SP, Brazil. ZC. 15385-000. (glaucia.a.faria@unesp.br).
Mucuna (Stizolobium pruriens) is widely used in agriculture as a green allowance and in crop rotation, due to its ability to fix nitrogen and recover degraded areas; without embargo, there is a slow and uneven germination. This study used some classical methods, together with the use of low-frequency ultrasound to accelerate and homogenize the germination and emergence of the seeds. The experiment was carried out at the Plant Tissue Cultivation Laboratory of the Ilha Solteira Campus, São Paulo, Brazil. The design used was a completely randomized one, with five replications, in a 3x6 factorial scheme, the factors being: three pre-treatments for latency break: mechanical scarification, thermal scarification, and without scarification with six levels of ultrasound exposure: 0, 1, 2, 3, 5, 8 min, totaling 18 treatments. For eight days the germination and the initial stages of the seedlings were controlled. The method without scarification subjected to 4.5 min of ultrasound can become an excellent alternative, since it presented greater germination vigor, while 3.14 min of exposure to ultrasound were enough to improve the emergence speed, regardless of the method used in the preparation of seeds. In conclusion, only with the use of low-frequency ultrasound, it is possible to improve both the germination speed index and the germination vigor, without the need for additional treatments.
Keywords mechanical scarification; thermal scarification; ultrasonic
La mucuna (Stizolobium pruriens) se usa ampliamente en agricultura como abono verde y en rotación de cultivos, debido a su capacidad para fijar nitrógeno y recuperar áreas degradadas; sin embargo, tiene una germinación lenta y desigual. Este estudio utilizó algunos métodos clásicos, junto con el uso de ultrasonidos de baja frecuencia para acelerar y homogeneizar la germinación y emergencia de las semillas. El experimento se realizó en el Laboratorio de Cultivo de Tejidos Vegetales del campus Ilha Solteira, São Paulo, Brasil. El diseño utilizado fue un completamente al azar, con cinco repeticiones, en un esquema factorial 3x6, siendo los factores: tres pre-tratamientos para ruptura de la latencia: escarificación mecánica, térmica, y sin escarificación con seis niveles de exposición a ultrasonido: 0, 1, 2, 3, 5, 8 min, totalizando 18 tratamientos. Durante ocho días se controló la germinación y las etapas iniciales de las plántulas. El método sin escarificación sometido a 4.5 min de ultrasonido puede convertirse en una excelente alternativa, ya que presentó mayor vigor de germinación, mientras que 3.14 min de exposición a ultrasonido fueron suficientes para mejorar la velocidad de emergencia, independientemente del método utilizado en la preparación de semillas. En conclusión, solo con el uso de ultrasonidos de baja frecuencia es posible mejorar tanto el índice de velocidad de germinación como el vigor de germinación, sin necesidad de tratamientos adicionales.
Palabras clave escarificación mecánica; escarificación térmica; ondas ultrasónicas
Introduction
The S. pruriens (Black mucuna) is native from the West Indies, and it is adapted to tropical and subtropical climates (Cruz et al., 2011). It is characterized as an annual legume, with climbing branches and low habit. Its growth is undetermined, with a life cycle lasting over 150 days (Ambrosano et al., 2016; Angeletti et al., 2016). The specie’s development has differential physiology that provides for rapid overlapping of vegetation, hindering growth and performance of other plant forms (Ramos et al., 2018). Black mucuna is commonly used as green manure, in the recovery of degraded areas, with active performance in crop rotation processes and soil decompaction. It also has a direct effect on nitrogen levels through biological fixation, suppressing some species of nematodes, it also acts as a weed controller, functioning mainly in grain production systems without direct seeding (Castro et al., 2011; Ragassi and Melo, 2017).
The species is classified in the the Fabaceae family, one of the largest families of Angiosperms with more than 650 genera (Mello et al., 2015), including species that stimulate the mineralization of some herbicides with phytoremediation effects. The Stizolobium pruriens is efficient in the processes of decontamination, showing some tolerance to herbicides (Silva et al., 2012). Widespread in Cerrado areas, due to the availability of seeds, S. pruriens is well adapted to water hortfall conditions, withstanding high temperatures, with no photoperiod restriction (Teodoro et al., 2015). However, even with all its potential, difficulties are still being faced due to the low and uneven seed germination (Oliveira et al., 2017).
Many plant species with viable seeds, do not absorb enough water to germinate, even under favorable conditions due to hardness and impermeability of tegument (Ramos et al., 2019). Several treatments are available to overcome this type of seed dormancy, such as immersion in acids, hot or cold water, alcohol, removal of the caruncle, mechanical scarification, among others. The set of treatments that will produce greater efficiency in germination depend on the particularities of each species (Oliveira et al., 2017; Pereira et al., 2020).
The use of low-frequency ultrasound in a liquid environment has a high potential to stimulate germination, as it contributes to water imbibition by seeds (Gordon, 1963; Yaldagard et al., 2008; Venâncio et al., 2016), and promotes development of living tissue (Venâncio and Martins, 2019). This work aims to study the behavior of S. pruriens seeds concerning the germination speed index and seedling emergence. Therefore, low-frequency ultrasound is associated with classical methods of dormancy breaking (wet thermal scarification and manual mechanical scarification).
Material and methods
The experiment took place in the Plant Tissue Culture Laboratory at UNESP campus of Ilha Solteira, São Paulo, Brazil, under controlled conditions. The seeds of S. pruriens used are from 2017 harvest under field cultivation, of those 94% are pure and 82% are viable, values informed by the seed producing company.
To avoid contamination, seeds were disinfected soaking them for 1 minute in a 50% water solution of Lysoform® (0.45% de Cocobenzyl Alkyl Dimethyl Ammonium Chloride/Didecyl Dimethyl Ammonium Chloride); after they were washed with distilled water.
Seeds were pre-treated, before applying the ultrasound, as follows: 1) wet thermal scarification, soaking in hot water until reaching 60 °C, drained, and cooling at room temperature for 24 hours; 2) manual mechanical scarification, which consists of sanding the seeds with 150 mm sandpaper; 3) and non-scarified seeds (control), and in each method, ten seeds were used.
After the process carried out with the three methods of scarification (pre-treated), seeds were exposed to the ultrasound treatment (application of sound waves via ultrasonic probe with frequency of 3 mHz, 120 volts ~ 50/60 Hz) for different periods of time (0, 1, 2, 3, 5 and 8 min). For ultrasound exposure, 10 seeds (replications) were placed in a 50 ml glass container with 20 ml of distilled water, where the ultrasonic probe was introduced, and the treatments (Table 1).
Table 1 Description of the treatments covered in the study.
| Treatments | Descriptions |
|---|---|
| WS | Control (seeds without scarification and no ultrasound exposure); |
| TS | Wet thermal scarification and no ultrasound exposure; |
| MS | Manual mechanical scarification with sandpaper and no ultrasound exposure |
| WSU1 | WS + 1 minute of ultrasound exposure |
| WSU2 | WS + 2 minutes of ultrasound exposure |
| WSU3 | WS + 3 minutes of ultrasound exposure |
| WSU5 | WS + 5 minutes of ultrasound exposure |
| WSU3 | WS + 8 minutes of ultrasound exposure |
| TSU1 | TS + 1 minute of ultrasound exposure |
| TSU2 | TS + 2 minutes of ultrasound exposure |
| TSU3 | TS + 3 minutes of ultrasound exposure |
| TSU5 | TS + 5 minutes of ultrasound exposure |
| TSU8 | TS + 8 minutes of ultrasound exposure |
| MSU1 | MS + 1 minute of ultrasound exposure |
| MSU2 | MS + 2 minutes of ultrasound exposure |
| MSU3 | MS + 3 minutes of ultrasound exposure |
| MSU5 | MS + 5 minutes of ultrasound exposure |
| MSU8 | MS + 8 minutes of ultrasound exposure |
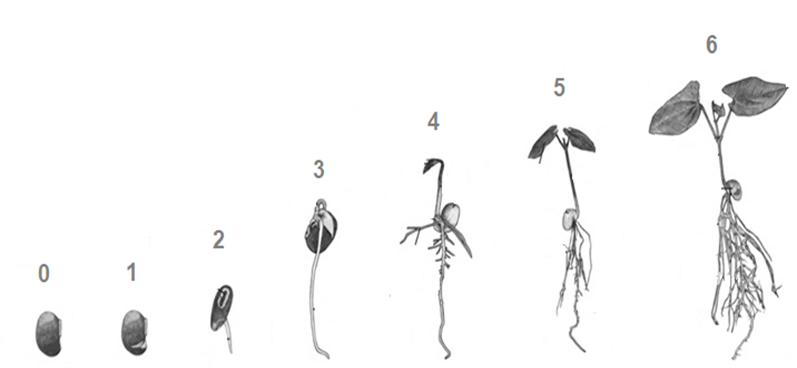
Treated seeds were introduced in containers with water and 20 g L-1 Phytagel, in a horizontal laminar flow chamber and placed in a growth room, with a constant temperature of 22 ºC and 18 h of photoperiod. Germination (G, in%) was evaluated daily, for 15 days, to obtain an average germination time (AGT) in equation 1, emergency speed index (ESI) in equation 2 (Maguire, 1962) and germination vigor (GV).
Table 2 Description of the phases observed in the study.
| Range | Descriptions |
|---|---|
| 0 | Did not germinate |
| 1 | Rupture of the integument |
| 2 | Radicle elongation |
| 3 | Differentiation of radicle, colleto and hypocotyl |
| 4 | Detachment of cotyledons from the seed coat, and beginning to opening |
| 5 | Open of cotyledons and emergence of the apical bud |
| 6 | Epicotyl and first pair of leaves opened |
A completely randomized designed was used, in a 3 x 6 factorial scheme, (without scarification -WS, wet thermal scarification -TS, mechanical scarification with sandpaper -MS) and six period of time of ultrasound incidence (0, 1, 2, 3, 5 and 8 minutes), producing 18 treatments with 5 replications each. The statistical analyses were performed using the SISVAR software. In the statistical analysis, the hypothesis of normality was initial tested using the Shapiro-Wilk test, after which an analysis of variance (Anova) was performed by F test at 5% probability to detect differences between factors and interactions.
The scarification factor effect was decomposed to verify the effect of the ultrasound time factor separately for each scarification method. In the presence of significant differences, the Tukey test (p< 0.05) was used to compare methods and the regression for ultrasound times. The regression model was verified via p-value of the regression deviation. The polynomial regression models with the higher determination coefficients (R2), were selected among the significant regressions by the F test.
Results and discussion
Seed germination differed (p< 0.05) among the methods used for breaking dormancy (Table 3), indicating that heating the seed is better than mechanical scarification, but both do not differ from the control without scarification. Ultrasound times alone and their interaction with dormancy-breaking methods did not show significant effects (p< 0.05) on seed germination (Table 3).
Table 3 Analysis of variance for germination (G) in percentage, average germination time (AGT) emergence speed index (ESI) and germination vigor (GV).
| Source of Variation | G | AGT | ESI | GV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSq1 | P-value | MSq | P-value | MSq1 | P-value | MSq | P-value | ||||
| Methods (M) | 4.44 | 0.012* | 4.39 | 0.01* | 4.55 | 0.139ns | 16.1 | 0.004** | |||
| Ultrasound (U) | 1.81 | 0.104ns | 0.4 | 0.812ns | 6.33 | 0.022* | 36.42 | 0** | |||
| M x U | 0.84 | 0.552ns | 1.81 | 0.046* | 4.23 | 0.062ns | 32.84 | 0** | |||
| CV (%) | 56 | 54 | 1 | 6 | |||||||
| Overall average | 0.55 | 1.78 | 1.01 | 2.88 | |||||||
| Methods | Average | ||||||||||
| WS | 0.51 ab | 1.413 ab | 1.014 | 2.873 ab | |||||||
| MS | 0.68 a | 1.75 a | 1.018 | 3.08 a | |||||||
| TS | 0.45 b | 2.176 b | 1.01 | 2.702 b | |||||||
| Ultrasound time (min) | |||||||||||
| 0 | 0.49 | 2.073 | 1.011 | 2.773 | |||||||
| 1 | 0.609 | 1.733 | 1.019 | 2.706 | |||||||
| 2 | 0.706 | 1.746 | 1.018 | 3.035 | |||||||
| 3 | 0.615 | 1.566 | 1.023 | 3.444 | |||||||
| 5 | 0.423 | 1.806 | 1.014 | 3.088 | |||||||
| 8 | 0.458 | 1.753 | 1.004 | 2.262 | |||||||
| Regression | MSq | p-value | MSq | p-value | |||||||
| Linear | - | - | 0.124 | 0.059ns | 24.625 | 0.003** | |||||
| Quadratic | - | - | 0.212 | 0.014* | 128.981 | 0** | |||||
| Cubic | - | - | 0.006 | 0.665ns | 2.083 | 0.392ns | |||||
| Deviation | - | - | 0.116 | 0.068ns | 13.205 | 0.01* | |||||
**, *, ns= significant at 1%, 5% and non-significant. By F test. Averages followed by the same letter in each column do not differ by Tukey test at p< 0.05.
The average germination time showed significant differences (p< 0.05) for scarification methods and for the interaction between both factors (Table 3). The main effect linked to scarification methods was disregarded, since the interaction effects allowed for the best responses. AGT showed significant differences for the methods and interaction. The variable emergency speed index did not show significant differences (p< 0.05) for methods of breaking dormancy (WS, TS and MS) and interaction. Therefore, there is no need manually to scarify or heat the seed for this purpose. However, ultrasound exposure had significant effects (p< 0.05) (Table 3) on this variable, with no significant interaction with the other factor.
It was expected that the TS treatment would accelerate the germination process, as its principle is to soften coat tissues favoring water absorption and gas exchange, accelerating the physiological reactions of seeds linked to germination (Câmara et al., 2015). However, this did not happen with the seeds of S. pruriens, which were indifferent to the scarification methods used. For ultrasound times, the levels of significance (p-value) of the equations and the deviation of the regression (Table 3) show significance for the quadratic model, with 0.014 of p-value (p< 0.05), explaining 86% of the data behavior (Table 3 and Figure 2), the maximum point for this regression model was estimated at 3.14 min of ultrasound exposure.
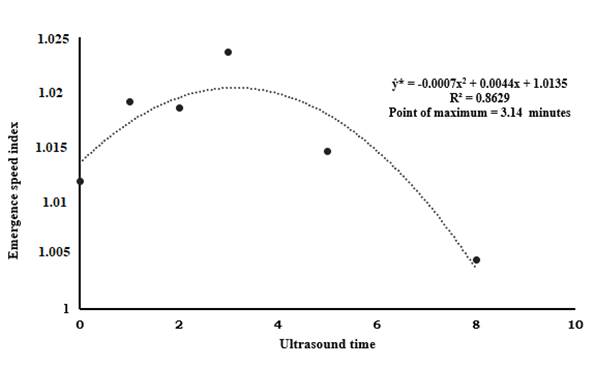
Figure 2 Quadratic regression for ultrasound incidence times regardless of the seed scarification method.
The variable ESI has statistical significance (p< 0.05) for ultrasound times (Table 3). In the quadratic regression model significant difference was found, with 0.014 p-value (p< 0.05), explaining 86% of the data. The maximum point for this regression model was estimated at 3.14 min of ultrasound exposure (Table 3 and Figure 2). For other models, the ESI was not significant (p< 0.05)
Seed germination vigor showed significant differences (p< 0.05) for scarification methods, ultrasound times and their interaction (Table 3). Thus, although the results main effects of individual factors were significant, the analysis of the interaction allowed a better assessment of the effects of these factors on germination vigor. The smallest vigor was obtained with TS, when compared WS and MS treatments (Table 4).
Table 4 Mean values of average germination time for different ultrasound exposure times and seed scarification methods.
| Ultrasound times scarification methods times (min) |
Control (no scarification) * |
Mecanical scarification ns |
Termal scarification ns |
||
|---|---|---|---|---|---|
| 0* | 2.72 b | 1.24 a | 2.26 ab | ||
| 1ns | 2.28 a | 2.72 a | 2.92 a | ||
| 2ns | 1.32 b | 3.693 a | 2.8 b | ||
| 3ns | 1.48 a | 3.92 a | 2.626 b | ||
| 5** | 0.78 a | 1.62 ab | 3.02 b | ||
| 8ns | 1.92 a | 2.666 a | 1.506 b | ||
| Regression | MSq | P-value | |||
| Linear | 2.502 | 0.101ns | - | - | |
| Quadratic | 8.667 | 0.003** | - | - | |
| Cubic | 0.143 | 0.693ns | - | - | |
| Deviation | 0.466 | 0.601ns | - | - | |
**, *, ns= significant at 1%, 5% and non-significant. By F test. Averages followed by the same letter in the lines do not differ by Tukey test at 5%.
For average germination time (AGT) a difference was found between the ultrasound incidence time (p< 0.05) only in treatments without scarification. The regression deviation was not significant (p> 0.05) and the data for adjusted only on the quadratic regression model (p< 0.05), with a very high coefficient of determination this 91%. This result suggests an excellent fit in the model.
The minimum AGT point occurred at the exposure time of 4.68 minutes; after that time there was an increase in average germination time. The time of ultrasound incidence showed a significant difference (p< 0.05) in all applied methods for germination vigor (GV), but this behavior varied within each method (Table 5). In the absence of scarification, the regression deviation was significant (p< 0.05), so the adjustment of any model must be accepted with restriction.
Table 5 Averages of the variable germination vigor in the interaction of ultrasound times and methods of breaking dormancy.
| Ultrasound times (minutes) | Methods of breaking dormancy | |||||
|---|---|---|---|---|---|---|
| WS** | MS** | TS** | ||||
| 0** | 2.4 b | 2.093 b | 3.826 a | |||
| 1ns | 2.48 a | 2.72 a | 2.92 a | |||
| 2** | 2.613 b | 3.693 a | 2.8 b | |||
| 3** | 3.786 a | 3.92 a | 2.626 b | |||
| 5** | 3.346 a | 3.386 a | 2.533 b | |||
| 8** | 2.613 a | 2.666 a | 1.506 b | |||
| Regression | MSq | P-value | MSq | P-value | MSq | P-value |
| Linear | 7.82 | 0.097ns | 4.557 | 0.206ns | 182.96 | 0** |
| Quadratic | 70.841 | 0** | 152.42 | 0** | 1.192 | 0.517ns |
| Cubic | 3.524 | 0.266ns | 16.496 | 0.016* | 21.941 | 0.006** |
| Deviation | 17.863 | 0.002** | 5.125 | 0.165ns | 1.385 | 0.614ns |
**, *, ns= significant at 1%, 5% and non-significant. Respectively. By F Test. Averages followed by the same letter in the lines do not differ by Tukey test at 5%.
In the absence of scarification, the quadratic regression model was the one that best fitted the data (p< 0.05), with a high coefficient of determination, but presented regression deviation significative. This result suggests that more studies would be necessary to determine the regression model (linear or non-linear), for this variable it is necessary to look for another model to explain this phenomenon. In the WS treatment, the maximum GV point occurred at the exposure time of 4.44 minutes, after that time there was no increase in germination vigor.
For the WS method, the regression deviation was not significant (p> 0.5) and the quadratic regression model was the one that best fitted the data (p< 0.05), with an adjustment (R²) of 85% to the data. In this case, the maximum germination vigor point occurred in 4.25 min of exposure of the seeds to the ultrasound. For both (WS and MS), the exposure time 5 and 8 min caused a decrease in GV. In the TS method, exposure to ultrasound was not beneficial for GV, obtaining decreasing scores as the time of exposure increased (Table 5 and Figure 3).

Figure 3 Average germination of S. pruriens subjected to different scarification methods (without scarification (WS), mechanical scarification (MS) and thermal scarification (TS)) associated with different ultrasound exposure.
In regard to the germination vigor variable, taking into account the effect of the methods when using the ultrasound, MS had better result than TS (Table 5), and no significant differences were found between WS and MS for the ultrasound times applied, except for 2 minutes when MS had greater vigor. At all incidence times to ultrasound, there were differences between the methods studied, except for 1 min. However, the WS + 4.5 minutes of exposure to ultrasound has an advantage, one operation less (without scarification), and the maximum vigor, what is very important if consider use the method as a commercial one (Figure 4).
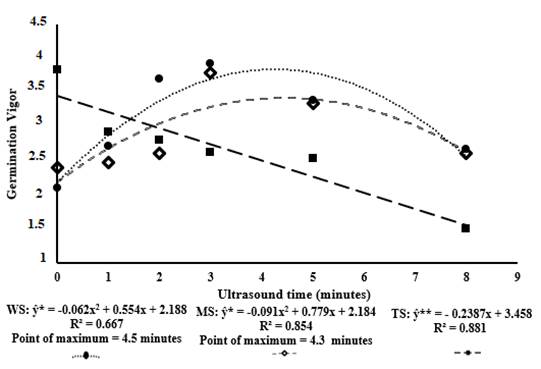
Figure 4 Germination vigor of S. prupriens seeds subjected to the different scarification methods (without scarification (WS), mechanical scarification (MS) and thermal scarification (TS)) associated with different ultrasound exposure times.
The use of ultrasound, until a certain time, can be promising to homogenize, accelerate and guarantee vigor in the germination process. The best ultrasound incidence times calculated by the regression equations were 3 (ESI), 4 (GV) and 5 (ATG) minutes (Figures 2, 3 and 4). After 3 min, there is a decrease in the ESI of the seeds of S. pruriens and the incidence of ultrasound for more than 3 min caused, probably, a deleterious effect on the seeds. With the incidence of ultrasound in 3 min, 87% of germination was obtained, while the control treatment showed an efficiency of 80%, the germination rate reported by the company producing the seeds used was 82%.
This effect was already expected, since it is known that the ultrasound provides vibrational energy, which can have a positive or negative effect on living tissues depending on the form that is applied, the intensity, the distance of application, frequency and time of exposure (Hebling et al., 1995). This effect combined with moist thermal scarification promoted a harmful effect on the seed, regardless of the exposure time.
In general, the dormancy breaking methods show a good increase in S. pruriens germination (Wutke et al., 1995; Fortes et al., 2010; Oliveira et al., 2017). However, in this study, the germination did not present significant difference (p< 0.05), only vigor was influenced by the scarification methods. The mechanical scarification processes were not promising in relation to the absence of scarification, WS and MS did not produce significant effects on germination (Table 3). For vigor, when the seeds were exposed to ultrasound (Table 5), there was no significant difference among treatments for breaking dormancy, except for 2 min of exposure, when MS had superior results.
Therefore, WS is simpler than WS and TS, which are operationally more expensive (requiring a lot of time; energy and labor), being viable only for small amounts of seeds, about 1 to 10 kg (Bianchetti et al., 1997), making it impossible for use in large areas. Also, in MS and TS methods, some care must be taken regarding the intensity, time and form of application, to avoid injuries that reduce vigor, causing seedling abnormality, seed mortality or serving as an entry point for infections by fungi and bacteria (Franke and Baseggio, 1998).
Conclusions
The wet thermal scarification associated with the use of ultrasound is not suitable for seeds of black mucuna, putting at risk the germination vigor. The use of ultrasound had positive effects for both emergence speed index and germination vigor of black mucuna seeds. Without considering seed preparation method (WS, MS or TS) the emergence speed index can be improved by applying ultrasound for 3.14 min. The best results for germination vigor of mucuna seeds was observed when applying ultrasound for 4.5 min, without scarification treatments. One advantage is that there is one less operation.
Literatura citada
Abud, H.; Reis, R. and Teófilo, E. 2009. Morphological characterization of fruits, seeds, seedlings and germination of Mucuna aterrima Piper & Tracy. Rev. Ciên. Agron. 40(4):563-569. https://www.researchgate.net/publication/236893302-Morphological-characterization-of-fruits-seeds-seedlings-and-germination-of-Mucuna-aterrima-Piper-Tracy. [ Links ]
Ambrosano, E. J.; Wutke, E. B.; Salgado, G. C.; Rossi, F.; Dias, F. L. F.; Tavares, S. and Otsuk, I. P. 2016. Caracterização de cultivares de mucuna quanto a produtividade de fitomassa, extração de nutrientes e seus efeitos nos atributos do solo. Cadernos de Agroecologia. 11(2):1-10. http://revistas.aba-agroecologia.org.br/index.php/cad/article/view/21087. [ Links ]
Angeletti, M. Dá, P.; Souza, J. L.; Costa, H.; Souza, G. S.; Ewald, M. C.; Brememkamp, C. and Bahiense, D. V. 2016. Utilização de espécies vegetais como cobertura de solo no sistema plantio direto e como adubação verde na região serrana do es Rev. Científica Intelletto Venda Nova do Imigrante. Espirito Santo. Brasil. 2(1):87-102. https://biblioteca.incaper.es.gov.br/digital/handle/123456789/2987. [ Links ]
Bianchetti, A.; Teixeira, C. A. D. y Martins, E. P. 1997. Tratamentos para superar a dormencia de sementes de pinho-cuiabano (Parkia multijuga Benth). EMBRAPA-CPAF. Rondonia. https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/700857. [ Links ]
Câmara, F. M. D. M.; Pereira, E. C.; Carneiro, J. V.; Oliveira, H. T. B.; Silva, R. M. and Pereira, G. A. 2015. Métodos alternativos na superação de dormência em sementes de flamboyant. Agropecuária Científica no Semiárido. 11(3):76-83. http://revistas.ufcg.edu.br/acsa/ index.php/ACSA/article/view/683. [ Links ]
Castro, G. S. A.; Crusciol, C. A. C.; Negrisoli, E. and Perim, L. 2011. Sistemas de produção de grãos e incidência de plantas daninhas. Artigos - Planta Daninha 29 (spe):1001-1010. https://www.scielo.br/scielo.php?pid=S0100-83582011000500006&script=sci-abstract &tlng=pt. [ Links ]
Cruz, J. C.; Pereira Filho, I. A.; Pimentel, M. A. G.; Coelho, A. M.; Karam, D.; Cruz, I. and Matrangolo, W. J. R. 2011. Produção de milho na agricultura familiar. (Embrapa milho e sorgo. Circular técnica, 159). Sete Lagoas: Embrapa Milho e Sorgo. 45 p. https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/905143. [ Links ]
Fortes, A. M. T.; Silva, P. S. S. and Brassal, V. A. 2010. Germinação de sementes de mucuna-preta após tratamentos para a superação da dormência. Rev. Varia Scientia Agrárias. 1(2):11-19. http://e-revista.unioeste.br/index.php/variascientiaagraria/article/view/2708. [ Links ]
Franke, L. B. and Baseggio, J. 1998. Superação da dormência de sementes de desmodium incanum. E lathyrus nervosus lam. Rev. Brasileira de Sementes. 20(2):182-186. https://www.scielo.br/scielo.php?script=sci-nlinks&ref=000088&pid=S01013122200900 040001500006&lng=en. [ Links ]
Gordon, A. G. 1963. The use of ultrasound in agriculture. Ultrasonics. 1(2):70-77. https://www.sciencedirect.com/science/article/abs/pii/0041624X6390057X. [ Links ]
Hebling, S. A. and Silva, W. R. 1995. Effects of low intensity ultrasound on the germination of corn seeds (Zea mays L.) under different water availabilities. Scientia Agrícola. 52(3):514-520. https://www.scielo.br/scielo.php?script=sci-arttext&pid=S010390161995000300017 &lng=pt&tlng=pt. [ Links ]
Maguire, J. D. 1962. Speed of germination aid in selection and evaluation for seedling emergence and vigor 1. Crop Sci. 2(2):176-177. https://acsess.onlinelibrary.wiley.com/doi/abs/10.2135/cropsci1962.0011183X000200020033x. [ Links ]
Mello, I. S.; Duarte, G. S. D. y Neto, G. G. 2015. Sinopse de fabaceae caesalpinioideae para a flora de Mato grosso, Brasil. Biodiversidade. 14(2):43-49. http://periodicoscientificos.ufmt.br/ ojs/index.php/biodiversidade/article/view/2892. [ Links ]
Oliveira, J. D.; Silva, J. B. y Alves, C. Z. 2017. Tratamentos para incrementar, acelerar e sincronizar a emergência de plântulas de mucuna-preta. Rev. Ciênc. Agron. 48(3):531-539. https://www.scielo.br/scielo.php?pid=S1806-66902017000300531&script=sci arttext&tlng=pt. [ Links ]
Pereira, G. F.; Porto, B. S. M.; Silva, W. J.; Mendonça, M. Z. M.; Aquino, J. D.; Sousa, N. S. y Vieira, T. C. 2020. Superação de dormência de três espécies vegetais nativas do cerrado brasileiro. Rev. GeTeC. 8(22):18-41. http://www.fucamp.edu.br/editora/index.php/getec/article/view/2036. [ Links ]
Ragassi, C. F. y Melo, R. A. C. E. 2017. Recomendações para manejo da compactação do solo no contexto da produção integrada do pimentão no distrito federal. (Embrapa hortaliças. Comunicado técnico, 115). Brasília, DF. 1-9 pp. https://www.embrapa.br/busca-de-publicacoes//publicacao/1067874/recomendacoes-para-manejo-da-compactacao -do-solo-no-contexto-daproducao-integrada-do-pimentao-no-distrito-federal. [ Links ]
Ramos, A. R.; Felisberto, P. A. C.; Timossi, P. C. y Costa Netto, A. P. 2018. Características agronómicas da mucuna-preta em diferentes épocas de sementeira. Rev. Cienc. Agrar. 41(4):1051-1058. http://www.scielo.mec.pt/scielo.php?script=sci-abstract&pid=S0871-018X2018000400020&lng=pt&nrm=iso. [ Links ]
Ramos, A. R.; Felisberto, P. A. C.; Costa-Netto, A. P. and Timossi, P. C. 2019. Épocas de colheita e semeadura afetam a germinação de mucuna-preta. Global Sci. Technol. 12(1). https://rv.ifgoiano.edu.br/periodicos/index.php/gst/article/view/1102. [ Links ]
Silva, G. B. F.; Azania, C. A. M.; Novo, M. C. S. S.; Wutke, E. B.; Zera, F. S. and Azania, A. A. P. M. 2012. Tolerância de espécies de mucuná a herbicidas utilizados na cultura da cana-de-açúcar. Planta Daninha. 30(3):589-597. https://www.scielo.br/scielo.php?script=sci-arttext&pid=S0100-83582012000300015&lng=en&nrm=iso. [ Links ]
Teodoro, M. S.; Santos, F. J.; Lacerda, S. M. N. and Araújo, L. M. S. 2015. Utilização de palhadas de adubos verdes em compostos orgânicos documentos embrapa meio-norte. ISSN 0104-866X; 234). Embrapa Meio-Norte. https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1053019/utilizacao-de-palhadas-de-adubos-verdes-em-compostos-organicos. [ Links ]
Venâncio, R. S. S. and Martins, A. C. G. 2019. Overcoming dormancy of senna multijuga seeds with an ultrasonic probe the comparison with ultrasound and sulfuric acid baths. Ciência rural, 49(9):1-9. e20180904. Epub september. https://www.scielo.br/scielo.php?script=sci-arttext&pid=S0103-84782019000900302. [ Links ]
Venâncio, R. S. S.; Piña-Rodrigues, F. C. M. and Martins, A. O. 2016. Técnicas alternativas de quebra de dormência: uso do ultrassom de baixa frequência em sementes de senna multijuga (Rich.) H. S. Irwin e Barneby: perspectivas em ciências tecnológicas. 5(5):28-42. http://fatece.edu.br/arquivos/arquivos%20revistas/perspectiva/volume5/2.pdf. [ Links ]
Yaldagard, M.; Mortazavi, S. A. and Tabatabaie, F. 2008. Application of ultrasonic waves as a priming technique for accelerating and enhancing the germination of barley seed: optimization of method by the Taguchi approach. Journal of the Institute of Brewing. 114(1):14-21. https://onlinelibrary.wiley.com/doi/abs/10.1002/j.2050-0416.2008.tb00300.x. [ Links ]
Wutke, E. B.; Maeda, J. A. and Pio, R. M. 1995. Superação da dormência de sementes de mucuna-preta pela utilização de “calor seco”. Sci. Agric. Piracicaba. 52(3):482-490. https://www.scielo.br/scielo.php?pid=S0103-90161995000300013&script=sci-abstract &tlng=pt. [ Links ]
Received: April 2021; Accepted: August 2021











 texto em
texto em